Abstract
Objective:
To determine if growth hormone (GH) and glutamine (Gln) might allow for a reduction in parenteral nutrition (PN) in individuals with short bowel syndrome.
Background Data:
Following massive intestinal resection, patients frequently sustain severe nutrient malabsorption and are dependent on PN for life. GH treatment with or without Gln might allow for a reduction in PN.
Methods:
A prospective, double-blind, randomized, placebo-controlled clinical trial performed in 41 adults dependent on PN. Following screening, patients were admitted to an in-house facility for 6 weeks. After 2 weeks of stabilization and dietary optimization, patients were randomized to one of 3 treatment arms (1:2:2 ratio): oral Gln (30 g/day) + GH placebo (control group, n = 9), Gln placebo + GH (0.1 mg/kg per day, n = 16), or Gln + GH (n = 16). Standard criteria based on clinical and laboratory measurements were followed to determine PN volume and content. After 4 weeks of treatment, patients were discharged and monitored; GH and GH placebo were discontinued, but the diet with Gln or Gln placebo was continued for 3 months.
Results:
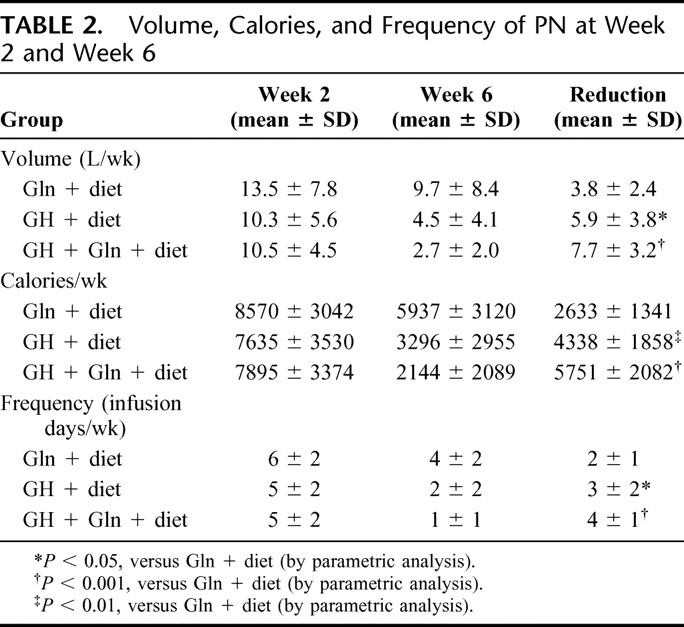
Patients receiving GH + Gln placebo + diet showed greater reductions in PN volume (5.9 ± 3.8 L/wk, mean ± SD), PN calories (4338 ± 1858 calories/wk), and PN infusions (3 ± 2 infusions/wk) than corresponding reductions in the Gln + diet group (3.8 ± 2.4 L/wk; 2633 ± 1341 calories/wk; 2 ± 1 infusions/wk, P < 0.05). Patients who received GH + Gln + diet showed the greatest reductions (7.7 ± 3.2 L/wk; 5751 ± 2082 calories/wk; 4 ± 1 infusions/wk, P < 0.001 versus Gln + diet). At the 3-month follow-up, only patients who had received GH + Gln + diet maintained significant reductions in PN (P < 0.005) compared with the Gln + diet.
Conclusions:
Treatment with GH + diet or GH + Gln + diet initially permitted significantly more weaning from PN than Gln + diet. Only subjects receiving GH + Gln + diet maintained this effect for at least 3 months.
In patients (n = 41) dependent on parenteral nutrition (PN), treatment with growth hormone (GH) ± glutamine (Gln) and an optimized oral diet allowed for significantly more weaning from PN than Gln + diet. Only those receiving GH + Gln maintained reductions at 3 months.
Short bowel syndrome is a malabsorptive disorder characterized by loss of intestinal length and consequent malnutrition. For those individuals who have less than one third of their small intestine remaining (<200 cm), parenteral nutrition (PN) is often necessary. This therapy carries a high morbidity, uses extensive healthcare resources, and diminishes the patient's quality of life. With time, many patients undergo intestinal adaptation and improve intestinal absorption, allowing them to be weaned from PN.1 However, in those individuals with extremely short segments of intestine and/or other complicating factors, independence from PN may be impossible.1,2 A program of intestinal rehabilitation has been proposed to enhance intestinal compensation and attenuate intestinal failure.3
A variety of growth factors and other substances are known to affect enterocyte function.4 Encouraged by findings in animal investigations5,6 and clinical studies7 evaluating growth hormone (GH) and glutamine (Gln), we initiated this randomized trial in PN-dependent patients with short bowel syndrome who were taking an optimal oral diet to determine whether PN requirements could be reduced through administration of GH with or without Gln.
METHODS
Patients
Patients were identified through notifying physicians and patient advocacy groups of the trial. Criteria for eligibility were: adults aged 18 to 75 years; body mass index (BMI) 17 to 28 kg/m2; small intestinal length ≤ 200 cm; the ability to ingest solid food while requiring ≥ 3000 PN calories/week; acceptable liver and kidney function; and normal or stable cardiovascular status. Patients had to have undergone bowel resection ≥ 6 months prior to study, and had intact stomach and duodenum and one or more of the following: 1) ≥ 30% of colon anastomosed to ≥ 15 cm jejunum–ileum; 2) < 30% colon anastomosed to ≥ 90 cm jejunum–ileum; or 3) < 3 L stool output/day. Total length of residual bowel was documented at the time of resection or, if operative reports were unavailable or unclear, from radiographic examinations, which were used in 2 individuals.
Exclusion criteria included pregnancy or lactation, a history of cancer within 5 years, major comorbidities, previous exposure to GH, and secretory diarrhea.
Those patients who met these screening criteria underwent a complete history and physical examination. If eligible, written informed consent was obtained. The study protocol had been reviewed and approved by the Western Institutional Review Board or the Institutional Review Board of the Nebraska Medical Center.
Study Design
The study was a randomized, double-blind, placebo-controlled, parallel-group phase III clinical trial. All participants were admitted to one of 2 treatment facilities for 6 weeks. The initial 2 weeks was a baseline period to allow observation, stabilization of medical and PN regimens using established guidelines, and education and optimization of the oral diet. If the patient was not receiving antidiarrheal or antisecretory medication, treatment with these agents was initiated during the baseline period and this fixed dose was maintained throughout the study.
After 2 weeks’ stabilization, patients were randomized to one of 3 groups (1:2:2 ratio): 1) oral Gln (30 g/day) + subcutaneous GH placebo; 2) oral Gln placebo + subcutaneous GH (0.1 mg/kg per day); or 3) oral Gln + GH (using the doses noted above).
The first group, which represented the effects of optimal dietary intake + oral Gln, served as a control. Data from previous studies3 suggested that supplementation with Gln in the absence of GH does not significantly affect nutrient absorption; thus, the use of Gln was not expected to affect the requirement for PN. In-house treatment continued for 4 weeks. Throughout the in-house period, patients were monitored by taking vital signs, weight, oral and parenteral intakes, stool and urine output, bioelectrical impedance (model 101A, RJL Systems, Clinton, MI), and selected blood concentrations at appropriate intervals. Based on pre-established weaning criteria that used these data, the PN was adjusted.
At the conclusion of the in-house period, patients were discharged home on the optimal diet with Gln or Gln placebo. Their discharge PN prescription was the same as that received during the last week of their in-house therapy. All patients were monitored by their physicians and the study team for the next 3 months. If indicated by changes in nutritional and hydration status, adjustments were made in PN. On week 18, a physical examination, body weight, nutritional history, and blood studies were obtained (Fig. 1).
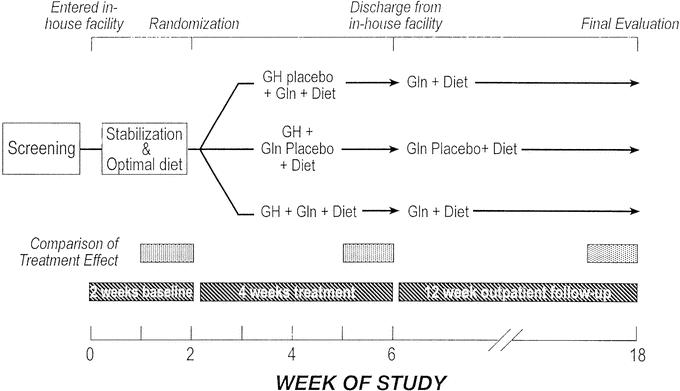
FIGURE 1. Study design.
Study Endpoints
The primary objective of this study was to evaluate the change in PN measured in week 2 (last week of baseline period) versus week 6 (last week of treatment). The primary endpoint was PN volume; secondary endpoints were changes in PN calories and frequency of administration. A second objective was to evaluate the durability of any treatment effect over time. Thus, the endpoints measured on week 18 were compared with those variables at baseline. A third objective was to evaluate the safety of treatment.
Diet and Drug Dosing
On admission to the study unit, patients received an individualized oral diet prescribed using clinical guidelines,8 which adjusted for the presence or absence of the colon. Overall, the diet was rich in protein (≈20%), low to moderate in fat (≈30%), and high in complex carbohydrate (≈50%). Simple sugars were restricted, essential fats were emphasized, and appropriate oral fluids were encouraged. Meals were provided as 6 feedings throughout the day. Calories were prescribed based upon the predicted basal energy expenditure of the individual derived from the Harris-Benedict formula. This quantity was then adjusted for activity and this total multiplied by a factor of 2 to account for malabsorption.9
Since deficiencies in some macronutrients and micronutrients are known to attenuate adaptive responses to resection and GH,10,11 assessment of vitamin (A, B12, C, folic acid, and E), mineral (magnesium, zinc, and selenium) and fatty acid concentrations was performed. If a deficiency was identified, a repletion regimen was instituted prior to randomization.
GH ∥ (somatropin of rDNA origin, 0.1 mg/kg per day, given to a maximal dose of 8 mg/day) or GH placebo was provided by Serono, Inc. (Rockland, MA). Both GH and placebo were reconstituted daily before subcutaneous administration. The protocol provided that GH or GH placebo administration could be suspended or reduced for up to 5 days if signs of toxicity occurred. Potential toxicities included severe edema, persistent headaches, and moderate-severe myalgia or arthralgia, all unresponsive to medical treatment. Clinical edema was treated symptomatically with diuretics and myalgia/arthralgia with anti-inflammatory agents, as necessary.
Gln # and Gln placebo were provided in individual packets (Nutritional Restart Pharmaceuticals, Durham, NC). Each packet contained 5 g Gln or placebo (maltodextrin, NF grade) and was indistinguishable in color, texture or taste. Each packet was mixed with a hypotonic beverage and consumed 6 times daily.
Criteria for Reducing PN
During weeks 3 to 5, PN requirements were reduced when patients demonstrated all 3 of the following: 1) maintenance of normal hydration, 2) stabilization of serum electrolyte and blood urea nitrogen (BUN), and 3) maintenance of a stable body weight. To assess these parameters objectively, enteral balance (defined as enteral fluid intake [mL] minus volume of stool output [mL]), urine volume, body weight, bioelectrical impedance analysis (used to evaluate changes in hydration), and oral calorie intake were measured daily. Serum electrolytes, BUN, and creatinine were assessed biweekly, or more frequently if indicated, to determine tolerance to the reduction in parenteral requirements. Adequate hydration was assessed by quantitating urine volume. A minimally adequate urine volume was defined as ≥ 0.5 mL/kg per hour over 24 hours on the nights the subjects did not infuse PN, or approximately 75% of their calculated minimum urine volume prior to nighttime infusion. Enteral balance, a parameter unaffected by diuretic use, was also used to determine a patient's ability to hydrate via the oral route. A minimally adequate enteral balance was defined as a positive value ≥ 500 mL/day, or the patient's calculated insensible fluid losses approximately ≥ 12 mL/kg per day.
Weaning only progressed if the patient maintained stable electrolytes and BUN, remained adequately hydrated as assessed by urine output and enteral balance, consumed adequate calories to accommodate the reduction or elimination of PN calories, and maintained a stable body weight and body water (reflected by a stable whole body resistance12) or gained weight while maintaining an appropriate ratio of body water (assessed by whole body resistance) to body weight. Reduction in frequency of infusion only occurred if the patient was capable of orally hydrating himself (herself), as defined above. During weeks 3 to 5, if a patient failed to maintain these parameters once PN had been reduced, parenteral support was increased to allow for adequate hydration and stabilization of body weight. During week 6, no additional reductions in PN were allowed.
Outpatient Follow-up
Throughout the follow-up period, PN was further adjusted, if indicated, based upon changes in weight, oral calorie intake, urine volume and serum albumin, electrolytes, BUN, and usual clinical signs of under hydration. This was performed by the patient's physician, in conjunction with the study and home-care teams. During the final week of study, the weekly infusion was based on the PN prescription, which was recorded.
Calculations
Intravenous fluid volume was determined from all measured volumes infused every 24 hours. Oral fluid volume was determined from measurement of liquid consumed from standard containers. The composition of oral intake was determined from standardized serving sizes and the portion of food consumed. The composition of the food was then determined either directly from the manufacture's product information or from a standardized computer program (Nutritionist Five, First Databank, Inc, San Bruno, CA).
Data Processing and Statistical Analysis
Based on the reduction of intravenous fluid volume, decrease in calories, and reduction in frequency observed in a previous study,13 a total of 40 patients (control, n = 8; GH, n = 16; GH + Gln, n = 16) were needed to yield 80% power for a significant F test using a one-way analysis of variance. Randomization was determined by a computer program that generated random assignment of patients entered sequentially into one of the 3 groups.
Primary and secondary variables were analyzed as a change from week 2 to week 6 using parametric methods if the normality assumptions were met; otherwise, nonparametric methods were used. A similar analysis compared the PN requirements at week 18 with week 2. An analysis of covariance model with the week 2 measurement as a single covariate was used to compare the primary efficacy endpoint (change in total PN volume) and 2 key secondary efficacy endpoints (change in PN calories and PN infusion frequency) between each of the 2 GH groups versus Gln + diet. An analysis of variance model was used to analyze other continuous efficacy endpoints between each of the 2 GH groups versus Gln + diet. The Dunnett multiple comparison procedure was used to control an overall 5% type I error rate. All statistical comparisons used 2-sided tests at the 0.05 alpha significance level.
RESULTS
Forty-six subjects entered the study. One patient withdrew consent and 4 others sustained complications during the baseline period, precluding randomization. Of the 41 subjects who were randomized, 40 completed the study. The single dropout occurred in a subject receiving GH + Gln placebo who developed an intercurrent illness unrelated to GH during active treatment. The data presented are based on an intent-to-treat analysis.
Study groups were well matched as to their characteristics, the causes of their resection and baseline PN requirements (Tables 1 and 2). During active treatment, all groups exhibited a reduction in PN dependence, with all groups significantly reducing volume, calories, and frequency of infusion (P < 0.02). However, both groups receiving GH had significantly greater reductions in intravenous support than the group receiving only Gln + diet, with the group receiving GH + Gln + diet having quantitatively the greatest change (Table 2).
TABLE 1. Comparison of the Three Groups at Initiation of Study
TABLE 2. Volume, Calories, and Frequency of PN at Week 2 and Week 6
During this period, oral intake of calories, protein, and diet constituents did not change significantly, although oral fluid intake increased similarly in all groups, presumably to compensate for the decrease in PN volume (Table 3). However, stool output did not change significantly. Urine output (mean ± SD) was 14.1 ± 4.7 L/wk at week 2 and 11.9 ± 4.6 L/wk at week 6 in the Gln + diet group; 13.4 ± 3.6 and 11.3 ± 3.9 L/wk, respectively, in the GH + diet group; and 14.7 ± 4.2 and 11.3 ± 4.1 L/wk, respectively, in the GH + Gln + diet group.
TABLE 3. Oral Intake at Baseline (Week 2) and During Treatment (Week 6)
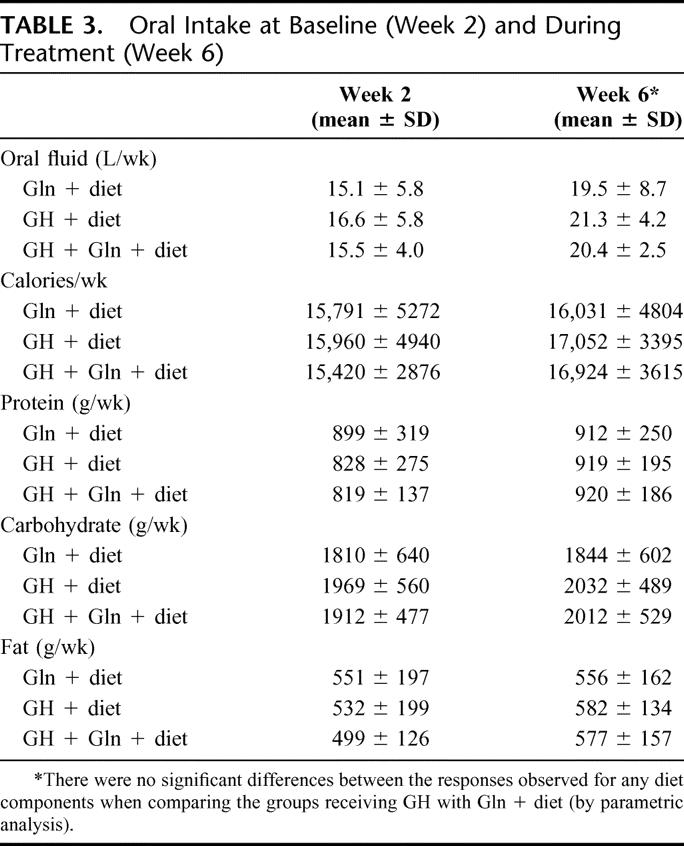
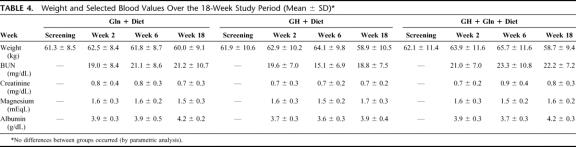
Hematologic, renal, and hepatic function studies were stable throughout the entire study period. Body weight decreased slightly (0.6 kg) in the Gln + diet group and increased in both GH-treated groups (1.16 kg in GH + diet group, P = 0.028; 1.8 kg in GH + Gln + diet group, P = 0.0024) during the 4-week treatment period.
Following discharge, volume, calories, and frequency of PN increased slightly but remained reduced relative to pretreatment values (Fig. 2). The reduction in PN requirements in the GH + Gln + diet group remained statistically significantly greater than the corresponding reduction in the Gln + diet control group (PN volume decrease 7.2 ± 4.0 L/wk for GH + Gln + diet versus 4.7 ± 4.2 L/wk for Gln + diet, P = 0.008). Serum indices of hydration and nutritional status were unchanged at 18 weeks compared with initial evaluation (Table 4). Body weight decreased from pretreatment (screening) to end of follow-up, but there was no significant difference between treatment groups. Decreases in weight were correlated with pretreatment BMI (R = 0.5, P = 0.001) and the average body weights of the groups remained within 3% of ideal body weight.
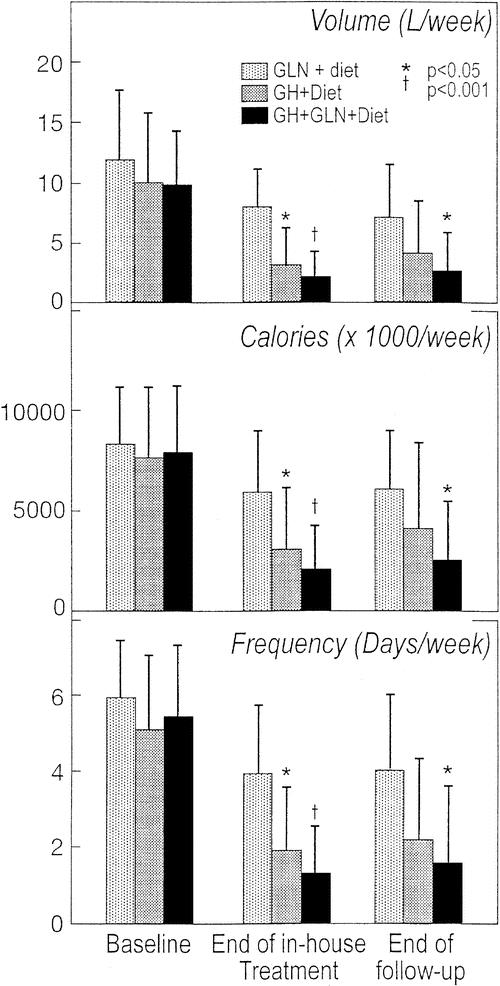
FIGURE 2. The volume, caloric content, and frequency of PN before treatment, after 4 weeks of treatment and after 3 months of home infusion.
TABLE 4. Weight and Selected Blood Values Over the 18-Week Study Period (Mean ± SD)
Minor adverse events occurred in many but not all patients. Peripheral edema occurred in 94% of subjects receiving GH versus 44% of controls, and musculoskeletal complaints were also more frequent (44% versus11%). As expected in this population, gastrointestinal complaints occurred in 67% of subjects receiving Gln + diet and in 75% of the GH groups. No serious adverse events related to GH occurred, but GH was held for several days (per protocol) in 4 patients who complained of chest pain (n = 2), severe edema and edema, headaches, and vomiting. After several days, the drug was then resumed at one-half dose and tolerated by all individuals. The dose was than returned to full dose in 2 individuals with no adverse effects. The other 2 subjects received the lower dose for approximately 1 week, until they completed the study. With discontinuation of GH, all symptoms related to the drug resolved in all subjects.
DISCUSSION
In this randomized, controlled trial, we studied the effect of GH, with or without Gln, on PN requirements to determine if patients with short bowel syndrome could be weaned from PN. Patients receiving this therapy decreased their PN requirements by 6 to 8 L/wk and 4000 to 6000 calories by week 4 of treatment compared with baseline. During treatment, patients maintained an appropriate body weight, stable electrolytes, and adequate hydration while PN was reduced suggesting that compensatory events in the residual bowel had occurred. Thus, this therapy appears useful to aid intestinal compensation in patients previously dependent on PN.
While other investigations have focused on the physiologic effects of such treatments, this study was directed toward the clinical application of this knowledge. For this reason, clinical endpoints related to the need for PN were used, and we did not directly measure the effects of treatment on nutrient absorption.
Standard criteria, such as body weight, urine volume, enteral balance, changes in body water from resistance measurements by bioelectrical impedance, renal function, and electrolyte concentrations were used to wean patients from PN. Using these criteria and the fact that all patients were taking adequate calories orally (adjusted for malabsorption), we observed a gradual reduction in PN dependence in all study subjects (Fig. 2). However, during the 4-week treatment period, patients receiving GH and GH + Gln demonstrated greater reductions in PN than those receiving Gln + diet, demonstrating the efficacy of GH and Gln in this situation. Since the oral nutrients were consumed in a relatively constant manner and weight remains stable throughout this period, these changes must represent compensation of the residual intestine. Others have demonstrated improved absorption with the administration of an appropriate diet,14 GH,15 and GH + Gln + diet.7,16,17
Following 4 weeks of in-house treatment, patients maintained their Gln + diet or Gln placebo + diet at home. Throughout the follow-up period, the patients remained adequately hydrated, with only 1 patient demonstrating signs and symptoms of dehydration related to a viral illness, rather than inadequate PN. Body weight was well maintained, with no differences observed between groups. All groups maintained their average weight between 97% and 103% of their ideal body weight.
Adjustment of the PN appropriate to the patients’ clinical condition occurred during the 3-month follow-up period. Only the group receiving GH + Gln + diet maintained a significant reduction in PN when compared with the control group. Thus, it may be possible to reduce requirements or discontinue PN in selected patients for extended periods after GH administration, if Gln + an optimal diet are provided.
Both open label3,16,17 and blinded studies15,19,20 have been performed, administering GH to patients with short bowel. Published studies vary in terms of patient selection, study design, endpoints, diet, drug dosage, use of Gln, and length of study. One investigation failed to observe a treatment effect,19 another noted improvement in some aspects of intestinal absorption,20 and the third reported improved absorption with GH.15 Patient selection may be important; in one of the negative trials, only 3 of the 8 patients studied would have satisfied entry criteria for this investigation.20 Nutritional state and diet have also been cited as key factors that must be normalized to achieve an adaptive response to GH.10,11 This was emphasized in patient preparation for our study and one other positive trial.15
The mechanisms involved in this response are multiple. Previous balance studies have shown a marked increase in fluid and sodium absorption with GH + Gln.7 In this study, enteral balance (calculated as the difference between oral fluid intake and stool output) was approximately 750 ± 1325 mL/day in the control group on week 6, a marginal quantity of fluid necessary to meet insensible losses in these 62-kg active subjects.21 In contrast, enteral balance averaged 1325 ± 1050 mL/day in the GH group and 1550 ± 700 mL/day in the subjects receiving GH + Gln. This augmented fluid absorption provided for additional water of hydration and accounted for the ability to reduce PN fluid volume by a proportional amount and maintain urine output and adequate hydration.
In humans, 3 days of GH administration increased transport velocity of amino acids in ileum,22 and GH also increased duodenal crypt cell proliferation of cultured human explants.23 These nutrient- and tissue-specific effects coupled with the known role of GH to enhance protein synthesis may have also played an important role in improved bowel function.
Gln is the primary fuel of enterocytes and in animal studies enhances the transport of sodium and water in the ileum,24 increases cellularity of intestinal mucosa in both animals5 and humans,25 and is necessary for cell signaling pathways when enterocytes are exposed to trophic agents.26 In animals, Gln and insulin-like growth factor-1 (stimulated by GH) interact synergistically to enhance adaptation of the small bowel following resection.27 Both GH and Gln cause cell swelling, a proposed mechanism for the up-regulation of intracellular protein synthesis.28
This relatively large, blinded, placebo-controlled study examined the clinical feasibility of using GH and Gln to reduce PN dependence in patients with short bowel syndrome. An earlier study has demonstrated that the single most important factor to enhance quality of life in PN-dependent patients is the elimination of 1 or more days required for nutrient infusion.29 In the current study, GH + Gln + diet reduced and maintained average infusion time to only 1 to 2 days/week from a previous schedule of 5 to 6 days/week. Earlier studies have shown that up to 40% of patients receiving this treatment can be free of PN over the long term.3 If further parenteral support is indicated at some future time, short periods of intermittent treatment with GH + Gln + diet may be desirable in selected patients.
ACKNOWLEDGMENTS
The authors thank the following: Suzanne Cox, RD, LDN, CNSD; Maria Karimbakas, RD, LDN, CNSD; Rebecca A. Weseman, RD, CNSD, LMNT; and Cindy Brown, APRN.
Footnotes
∥Zorbtive is the newly granted trade name for Serono's GH for this indication.
#NutreStore is the newly granted trade name for Gln for this indication.
Supported by Serono, Inc., Rockland, MA, and Nutritional Restart Pharmaceutical, LP, Durham, NC.
Reprints: Douglas W. Wilmore, MD, P.O. Box 1245, Kilauea, HI 96754. E-mail: dwilmore@partners.org.
REFERENCES
- 1.Gouttebel MC, Saint Aubert B, Colette C, et al. Intestinal adaptation in patients with short bowel syndrome: measurement by calcium absorption. Dig Dis Sci. 1989;34:709–715. [DOI] [PubMed] [Google Scholar]
- 2.Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043–1050. [DOI] [PubMed] [Google Scholar]
- 3.Byrne TA, Persinger RL, Young LS, et al. A new treatment for patients with short-bowel syndrome: growth hormone, glutamine, and a modified diet. Ann Surg. 1995;222:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drucker DJ. Epithelial cell growth and differentiation: I. Intestinal growth factors. Am J Physiol. 1997;273:G3–G6. [DOI] [PubMed] [Google Scholar]
- 5.O'Dwyer ST, Smith RJ, Hwang TL, et al. Maintenance of small bowel mucosa with glutamine-enriched parenteral nutrition. JPEN. 1989;13:579–585. [DOI] [PubMed] [Google Scholar]
- 6.Ulshen MH, Dowling RH, Fuller CR, et al. Enhanced growth of small bowel in transgenic mice over expressing bovine growth hormone. Gastroenterology. 1993;104:973–980. [DOI] [PubMed] [Google Scholar]
- 7.Byrne TA, Morrissey TB, Nattakom TV, et al. Growth hormone, glutamine and a modified diet enhance nutrient absorption in patients with severe short bowel syndrome. JPEN. 1995;19:296–302. [DOI] [PubMed] [Google Scholar]
- 8.Byrne TA, Veglia L, Camelio M, et al. Beyond the prescription: optimizing the diet of patients with the short bowel syndrome. Nutr Clin Pract. 2000;25:306–311. [Google Scholar]
- 9.Lennard-Jones JE. Practical management of the short bowel. Aliment Pharmacol Ther. 1994;8:563–577. [DOI] [PubMed] [Google Scholar]
- 10.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. [DOI] [PubMed] [Google Scholar]
- 11.Wilmore DW. Impediments to the successful use of anabolic agents in clinical care. JPEN. 1999;23(suppl):210–213. [DOI] [PubMed] [Google Scholar]
- 12.Scheltinga MR, Jacobs DO, Kimbrough T, et al. Identifying body fluid distribution by measuring electrical impedance. J Trauma. 1992;33:665–670. [DOI] [PubMed] [Google Scholar]
- 13.Byrne TA, Nompleggi DJ, Wilmore DW. Advances in the management of patients with intestinal failure. Transplant Proc. 1996;28:2683–2690. [PubMed] [Google Scholar]
- 14.Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet. 1994;343:373–376. [DOI] [PubMed] [Google Scholar]
- 15.Seguy D, Vahedi K, Kapel N, et al. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: a positive study. Gastroenterology. 2003;124:293–302. [DOI] [PubMed] [Google Scholar]
- 16.Wilmore DW, Lacey JM, Soultanakis RP, et al. Factors predicting a successful outcome after pharmacologic bowel compensation. Ann Surg. 1997;226:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu W, Li N, Ren J, et al. Rehabilitation therapy for short bowel syndrome. Chin Med J (Engl). 2002;115:776–778.12133556 [Google Scholar]
- 18.Deleted in proof.
- 19.Szkudlarek J, Jeppesen PB, Mortensen PB. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: a randomized, double blind, crossover, placebo controlled study. Gut. 2000;47:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scolapio JS, Camilleri M, Fleming CR, et al. Effect of growth hormone, glutamine, and diet on adaptation in short-bowel syndrome: a randomized, controlled study. Gastroenterology. 1997;113:1074–1081. [DOI] [PubMed] [Google Scholar]
- 21.Merck Manual of Diagnosis and Therapy. Water, Electrolyte, Mineral, and Acid-Base Metabolism, section 2, chapter 12. Available at: http://www.merck.com/mrkshared/mmanual/section2/sec2.jsp. Accessed June 23, 2004.
- 22.Inoue Y, Copeland EM, Souba WW. Growth hormone enhances amino acid uptake by the human small intestine. Ann Surg. 1994;219:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Challacombe DN, Wheeler EE. The trophic action of human growth hormone on human duodenal mucosa cultured in vitro. J Pediatr Gastroenterol Nutr. 1995;21:50–53. [DOI] [PubMed] [Google Scholar]
- 24.Rhoads JM, Chen W, Chu P, et al. L-glutamine and l-asparagine stimulate Na+ -H+ exchange in porcine jejunal enterocytes. Am J Physiol. 1994;266:G828–G838. [DOI] [PubMed] [Google Scholar]
- 25.van der Hulst RRWJ, van Kreel BK, von Meyenfeldt M, et al. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1336–5. [DOI] [PubMed] [Google Scholar]
- 26.Rhoads JM, Argenzio RA, Chen W, et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G43–G53. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler TR, Mantell MP, Chow JC, et al. Gut adaptation and the insulin-like growth factor system: regulation by glutamine and IGF-1 administration. Am J Physiol. 1996;271:G866–G875. [DOI] [PubMed] [Google Scholar]
- 28.Haussinger D, Roth E, Lang F, et al. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet. 1993;341:1330–1332. [DOI] [PubMed] [Google Scholar]
- 29.Rovera GM, Anderson S, Camelio N, et al. Enhancing outcome in patients receiving long term TPN. Clin Nutr. 2000;19:2. [Google Scholar]