Abstract
Background and Aims:
This is the first double-blind multicenter study examining the effectiveness of sacral nerve stimulation in a significant number of fecally incontinent patients.
Methods:
A total of 34 consecutive patients (31 women), median age 57 years (range, 33–73 years), underwent sacral nerve stimulation for fecal incontinence. After implantation, 27 of 34 patients were randomized in a double-blind crossover design to stimulation ON or OFF for 1-month periods. While still blinded, the patients chose the period of stimulation (ON or OFF) that they had preferred. The mode of stimulation corresponding to the selected period was continued for 3 months (final period). Outcome measures were frequency of fecal incontinence and urgency episodes, delay in postponing defecation, score severity, feeling of improvement, preference for ON or OFF, quality of life, and manometric measurements.
Results:
In the crossover portion of the study, the self-reported frequency of fecal incontinence episodes was significantly reduced during the ON versus the OFF period (P = 0.03), and this symptomatic improvement was consistent: 1) with the patients feeling of greater improvement during the ON versus OFF period (P = 0.02); 2) with the significant preference of patients (P = 0.02) for the ON versus OFF period. In the final period of the study, the frequency of fecal incontinence episodes decreased significantly (P = 0.005) in patients with the stimulator ON. The ability to postpone defecation (P = 0.01), the score for symptom severity (P = 0.0004), and the quality of life (P < 0.05) as well as anal sphincter function significantly improved.
Conclusions:
The significant improvement in FI during the ON versus OFF period indicated that the clinical benefit of sacral nerve stimulation was not due to placebo.
This double-blind multicenter study, examining the effectiveness of sacral nerve stimulation in patients with fecal incontinence, demonstrated significant improvement in fecal incontinence during the ON versus the OFF period, indicating that clinical benefit of sacral nerve stimulation was not due to placebo.
Fecal incontinence (FI) remains a therapeutic problem in many patients when conservative measures (ie, medical treatment, biofeedback) fail and sphincter repair is unsuccessful or inappropriate. Biologic or artificial neosphincters are a therapeutic option in these cases, but these treatments have a significant failure rate and high associated morbidity.1,2 Sacral nerve stimulation (SNS), which has been successfully used for urologic incontinence,3,4 is an alternative approach. Its clinical results appear to be excellent, with an approximate overall 80% success rate, in fecal incontinent patients with a neurologically intact sacral plexus and an anatomically intact anal sphincter and rectum.5–13 Despite the clinical benefit of this technique, the mechanism of action of SNS is unclear; it does not seem to be due to a placebo effect because of the absence of deterioration in clinical benefit over time in the medium term.7,11,12 However, until now, all studies, except one, were performed without a randomized control group, making it impossible to completely exclude a placebo effect. In addition, the double-blind crossover trial was performed in only 2 patients.14
Therefore, the goal of our study was to investigate the efficacy of permanently implanted SNS in a significant number of patients with FI in a randomized, double-blind, crossover trial.
PATIENTS AND METHODS
Patients
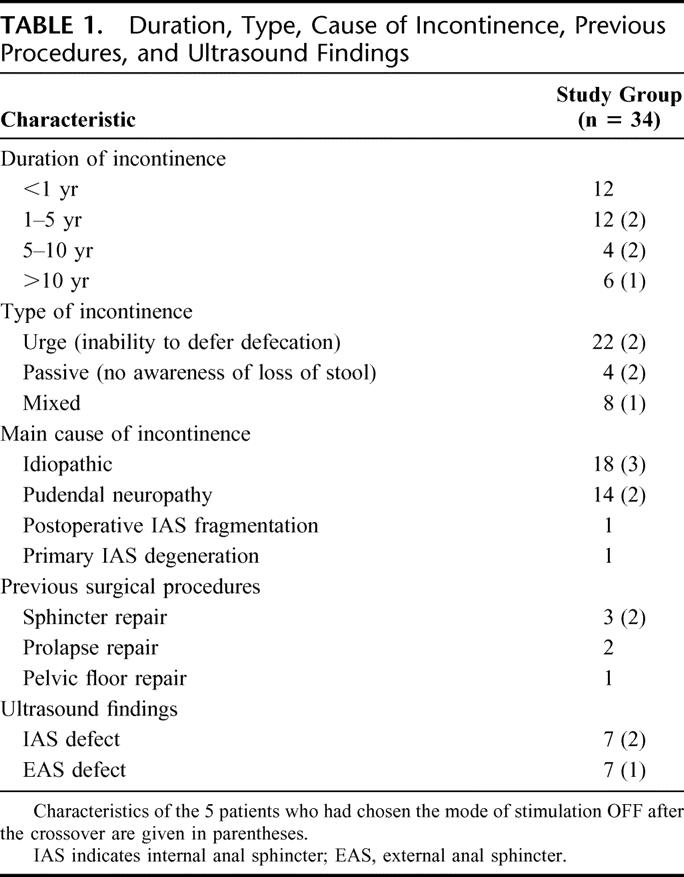
From February 2000 to February 2003, a multicenter, prospective randomized trial was performed. Thirty-four patients (31 women; median age, 57 years; range, 33–73 years), underwent implantation of a permanent sacral nerve electrode and stimulator. All patients had FI to solid or liquid stools (or urgency episodes causing patients to remain at home to avoid incontinence accidents) at least once per week, documented on a prospectively recorded diary card, for at least 3 months. Conservative treatment (ie, medical treatment or biofeedback) had failed in all patients. Patient characteristics are presented in Table 1. Patients with external anal sphincter damage on ultrasound were included in the study if the defect was not considered to be the main cause of FI (ie, limited defect, ≥30° or limited to 1 part, superficial, middle or deep part, of the external anal sphincter). All patients had at least a demonstrable unilateral bulbo(clitorido)-cavernosus reflex, indicating existing conducting pathways between the sacral plexus and the pelvic floor. Written informed consent was obtained from all patients before entry into the study. The protocol was approved by the Ethics Committee of Haute-Normandie (France).
TABLE 1. Duration, Type, Cause of Incontinence, Previous Procedures, and Ultrasound Findings
Methods
Before permanent implantation, patients underwent temporary percutaneous stimulation to assess their probable response to treatment. At the discretion of the surgeon, temporary stimulation was performed with a temporary, percutaneously placed, test stimulation lead (Medtronic Interstim model 3057, Minneapolis, MN) or by placement of a permanent quadripolar lead (Medtronic Interstim model 3093). Both types of leads were connected to an external pulse generator (Medtronic Interstim model 3625). All patients were tested for between 8 and 15 days. The decision to progress from temporary to permanent stimulation with the implantation of a stimulating system was made on the basis of at least a 50% reduction in the number of episodes of incontinence per week and/or a 50% reduction in the number of fecal urgencies per week. This was documented in a symptom diary card that described the number of FI episodes, urgencies, and the ability to postpone defecation before and during the test. All patients satisfied the criteria for permanent stimulation. The surgical procedure and equipment used for permanent electrode and stimulator implantation have been described previously.8 Patients with temporary test stimulation lead underwent simultaneous implantation of the quadripolar lead and the pulse generator; those with a lead already in place underwent removal of the percutaneous extension (Medtronic Interstim model 3095) before placement of the pulse generator (Medtronic Interstim model 3023) subcutaneously, below the superficial fascia, in the upper part of buttocks ipsilateral to the permanent electrode. The lead contained 4 contact electrodes. The electrode combination, which allowed the patient to have the best perception of the perineum muscle and anal sphincter contraction, was chosen for permanent stimulation. Stimulation was continuous with a pulse width of 210 microseconds, a frequency of 14 pulses per second, and a current amplitude adapted to the patient's perception of perineal and anal sphincter muscle contraction. The stimulator was left on during defecation and urinary voiding.
The study design is given in Figure 1. After permanent implantation, each patient had a 1- to 3-month phase when the stimulator was turned ON (postimplantation period) to optimize the effectiveness of stimulation by determining the most effective parameters of stimulation (choice of stimulation electrodes, intensity of stimulation) for each patient. At the end of the postimplantation period, patients were randomized in a double-blind crossover design to ON or OFF stimulation for a 2-month period. Each patient was randomized by using a random number table. One investigator, who was not involved in assessment of the clinical outcome, turned the stimulator ON or OFF at the beginning of the first 1-month period, without the patient's or other investigator's knowledge. At the end of the first month, the neurostimulator was programmed to the opposite mode (OFF or ON), and monitoring continued for a second month. There was no interval between the 2 treatment periods. At the end of the double-blind crossover period, while still blinded, the patients chose the period of stimulation (ON or OFF) they had preferred. The mode of stimulation corresponding to the selected period (ON or OFF) was then continued for 3 months (final period). If the patient could not choose 1 of the 2 periods, the stimulator was turned ON. The total duration of the study was between 6 and 8 months. In our opinion, patients were really blinded to the kind of stimulation (ON or OFF) for 2 reasons: first, the patients did not know that the stimulator was turned OFF during the study. The information given to the patient was that 2 different stimulations would be tested during 2 periods of 1 month each. The patient was told that for this reason: it was not always possible to perceive stimulation. Second, SNS is thought to function like neuromodulation and not by direct stimulation. Direct stimulation of the third sacral nerve evokes contraction of perineal muscles and requires high levels of electrical current. Neuromodulation acts via afferent neurons to evoke recovery of the control and coordination system and uses a low level of electrical current. Consequently, stimulation was set at a level that corresponded to a sensory threshold. At this level, it is usual that feeling is vague and that it disappears in a few minutes even though stimulation remains effective.14 To have the same conditions for the ON and OFF periods, the parameters were set the same way, first with perceptible stimulation to determine the sensory threshold and then, for the OFF period, the stimulator was switched off at the end of the session. For these 2 reasons, it was not possible for the patient to suspect that stimulation was switched off; and if results were ineffective, the patient considered that there had been an unsatisfactory adjustment simulation parameter. Throughout the study and for ethical reasons, the patient had a handheld programmer (to interrupt or to start stimulation, to increase or decrease stimulation) (Medtronic, Interstim model 3031A), but he was asked not to use it except in case of urgency. If the patient used his programmer, he was supposed to inform the investigator. In addition, the physicians in charge of setting the stimulator could check, at the end of each period (ON and OFF period), that the stimulator was still switched ON or OFF depending on the period concerned and consequently that no change had occurred in the kind of stimulation (ON or OFF).
FIGURE 1. Design of the study and number of patients enrolled and randomized.
All patients kept a diary card before stimulation (baseline period), during the temporary test stimulation period, during the postimplantation period, during each of the 2 1-month periods, and during the final period. Each assessment period, except for the temporary test, lasted at least 3 weeks. Patients recorded episodes of FI, fecal urgency, delay in postponing defecation, and bowel movements. The delay in postponing defecation was coded in the following way: 1, delay less than 5 minutes; 2, delay between 5 and 15 minutes; 3, delay more than 15 minutes. At the end of the postimplantation period, of each crossover periods, and of the final period, the patients had to give their opinion by indicating, in a binary way, if they felt they had improved or not.
Severity of incontinence was graded by the Cleveland Clinic Continence Scoring System15 obtained before stimulation (baseline period), at the end of the postimplantation period, of each crossover periods, and of the final period. The score ranged from 0 (normal continence) to 20 (maximum incontinence).
We assessed quality of life (QOL) with the French version of the American Society of Colon and Rectal Surgeons quality-of-life questionnaire for FI (FIQL).16 The French version of the FIQL questionnaire has been recently validated transculturally.17 In the questionnaire, 4 separate QOL domains were explored, including lifestyle, coping/behavior, depression/self-perception, and embarrassment.
Anal manometry was done according to our standard technique with a balloon catheter.18 Measurements of maximum resting pressure and maximum squeeze pressure were recorded at the baseline period, at the end of each crossover periods, and of the final period. Rectal sensation to balloon distension with air was performed at the baseline and final periods. The smallest amount of distension felt by the patient (ie, the threshold of conscious rectal sensation), the distending volume eliciting a call to defecate (constant sensation), the maximum tolerable volume were then determined.
Statistics
The primary outcome measured during the crossover period was the difference in symptomatic and manometric data between stimulation ON compared with OFF. The mean number of weekly incontinence and urgency episodes, the mean delay for postponing defecation were determined for each patient diary, and these data, in addition to the Cleveland Clinic score and to manometric data, were then further assessed by the Wilcoxon signed rank test. Each clinical outcome measurement was considered for a treatment effect, a period effect (order of period ON and OFF of crossover), a time effect by performing a non parametric test of Wilcoxon type. Correlations between symptomatic and manometric data were measured by the Spearman correlation test. A secondary outcome, the patient's preference for stimulation ON or OFF, was analyzed by using the McNemar test. In addition, we used the Mainland-Gart test to compare the percentages of patients who felt they had improved at each period of the crossover (stimulation ON and OFF). The results were reported as a median and range and were considered significant for P < 0.05.
For postimplantation and final periods, the primary outcome measurements were the changes between baseline and the postimplantation period and the final period for symptomatic and manometric data (only for the final period). The Wilcoxon signed rank test was used for paired comparisons of frequency of FI and urgency episodes, mean delay in postponing defecation, Cleveland Clinic score, manometric data, and FIQL scores. Correlation analyses between frequency of symptoms, Cleveland Clinic score, and manometric results or FIQL scores used the Spearman's correlation coefficient. We used the Mainland-Gart test to compare the percentages of patients who felt that they had improved when the stimulator was ON. The results were reported as a median and range and were considered significant for P < 0.05. During the final evaluation, the patients who chose the mode of stimulation OFF were not taken into account for the statistical analysis because they were too few of them. The analysis was performed with the Statview program (SAS Institute, Berkeley, CA).
RESULTS
Discontinuations
In the randomized control trial, 27 patients (79%) were randomized and completed the 2-month crossover treatment. Subsequently, 24 (89%) completed the study (Fig. 1). Ten of 34 patients prematurely discontinued the trial: 7 before the crossover period and 3 during the final period. The 2 main reasons for discontinuation were device-related adverse events (4 device explantations: 3 for unresolved pain and 1 for recurrent infection) before the crossover period and protocol violation in 3 after the crossover period (patients used the handheld programmer). Other reasons for discontinuation (before the crossover period) were insufficient therapeutic response in 1, no return to follow-up in 1, and adverse event not related to the SNS in 1 (stroke).
Crossover Period
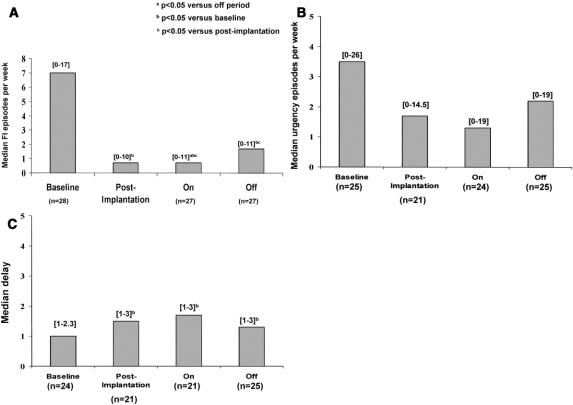
Only 1 of the 27 patients who participated in the crossover period asked to cross from OFF to ON prematurely because of poor results during the OFF period. There was a significant treatment effect with a decrease in median frequency of FI episodes between stimulation ON and OFF (P = 0.03), without any interaction between treatment and order effect (P = 0.6) or time effect (P = 0.5) (Fig. 2A). There was no significant change in the frequency of urgency episodes (Fig. 2B), the delay in postponing defecation (Fig. 2C), and the number of bowel movements per week (10.2; range, 2–32 during the ON period versus 11.1; range, 5–33) during the OFF period) between the 2 modes of stimulation. Cleveland Clinic score fell from a median of 16 at baseline (range, 8–20) to 9 (range, 0–19) after implantation (P = 0.0002) and tended toward an improvement when the stimulation was ON (8.5; range, 3–18) versus OFF (10.5; range, 4–17), although this did not reach statistical significance (P = 0.2). Despite a more marked improvement in symptoms during the ON crossover period, patients also seem to have improved during the OFF period. When crossover data were compared with baseline, the median frequency of FI episodes decreased by 90% during the ON period (P = 0.0003) but also by 76% during the OFF period (P = 0.001) (Fig. 2A). Defecation postponement (Fig. 2C) and the Cleveland Clinic score improved markedly between the baseline and crossover periods whatever the mode of stimulation ON (P = 0.008 and P = 0.0005, respectively) or OFF (P = 0.003 and P = 0.0004, respectively). The significant symptomatic improvement observed during the OFF crossover period could not be explained by a time effect during the crossover trial (delay to postpone defecation, P = 0.7; Cleveland Clinic score, P = 0.2).
FIGURE 2. Clinical results according to the different periods of the study (baseline, postimplantation, crossover). Number of patients and ranges are given in brackets. A, Fecal incontinence episodes per week. B, Urgency episodes per week. C, Delay to postpone defecation.
Twenty-four patients (89%) felt that they had improved during the ON crossover period compared with 17 (63%) during the OFF period. Four patients (0.1%) could not decide if they had improved or not (3 during the OFF period and 1 during the ON period) (P = 0.02). Before the end of the crossover period and always in a double-blind manner, 18 patients expressed a preference for the stimulation ON, whereas 6 preferred stimulation OFF and 3 had no preference (P = 0.02). Symptomatic results of patients throughout the study, according to their choice of stimulation (stimulation ON or OFF at the end of crossover), are shown in Table 2. Patients' choice for stimulation ON could be justified by a more marked symptomatic improvement during the ON than the OFF crossover period. That was not the case for those who had chosen the OFF stimulation (Table 2).
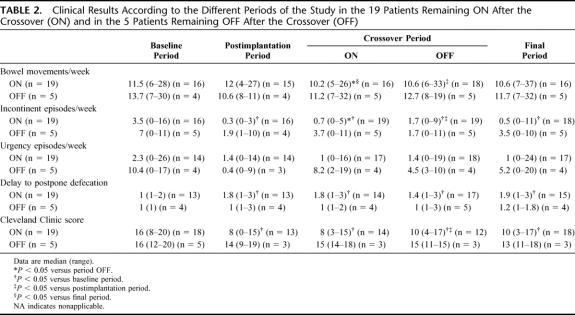
TABLE 2. Clinical Results According to the Different Periods of the Study in the 19 Patients Remaining ON After the Crossover (ON) and in the 5 Patients Remaining OFF After the Crossover (OFF)
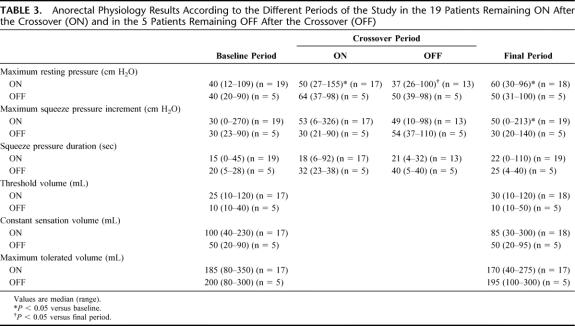
Anorectal physiology results during the crossover period are shown in Figure 3. Maximum anal resting pressure, squeeze pressure increment, and duration of voluntary contraction did not change significantly during the ON period compared with the OFF period of the crossover. However, maximum anal resting pressure only increased significantly compared with baseline during the ON period (P = 0.02). There was a significant increase in maximum squeeze pressure increment during both the ON (P = 0.004) and the OFF (P = 0.01) periods of the crossover compared with baseline results. There was no correlation between changes in symptomatic data (ie, frequency of FI and urgency episodes, delay to postpone defecation, Cleveland Clinic score) and changes in manometric parameters (ie, anal resting pressure, amplitude and duration of anal voluntary contraction), whatever the mode of stimulation.
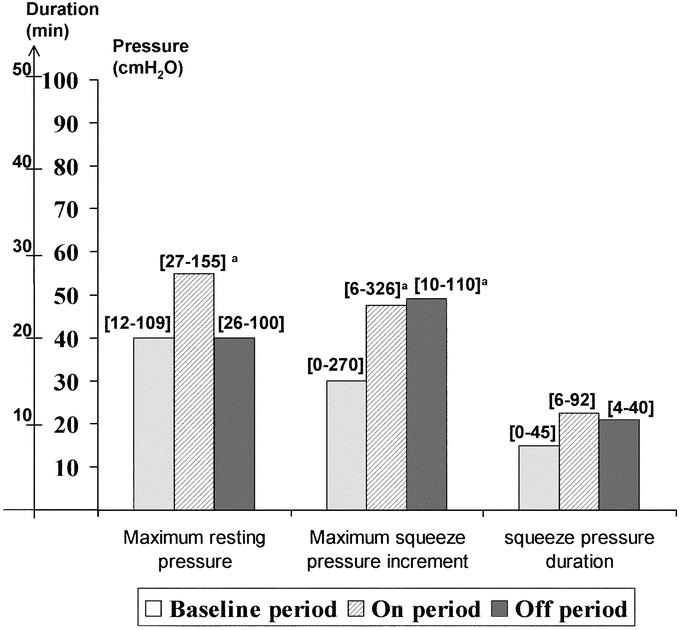
FIGURE 3. Anorectal physiology results after implantation and during the crossover period. Ranges into brackets.
Global Effectiveness of the SNS (Comparison Between Baseline, Postimplantation, and Final Periods in Patients Remaining ON After the Crossover)
Nineteen patients had their stimulator left ON after the crossover: 18 chose this mode of stimulation, 1 was indifferent to the mode of stimulation and was left ON according to the protocol. The 2 other patients who expressed no preference between the 2 modes of stimulation and consequently who should have been included in the stimulated patients group stopped the protocol prematurely because of protocol violation.
All patients except 2 of the 19 who had their stimulator left ON had improved continence during the final period. Continence was fully restored in 5 (26%) patients. Two patients improved during the postimplantation period and the ON period of crossover, but then their symptoms recurred and were similar to those experienced before implantation for unknown reasons. Postimplantation and final periods results are summarized in Table 2. Episodes of FI during the postimplantation period (P = 0.001) and the final period (P = 0.005) decreased compared with baseline. The ability to postpone defecation increased during these periods compared with baseline (P = 0.01), whereas the frequency of urgency episodes was not significantly different. Cleveland Clinic scores improved during postimplantation (P = 0.002) and final visits (P = 0.0004).
Seventeen patients (89%) felt they had improved during the final evaluation compared with the baseline period (P = 0.001). Additionally, assessment with the FIQL questionnaire showed significant improvement in the 4 QOL domains that were explored between the baseline and the final follow-up periods. Median (range) baseline and final values for lifestyle, coping/behavior, depression/self-perception, and embarrassment were 1.7 (1–3.8) and 3.2 (1.9–4; P = 0.001), 1.5 (1–2.8) and 2.7 (1–4; P = 0.002), 2.2 (1–4.1) and 3.6 (1.8–4.2; P = 0.009), and 1.3 (1–3) and 2.3 (1–4; P = 0.002), respectively. Some categories of the FIQL questionnaire were correlated with an improvement of FI symptoms: depression/self-perception and frequency of urgency, r = −0.5, P = 0.049; depression/self-perception and Cleveland Clinic score, r = −0.5, P = 0.03; embarrassment and frequency of episodes of FI, r = −0.5, P = 0.047; embarrassment and delay to postpone defecation, r = 0.6, P = 0.03; embarrassment and Cleveland Clinic score, r = −0.6, P = 0.02.
Manometric results at baseline and during the final period in patients with their stimulators turned ON are shown in Table 3. Median (range) maximal anal resting pressure (P = 0.006) and maximum squeeze pressure (P = 0.05) increased significantly during the final visit compared with baseline. There was no significant change in the rectal sensation to balloon distension. There was no correlation between baseline frequency of FI and urgency episodes, delay in postponing defecation, Cleveland Clinic score and anal resting pressure, maximal squeeze pressure, squeeze pressure duration, threshold, constant sensation, and maximum tolerated volumes. Additionally, there was no correlation between changes in the frequency of urgency episodes, delay in postponing defecation, Cleveland Clinic score, and changes in anal resting pressure, maximal squeeze pressure, squeeze pressure duration, threshold, constant sensation, and maximum tolerated volumes between the baseline and final periods. However, the decrease in the frequency of FI episodes was correlated with the increase in squeeze pressure duration between baseline and the final period (r = −0.6, P = 0.02). This was not true for changes in the frequency of FI episodes and other manometric parameters.
TABLE 3. Anorectal Physiology Results According to the Different Periods of the Study in the 19 Patients Remaining ON After the Crossover (ON) and in the 5 Patients Remaining OFF After the Crossover (OFF)
Details About 5 Patients Remaining OFF After the Crossover and Comparison With Patients Remaining ON
Among the 6 patients who had chosen the OFF mode of stimulation at the end of the crossover, 5 continued the study and 1 was excluded because of a protocol violation. Patient characteristics are given in Table 1. There was no clear difference between baseline anorectal symptoms, FIQL score and manometric data in the 5 patients who had chosen the OFF mode of stimulation and those who had chosen the ON mode of stimulation at the end of crossover (Table 2).
All of the 5 patients who had chosen the OFF mode of stimulation except 1 had improved continence at 6 months and 2 became fully continent. However, the stimulator of 2 of the patients who felt they had improved during the final period was switched ON later because of a reappearance of symptoms. Clinical results of these 5 patients throughout the study are given in Table 2. The number of FI and urgency episodes per week decreased after implantation (Table 2).
During the final evaluation, 3 of 5 patients stated that they had improved compared with baseline. Only 2 of 5 patients were assessed with the FIQL questionnaire during the final visit. An improvement was seen in each of the 4 domains of QOL (lifestyle, coping/behavior, depression/self-perception, and embarrassment) in only 1 of these 2 patients.
Manometric results at baseline, the ON and OFF periods of crossover, and the final period are shown in Table 3. The manometric variables did not change between the baseline and the final period.
DISCUSSION
This randomized, double-blinded, multicenter crossover study demonstrated a significant efficacy of SNS on FI in 27 patients. To our knowledge, only 1 randomized, placebo-controlled trial (stimulation versus sham stimulation) has been reported, to date, on the efficacy of the SNS for the treatment of FI, but this was only performed in 2 patients.14 The results of the crossover period of our study showed a consistently positive outcome (significantly decreased frequency of FI episodes, a feeling of improvement, and a preference for ON stimulation). The results of the crossover were less marked for other symptoms of anorectal disorders (ie, there was no significant difference in either the frequency of urgency or delay to postpone defecation and consequently in the Cleveland Clinic score, between ON and OFF periods) or manometric parameters (ie, anal resting pressure, squeeze pressure). Several factors may explain these findings. If more patients had been enrolled, the statistics would have been more robust. Second, symptomatic and manometric improvement, which was also observed during the OFF period, may have biased the results of the crossover period. Indeed, all the OFF periods considered (crossover OFF period or patients who chose an OFF mode of stimulation to finish the study) showed a symptomatic and manometric improvement (only during the crossover OFF period) compared with baseline, even if it was less significant than during ON stimulation. We cannot completely exclude the possibility of a placebo effect to explain this result. However, whereas some studies indicate that stimulation rapidly elicits a clinical effect and benefit rapidly disappears if stimulation is stopped,14,19 others have suggested that SNS involves modifications of neuronal plasticity.20,21 These modifications could be a the cause of a delayed effect, which would explain persistent improvement despite OFF stimulation. Thus, it is not rare to observe patients who have long-term improvement after temporary nerve stimulation, who prefer not to have implantation. This hypothesis is supported by the case of the 2 patients who felt they had improved during the final period even though stimulation had been stopped, but who later asked to change their stimulation parameters because of recurrent symptoms. The duration of crossover may not have been long enough to obtain a significant difference between the ON and OFF periods for all symptoms of FI (ie, urgency and delay to postpone defecation) and for manometric changes.
During the final period, sustained improvement occurred in multiple variables of symptom outcome (ie, significant decrease in the frequency of FI episodes, increase in delay to postpone defecation, and improvement of the Cleveland Clinic score) in patients who chose the ON mode of stimulation, compared with baseline period. These results are consistent with earlier studies of SNS in FI.5–13 We did not confirm the reduction in the frequency of urgency that has previously been described,8 whereas there was a significant improvement in the ability to postpone defecation.
In addition to the feeling of improvement in 89% of patients in the ON mode during the final period, patients also felt that there was a significant improvement of their QOL, which supports other studies.10–13,22,23 The FIQL scores improved significantly in all domains compared with baseline. In addition, the correlation between variables such as depression and embarrassment and symptoms (ie, frequency of FI episodes, of urgency and delay to postpone defecation) and Cleveland Clinic score emphasized the emotional improvement that accompanies recovery from incontinence. However, Rothbarth et al24 determined that the QOL is altered when the Cleveland Clinic score is above the threshold value of 9/20. There was a contradiction between the Cleveland Clinic score, which remained relatively high during the final period (10/20), and the FIQL scores, which were significantly improved during the same period. The significant but relatively weak improvement of the Cleveland Clinic score could be because gas incontinence is rarely improved by SNS, or by most of the treatments of the IF. In addition, 2 other criteria, used to calculate the Cleveland Clinic score, wearing protection and the impact on social life (probably less sensitive than an evaluation by the QOL score), often require more than 6 months to change, even if the treatment is effective, because the patient takes time to gain confidence in himself. Nevertheless, these results raise the problem of the choice of criteria to evaluate treatment and the need for systematic assessment of QOL to assess the real impact of any treatment of FI.25 For this reason, we recommend that future placebo-controlled protocols include an evaluation of QOL even during the crossover period.
The most consistent physiologic finding found in later studies with larger numbers of incontinent patients treated by SNS is an increase in anal squeeze pressure.10,11,23 The effect on the internal anal sphincter and on rectal sensation remains unclear: some studies report modifications in resting pressure9,10 or rectal sensation,11,23 whereas others show no significant change.6,8,10 In our study, during the final period, patients with the stimulator ON showed a significant increase in resting and anal squeeze pressures, but there was no change in rectal sensation to balloon distension compared with baseline. There was a weak correlation between symptomatic changes and manometric data. The only manometric parameter that was correlated with the frequency of FI episodes was the duration of voluntary contraction. Although some patients may have a clinical benefit without any improved external or internal anal function, the manometric modifications observed and the correlation between these modifications and the manometric parameters provide additional arguments against a placebo effect of SNS.
Our trial has shown that the clinical benefit derived of SNS was not due to a placebo effect and has confirmed the short-term efficacy of this technique on continence and the QOL in patients with FI.
ACKNOWLEDGMENTS
The authors thank Jean-Francois Menard for expert statistical advice.
Footnotes
Supported by Medtronic, but study design, performance, analysis, and reporting were not influenced by Medtronic.
Drs. Leroi and Michot designed the study. All authors were involved in patient recruitment, selection, and follow-up; enrollment; data for completion; and manuscript review.
Reprints: Anne-Marie Leroi, PhD, Physiology Unit, Hôpital Charles Nicolle, 1 rue de Germont, 76031 Rouen Cedex, France. E-mail: anne-marie.leroi@chu-rouen.fr.
REFERENCES
- 1.Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45:1139–1153. [DOI] [PubMed] [Google Scholar]
- 2.Michot F, Costaglioli B, Leroi AM, et al. Artificial anal sphincter in severe fecal incontinence: outcome of prospective experience with 37 patients in one institution. Ann Surg. 2003;237:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch JL, Groen J. Sacral (S3) segmental nerve stimulation as a treatment for urge incontinence in patients with detrusor instability: results of chronic electrical stimulation using an implantable neural prosthesis. J Urol. 1995;154:504–507. [DOI] [PubMed] [Google Scholar]
- 4.Shaker HS, Hassouna M. Sacral nerve root neuromodulation: an effective treatment for refractory urge incontinence. J Urol. 1998;159:1516–1519. [DOI] [PubMed] [Google Scholar]
- 5.Matzel KE, Stadelmaier U, Hohenfellner M, et al. Electrical stimulation of sacral spinal nerves for treatment of fecal incontinence. Lancet. 1995;346:1124–1127. [DOI] [PubMed] [Google Scholar]
- 6.Malouf AJ, Vaizey CJ, Nicholls RJ, et al. Permanent sacral nerve stimulation for fecal incontinence. Ann Surg. 2000;232:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matzel KE, Stadelmaier U, Hohenfellner M, et al. Chronic sacral spinal nerve stimulation for fecal incontinence: long-term results with foramen and cuff electrodes. Dis Colon Rectum. 2001;44:59–66. [DOI] [PubMed] [Google Scholar]
- 8.Leroi AM, Michot F, Grise P, et al. Effect of sacral nerve stimulation in patients with fecal and urinary incontinence. Dis Colon Rectum. 2001;44:779–789. [DOI] [PubMed] [Google Scholar]
- 9.Ganio E, Ratto C, Masin A, et al. Neuromodulation for fecal incontinence: outcome in 16 patients with definitive implant. The initial Italian Sacral Neurostimulation Group (GINS) experience. Dis Colon Rectum. 2001;44:965–970. [DOI] [PubMed] [Google Scholar]
- 10.Rosen HR, Urbarz C, Holzer B, et al. Sacral nerve stimulation as a treatment for fecal incontinence. Gastroenterology. 2001;121:536–541. [DOI] [PubMed] [Google Scholar]
- 11.Kenefick NJ, Vaizey CJ, Cohen RC, et al. Medium-term results of permanent sacral nerve stimulation for fecal incontinence. Br J Surg. 2002;89:896–901. [DOI] [PubMed] [Google Scholar]
- 12.Matzel KE, Kamm MA, Stosser M, et al. Sacral spinal nerve stimulation for fecal incontinence: multicentre study. Lancet. 2004;363:1270–1276. [DOI] [PubMed] [Google Scholar]
- 13.Altomare DF, Rinaldi M, Petrolino M, et al. Permanent sacral nerve modulation for fecal incontinence and associated urinary disturbances. Int J Colorectal Dis. 2004;19:203–209. [DOI] [PubMed] [Google Scholar]
- 14.Vaizey CJ, Kamm MA, Roy AJ, et al. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2000;43:298–302. [DOI] [PubMed] [Google Scholar]
- 15.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood TH, Church JM, Fleshman JW, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16; discussion 16–17. [DOI] [PubMed]
- 17.Rullier EZF, Marel A, Amouretti M, et al. Validation of the French version of the Fecal Incontinence Quality of Life scale (FIQL). Gastroenterol Clin Biol. 2004;28:562–568. [DOI] [PubMed] [Google Scholar]
- 18.Leroi AM, Berkelmans I, Denis P, et al. Anismus as a marker of sexual abuse: consequences of abuse on anorectal motility. Dig Dis Sci. 1995;40:1411–1416. [DOI] [PubMed] [Google Scholar]
- 19.Kenefick NJ, Vaizey CJ, Cohen CR, et al. Double-blind placebo-controlled crossover study of sacral nerve stimulation for idiopathic constipation. Br J Surg. 2002;89:1570–1571. [DOI] [PubMed] [Google Scholar]
- 20.Hamdy S, Enck P, Aziz Q, et al. Spinal and pudendal nerve modulation of human corticoanal motor pathways. Am J Physiol. 1998;274:G419–G423. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Hassouna MM. Neuromodulation reduces c-fos gene expression in spinalized rats: a double-blind randomized study. J Urol. 2000;163:1966–1970. [PubMed] [Google Scholar]
- 22.Ripetti V, Caputo D, Ausania F, et al. Sacral nerve neuromodulation improves physical, psychological and social quality of life in patients with fecal incontinence. Tech Coloproctol. 2002;6:147–152. [DOI] [PubMed] [Google Scholar]
- 23.Jarrett ME, Varma JS, Duthie GS, et al. Sacral nerve stimulation for fecal incontinence in the UK. Br J Surg. 2004;91:755–761. [DOI] [PubMed] [Google Scholar]
- 24.Rothbarth J, Bemelman WA, Meijerink WJ, et al. What is the impact of fecal incontinence on quality of life? Dis Colon Rectum. 2001;44:67–71. [DOI] [PubMed] [Google Scholar]
- 25.Damon H, Dumas P, Mion F. Impact of anal incontinence and chronic constipation on quality of life. Gastroenterol Clin Biol. 2004;28:16–20. [DOI] [PubMed] [Google Scholar]