Abstract
Objective:
To identify the most efficient parenchyma transection technique for liver resection using a prospective randomized protocol.
Summary Background Data:
Liver resection can be performed by different transection devices with or without inflow occlusion (Pringle maneuver). Only limited data are currently available on the best transection technique.
Methods:
A randomized controlled trial was performed in noncirrhotic and noncholestatic patients undergoing liver resection comparing the clamp crushing technique with Pringle maneuver versus CUSA versus Hydrojet versus dissecting sealer without Pringle maneuver (25 patients each group). Primary endpoints were intraoperative blood loss, resection time, and postoperative liver injury. Secondary end points included the use of inflow occlusion, postoperative complications, and costs.
Results:
The clamp crushing technique had the highest transection velocity (3.9 ± 0.3 cm2/min) and lowest blood loss (1.5 ± 0.3 mL/cm2) compared with CUSA (2.3 ± 0.2 cm2/min and 4 ± 0.7 mL/cm2), Hydrojet (2.4 ± 0.3 cm2/min and 3.5 ± 0.5 mL/cm2), and dissecting sealer (2.5 ± 0.3 cm2/min and 3.4 ± 0.4 mL/cm2) (velocity: P = 0.001; blood loss: P = 0.003). Clamp crushing technique was associated with the lowest need for postoperative blood transfusions. The degree of postoperative reperfusion injury and complications were not significantly different among the groups. The clamp crushing technique proved to be most cost-efficient device and had a cost-saving potential of 600 € to 2400 € per case.
Conclusions:
The clamp crushing technique was the most efficient device in terms of resection time, blood loss, and blood transfusion frequency compared with CUSA, Hydrojet, and dissecting sealer, and proved to be also the most cost-efficient device.
Clamp crushing technique for liver transection was associated with a shorter resection time, less blood loss, and a lower blood transfusion frequency compared to CUSA, Hydrojet, and dissecting sealer in noncirrhotic and noncholestatic patients. Furthermore, the clamp crushing technique proved to be the most cost-efficient device of all 4 tested transection devices regardless of the case load.
Liver resection has been increasingly performed over the last 2 decades worldwide because of improved postoperative outcomes and evidence that this approach offers the only chance of cure in many patients.1–4 Technical innovations have mainly focused on minimizing bleeding during transection of the hepatic parenchyma5,6 because excessive hemorrhage and the need for blood transfusion are associated with increased postoperative morbidity and mortality,7 as well as reduced long-term outcome.7–9 Inflow occlusion (Pringle maneuver) has been used since the early 20th century10 to prevent bleeding during parenchyma transection.11–14 The concomitant use of low central venous pressure (CVP) anesthesia further minimizes blood loss by preventing retrograde bleeding from the hepatic veins.15,16 Assuming that inflow occlusion and low CVP anesthesia cause significant damages by ischemia and reperfusion, there has been a growing interest in using new devices that facilitate bloodless transection, obviating the need for inflow occlusion.
The most popular devices facilitating bloodless transection include the ultrasonic dissector (CUSA, Tyco Healthcare, Mansfield, MA) using ultrasonic energy, the Hydrojet (Hydro-Jet, Erbe, Tubingen, Germany) using a pressurized jet of water and the dissecting sealer (TissueLink, Dover, NH) using radiofrequency energy. Parenchymal transection under routine inflow occlusion has been performed with finger fracture technique (digitoclasy),17 where liver parenchyma was crushed between the thumb and one finger isolating vessels and bile ducts, which can then be ligated and divided. This technique was subsequently improved through the use of surgical instruments such as small Kelly or Péan clamps (clamp crushing) for blunt transection.18
Some of these devices have gained wide acceptance for hepatectomy, although, to our knowledge, their efficacy has been tested in only 2 randomized controlled trials (RCTs) comparing clamp crushing technique versus CUSA19 and CUSA versus Hydrojet,20 using inflow occlusion in all cases. Both RCTs had critical limitations as Takayama et al19 included normal and cirrhotic livers and the RCT by Rau et al20 was not based on a sample size and power calculation. There is no RCT that compares the most commonly used devices to each other, such as the clamp crushing technique, CUSA, Hydrojet, and dissecting sealer. Further experience with transection devices has only been reported by lower evidence retrospective studies.21–30
It is surprising that often expensive devices are introduced in routine surgical practice without proper proof of their efficacy or superiority over simpler techniques. Especially, the economic pressure on health care costs scrutinizes the appropriateness of expensive transection devices if simpler techniques with comparable efficacy profile are available.
Therefore, in the lack of available convincing data, we designed an RCT in noncirrhotic and noncholestatic patients undergoing liver resection comparing 4 different techniques of parenchyma transection, including clamp crushing technique under inflow occlusion, CUSA, Hydrojet, and dissecting sealer. Inflow occlusion was used in the 3 latter groups only when needed (see experimental design). Endpoints, such as intraoperative blood loss, transection time, degree of reperfusion injury (as assessed by postoperative transaminase and bilirubin levels), and postoperative complications, were determined to identify the most efficient device for liver parenchyma transection in terms of safety and costs.
MATERIALS AND METHODS
Experimental Design
Between June 2003 and September 2004, 100 consecutive patients were randomized to undergo liver resection, with 1 of 4 different parenchyma transection strategies. Living donors for liver transplantation were not included in the study. The transection strategies included: 1) the clamp crushing technique under routine inflow occlusion (Pringle maneuver) and 3 other frequently used techniques without routine Pringle maneuver; 2) ultrasonic dissector (CUSA Excel, Tyco Healthcare); 3) Hydrojet (Helix Hydro-Jet, Erbe); and 4) DS3.0 dissecting sealer (TissueLink). Each patient was operated by the same hepatobiliary surgeon (P.-A.C.), and more than 30 patients were operated with each transection device prior to the initiation of the study. Eligibility criteria included partial hepatectomy (≥2 segments) for benign and malignant tumors, and acceptable clotting profile (platelet count >100 × 109/L and prothrombin activity >60%). Cirrhotic and cholestatic (serum bilirubin >100 μmol/L) patients were excluded from the study.
Surgical Procedure
The procedure was approved by the ethic committee of the University of Zurich, and an informed consent was obtained from each patient prior to surgery. Each patient was randomized the night prior to surgery using sealed envelopes (25 for each technique). The reason to randomize the day before relies on the need for the nursing staff to prepare the material when CUSA and Hydrojet were selected. The surgical team was, however, informed of the allocation only in the operating room. When liver resection could not be performed, the envelope was sealed again and returned in the pool of sealed envelopes. Major hepatectomy was defined as a liver resection ≥3 segments. All resections were performed with the requirement of a low CVP (0–5 mm Hg). Each patient underwent an intraoperative ultrasound (US) to define the tumor localization in relation to the major vascular and biliary structures. Anatomic hemi-hepatectomy was performed in a standardized manner with a selective devascularization of the resected specimen as previously described.31 A stapler device was only used for the transection of the hepatic veins. The 4 different transection techniques are defined below:
Clamp Crushing Technique
A continuous Pringle maneuver was routinely used with the clamp crushing technique. The tourniquet technique around the portal triad with a 4-mm mersilene tape was used to achieve inflow occlusion. Liver transection was performed by parenchyma crushing using a small Kelly clamp (3-mm-diameter tip). Small vessels (<2 mm) were coagulated with the irrigated bipolar forceps set at 120 Watts. All other structures including major intrahepatic bile ducts were ligated or clipped.
Ultrasonic Dissector
An ultrasonic dissector (Cavitron Ultrasonic Surgical Aspirator, CUSA) (23 kHz standard tip, cauter 70 Watt, flush 4 mL/min, sensitivity 100%) was used for parenchyma transection. Inflow occlusion was performed only in the presence of significant bleeding preventing selective coagulation or ligation of small structures or when blood loss exceeded 500 mL. The use of bipolar forceps, ligatures, and clips was identical to the clamp crushing technique.
Hydrojet
The soft liver tissue was washed off the more resistant vessels and bile ducts by a jet of water, which was pressurized by a high pressure pump and conducted by a high pressure hose to the nozzle (Helix Hydro-Jet) (30–40 bar water pressure). The use of the Pringle maneuver, bipolar forceps, ligatures, and clips were similar to the CUSA technique.
Dissecting Sealer
This device (TissueLink) couples radiofrequency with a conductive fluid to seal liver tissue to precoagulate parenchyma and isolate small and larger intrahepatic structure. The use of the Pringle maneuver, ligatures, and clips were similar to the CUSA and Hydrojet techniques. In the liver hilus and 5 mm around biliary structures, we avoided the use of dissecting sealer because of the risk of biliary leaks.
Neither intermittent clamping nor ischemic preconditioning was applied in any technique. Prophylactic postoperative drainage was only used when a bilioenteric anastomosis was performed.
Outcome Measures
Each patient was followed for 3 months. The primary endpoints were blood loss during parenchyma transection, resection time, and postoperative hepatocyte injury. The degree of postoperative hepatic injury was assessed by daily measurements of postoperative AST and ALT levels, bilirubin levels, and prothrombin times until hospital discharge. Resection time was defined as the duration between the beginning and the end of parenchyma transection. Another time was recorded until complete satisfaction with the hemostasis. Blood loss was carefully monitored until the beginning of the parenchyma transection, during transection, and after hepatectomy. The amount of blood loss was estimated by including the suction volume after subtraction of rinse fluids and weighting the swabs that were used during transection (assuming that 1 mL of blood = 1 g). As soon as liver resection was completed, the cut surface was put on a sponge and the area of liver transection surface was drawn on a sheet of paper. The paper area was cut and weight to calculate area surface according to the paper density (80 mg/m2). The data for blood loss were standardized to the transection area expressed as cm2. The speed of transection was calculated as transection area divided by transection time (cm2/min).
Secondary endpoints included the duration of inflow occlusion, the degree of liver damage by serial postoperative bilirubin and prothrombin levels, the need of blood transfusion, and morbidity and mortality according to a new classification of complications by severity,32 with an additional emphasis on bile leaks, hospital stay, and cost. The indications for blood transfusion were a massive hemorrhage (>1500 mL) during surgery or a hemoglobin level <7 g/dL within 24 hours following surgery. Each negative or adverse intraoperative and postoperative event was recorded. An abdominal US was performed at postoperative day 5 and a CT scan at 3 months to assess fluid collections.
Cost Analysis
We performed a comprehensive actual cost analysis focusing on the cost of liver resection since the study did not show significant differences in postoperative complications, ICU, and hospital stay among the groups (see below). The cost of each case was estimated considering 4 items: the costs of the device (ie, clamp crushing technique, CUSA, Hydrojet, and dissecting sealer), costs of the operating room time, costs of additional methods to control bleeding, and costs of transfused red blood cell units. The cost of each device was calculated on the basis of capital charge, maintenance of material, and disposal material. Capital charge per case was based on capital equipment with a 5-year depreciation. Since costs of capital equipment and maintenance depend on the number of cases performed per year, we provided a cost analysis on a basis of 3 different scenarios according to the center volume of 10, 50, or 100 liver resections performed per year, respectively. The price of the operating room was 466€ per hour, excluding physicians’ fees. Physician fees are calculated on the basis of the type of operation, not the duration, and are therefore equivalent among the groups. Each clip stapler device contained 20 clips per stapler (Ligaclip MCM 20 Medium clips, Ethicon Endo-Surgery, Cincinnati, OH) and purchased for 113€ per device. The price of a red blood cell unit was 127€. The costs of operating room time, clip staplers, and red blood cell transfusion were provided by our accounting department and reflect actual costs for the year 2004. Costs are expressed in Euros (€) (∼1.3 U.S.$).
Statistical Analysis
The analysis was performed in “an intention-to-treat” manner. Summary statistics are expressed as mean ± SEM or median (range), as appropriate. According to previous published data19,20 and previous experience with each device, the sample size calculation was performed with the expectation of a 30% difference in blood loss or resection time during parenchyma transection in at least 1 group with a level of statistical significance of 0.05 and a power of 0.80, using a posthoc 2-sample t test with a Bonferroni correction. We also calculate a sample size to detect a 100 IU/L difference in the opening (6 hours after surgery) or peak AST levels with a SD of 100 IU/L. These calculations included 25 patients in each group. Comparisons between groups were performed by using the ANOVA test followed by the post hoc Bonferroni test when needed. For qualitative variables, comparisons between groups were performed by the χ2 test followed by the posthoc 2 × 2 Fisher exact test, when needed. All the calculations were performed with the SPSS 12.0 statistical package (SPSS, Inc., Chicago, IL).
RESULTS
Was the Randomization Process Adequate and Were the 4 Groups Comparable?
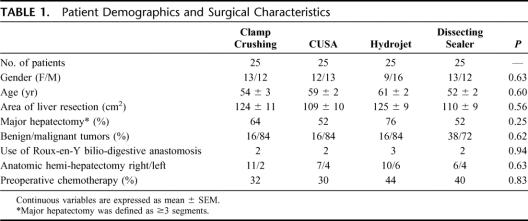
A total of 110 consecutive patients were considered for this study. Four patients refused to sign the consent form; and in 6 cases, liver resection was not performed because of extrahepatic disease or unresectable tumor found at the time of laparoscopy or laparotomy. No patient was excluded thereafter. Of the 100 randomized patients (25 each group), 53 were men and 47 were women, with a mean age of 56 ± 1 years. The mean area of liver resection was 117 ± 10 cm2. Prior to liver resection, chemotherapy was applied within 2 months of surgery in 38 (38%) of patients. Sixty-one patients (61%) underwent a major (≥3 segments) hepatectomy, of whom 34 had an anatomic right (SV–VIII) hemi-hepatectomy, 16 had a left (SI–IV) hemi-hepatectomy, 5 had extended right hemi-hepatectomy (SIV–VIII), 5 had an extended left liver resection (SI–IV), and 1 had a central hepatectomy (SIV, SV, SVIII). Thirty-three patients underwent a bi-segmentectomy, including 9 patients with anatomic SII–III bisegmentectomy. Six patients underwent an atypical wedge resection of ranging about 20% and 30% of the liver mass. Patient age, gender, the surface of liver resection, the use of chemotherapy prior resection, and the type of hepatectomy were comparable among the groups (Table 1).
TABLE 1. Patient Demographics and Surgical Characteristics
Thirty-three patients were operated for liver metastases from a colorectal carcinoma, 13 for cholangiocarcinoma, 8 for hepatocellular carcinoma, 7 for gallbladder carcinoma, and 20 for various kinds of metastatic diseases. In 19 patients, liver resection was performed for benign diseases, such as liver adenoma, focal nodular hyperplasia, echinococcosis cyst, or hemangioma. The different diagnoses were distributed homogeneously among the 4 groups.
Are Transection Time, Blood Loss, and Need for Blood Transfusion Influenced by the Resection Technique?
The overall mean transection time was 46 ± 4 minutes (median, 42 minutes; range, 14–128 minutes). The mean transection speed was 2.8 ± 1.6 cm2/min. The clamp crushing technique was associated with a significantly faster transection speed (about twice) than any of the 3 other techniques (P = 0.001) (Table 2). The overall mean blood loss was 326 ±52 mL (median, 300 mL; range, 10–1200 mL). The blood loss per resection surface was significantly lower for the clamp crushing technique than for the 3 other resection devices (about 2-fold lower; P = 0.003). Twenty-two patients (22%) received blood transfusion during or within 24 hours following surgery. There was an about 5-fold less requirement for blood transfusion in the clamp crushing group, although this did not reached significance (P = 0.06). Clips, sutures, or ligatures were used during parenchyma transection as additional methods to avoid hemorrhage and bile leaks. As shown in Table 2, the cumulative number of clips, sutures, and ligatures used per area of liver resection surface was not significantly different among groups (P = 0.53). The hemostasis time from the end of transection until completion of hemostasis was not different among the groups.
TABLE 2. Intraoperative Transection-Related Features
Does the Resection Technique Impact on the Use of Inflow Occlusion (Pringle Maneuver) and Postoperative Liver Injury?
The data are summarized in Table 3. The Pringle maneuver was applied in each patient receiving the clamp crushing technique according to the study protocol, while it was applied in only 5 patients (20%), 7 patients (28%), and 9 patients (36%) in the CUSA, Hydrojet, and dissecting sealer groups, respectively (P < 0.001 clamp crushing versus other techniques). In patients in whom Pringle was applied, the overall mean Pringle time was 28 ± 2 minutes (median, 28 minutes; range, 10–60 minutes). Evaluating only patients with the Pringle maneuver in the last 3 groups, the ischemic time was about half of the resection time.
TABLE 3. Use of Inflow Occlusion (Pringle Maneuver)
The peak of the transaminases occurred between 6 hours after surgery and the third postoperative day in each case. In most patients, AST and ALT levels returned to normal values within 7 days. There was no significant difference among the 4 groups. Postoperative peak values of AST and ALT are presented in Figure 1. Similarly, there was no significant difference among the groups regarding the postoperative prothrombin time (70% ± 4%, 71% ± 4%, 68% ± 4%, and 71% ± 13% for clamp crushing technique, CUSA, Hydrojet, and dissecting sealer, respectively, P = 0.69). Similarly, no differences were found in bilirubin levels (43 ± 7, 38 ± 7, 56 ± 8, and 43 ± 7 μmol/L for clamp crushing technique, CUSA, Hydrojet, and dissecting sealer, respectively, P = 0.12).

FIGURE 1. Peak AST and ALT levels (n = 25 in each group, P = 0.71 for AST and P = 0.80 for ALT).
Do the Respective Techniques Impact on Postoperative Complications, ICU, and Hospital Stay?
Four patients died within 30 days following major hepatectomy. Two of these patients were operated for cholangiocarcinoma and the 2 others for gallbladder carcinoma. Postoperative deaths were due in 1 case to mesenteric arterial infarction at the fifth postoperative day with an otherwise normal course, and in 3 patients to sepsis and multiorgan-failure. Death occurred in these cases between the 10th and 28th postoperative days. Two of the patients who died underwent liver resection with the CUSA, whereas the other 2 patients were operated with the Hydrojet.
Asymptomatic intraperitoneal collections were assessed with an abdominal ultrasound at postoperative day 5 and CT scan at 3 months. Only collections ≥3 cm were taken into account. The overall rate of collections was 31% and were documented in 8 (32%), 9 (36%), 6 (24%), and 8 (32%) patients in clamp crushing, CUSA, WaterJet, and dissecting sealer groups, respectively (P = 0.82). Additionally, percutaneous drainage (symptomatic biloma) was necessary in 5 patients, and 1 patient was reoperated for an infected biloma.
Complications were ranked according to our recently reported classification of surgical complications.32 The overall complication rate was 32%, including the 4 deaths (grade 5) (Table 4). There was no significant difference among the 4 groups regarding postoperative minor (grade 1 and 2) or major (grade 3, 4, and 5) complications. The overall median ICU and hospital stay were 1 day (range, 0–26 days) and 9 days (range, 4–34 days), respectively, and were not significantly different among the groups (Table 4).
TABLE 4. Postoperative Complications, ICU, and Hospital Stay in the 4 Groups
Do the Respective Transection Techniques Influence Costs?
The cost of each device is given in Table 5 according to capital equipment with a 5-year depreciation, costs of maintenance, and costs of disposal material.
TABLE 5. Material-Related Cost of Each Device (Euro [€])
The mean price for a liver resection per case is given in Table 6. The clamp crushing technique was the least expensive device and appeared to provide significant cost savings regardless of the volume of liver resections performed per year. Inversely, CUSA was the most expensive technique, but its cost equaled the cost of the dissecting sealer for high-volume centers performing 100 liver resections per year.
TABLE 6. Cost (Euro [€]) of Liver Resection per Case According to the Transection Technique
DISCUSSION
This is the first randomized controlled trial investigating the efficacy, safety, and cost-effectiveness of 4 different parenchyma transection strategies in noncirrhotic and noncholestatic patients undergoing liver resection. We found that clamp crushing technique with routine inflow occlusion was significantly associated with decrease blood loss and shorter resection time when compared with transection with CUSA, Hydrojet, or dissecting sealer. The degree of postoperative (reperfusion) liver injury as measured by serial serum levels of transaminases, bilirubin, or prothrombin times, as well as the incidence and severity of complications were comparable among all groups. Furthermore, the actual cost analysis of the use of each respective device indicated that the clamp crushing technique is the most cost-efficient strategy regardless of the volume of liver resection performed.
The study protocol was designed with careful attention to minimize potential bias. Each procedure was performed by the same surgical team with a standardized resection technique. Prior to the initiation of the study, at least 30 liver resections were performed with each transection device to exclude the negative effects of a potential learning curve. A single anesthesia team experienced in liver resection, especially in low CVP anesthesia, was involved in all cases, which enabled performance of each case under a CVP maintained between 0 and 5 mm Hg. The use of the Pringle maneuver and blood transfusion was standardized to prevent individual variations. It can also be argued that sponsoring from companies may potentially influence on the study protocol. We accepted sponsorship from the 3 device companies (CUSA, Hydrojet, and dissecting sealer) at the same amount, and the study protocol was solely prepared by the authors. The surgeons were informed about the randomization only after the decision to proceed with hepatectomy was made intraoperatively. Finally, the analysis of the data indicated excellent matching in terms of patient demographics and type of resection among the 4 groups (Table 1). The exclusion of patients with liver cirrhosis and cholestasis further secured homogeneity among the groups.
However, the experimental design of such trial is inherently associated with a few limitations. The study was only powered to evaluate blood loss, resection time, and reperfusion injury; thus, the sample size was not sufficient to detect differences in postoperative mortality and morbidity, as well as bile leak. To overcome the failure of detecting bile leak, we included routine US at the fifth postoperative day and a CT scan at 3 months. The study did not look at long-term outcome as data were collected only for 3 months after surgery. While we failed to show significant differences among the groups in terms of ischemia and reperfusion injury, ie, postoperative levels of serum liver transaminases and bilirubin were comparable in patients subjected or not to inflow occlusion, other more subtle changes were not investigated. For example, we did not look at hepatocyte apoptosis, area of necrosis, and activation of pathways of injury as postoperative liver biopsy was not included in the study protocol due to safety reason.
The central finding in this study was the significantly lower blood loss in patients undergoing liver transection with the clamp crushing technique under inflow occlusion compared with CUSA, Hydrojet, and dissecting sealer (Table 2). Although blood loss and the need of blood transfusion (mean, 300 mL; only 22% of patients received blood transfusion) compared favorably to many other series,1,2,15 the clamp crushing technique resulted in about 3- to 5-fold lower blood loss during hepatectomy and transfusion frequency than any of the other techniques. Our interpretation for the favorable results of the clamp crushing technique is that the associated inflow occlusion and low CVP very efficiently prevented bleeding enabling optimal identification of all tiny intrahepatic structures, which could be selectively coagulated, ligated, or clipped. Hemorrhage after release of the inflow occlusion was also minimal and comparable to the other techniques supporting the safety of the clamp crushing technique. Although CUSA, Hydrojet, and dissecting sealer theoretically divide liver parenchyma without vessel injury, bleedings from smaller vessels, particularly veins, do occur impairing optimal visibility of the transection plane. The finding that inflow occlusion was needed in about 20% to 36% of patients further supports the significance of hemorrhage during transection (Table 3). Similar experience was also reported by others; for example Rau et al compared liver resections with CUSA and Hydrojet in an RCT.20 Although inflow occlusion was applied to all resections, the authors reported a significant intraoperative blood loss of 1800 mL for CUSA and 1500 mL for Hydrojet. Over the recent years, we, as many others, have learned to perform hemi-hepatectomy for living donation with the Hydrojet or CUSA without inflow occlusion and intraoperative blood loss of less than 500 mL. However, these procedures are performed exclusively in healthy and usually young patients with the ability to respect perfect anatomic plan as safety margins are not an issue.
We found a comparable degree of postoperative injury (transaminase and bilirubin levels, and prothrombin time) in patients resected with the clamp crushing technique and inflow occlusion and those without inflow occlusion. The majority of patients (65%) had low AST levels (<400 U/mL) after surgery. This observation is likewise related to several factors. First, the clamp crushing technique enabled a fast resection requiring inflow occlusion for less than 30 minutes in most cases. We previously showed that ischemic time less than 30 minutes is associated with minimal reperfusion injury.33 Second, patients with particularly sensitive livers to ischemic injury, such as those with cirrhosis and cholestasis, were not included in the study. Third, the decreased blood loss in the clamp crushing technique group might result in improved liver perfusion and therefore decreased reperfusion injury. Assuming that the current data (mean AST level of 400 IU/L, SD 350 IU/L) would be used to calculate a new sample size, we would need 250 patients per group to show a significant difference between the clamp crushing technique and the other devices.
Postoperative mortality and morbidity were evaluated using a standardized classification system enabling to stratify complications by severity.32 The 4% mortality is in the range of previous reports1–3 and was related to the nonselectivity of the population studied including extensive liver resection (eg, for cholangiocarcinoma) and many patients at risk. Complications were documented in 32% of patients, with an incidence of severe complications (grades 3–5) of 16% (Table 4). The rate of minor and major complications was not significantly different among the 4 groups. This observation corresponds to the findings of the 2 published RCTs, which reported a similar overall complication profile between the use of clamp crushing, CUSA, and Hydrojet.19,20 Bile leaks remain a challenging complication, occurring in 5% to 14% of patients undergoing hepatectomy.29,34,35 Symptomatic bile leaks requiring an intervention occurred in 6% of our patients without difference among the groups. Of note, our sample size was not calculated to identify a difference in bile leaks or other complications. Since we do not use prophylactic drains, except in presence of hepato-jejunostomy, we performed an US at the fifth postoperative day and a CT scan at 3 months. The incidence of asymptomatic fluid collection was comparable among the groups ranging from 24% to 36%. Therefore, our data suggest that each technique is suitable to perform safe liver resections with a similar complication profile and a minimal risk for bile leaks.
Another important finding was the significant faster parenchyma transection with the clamp crushing technique compared with the other devices. Sakamoto et al reported similar data in a retrospective analysis comparing the clamp crushing technique with the floating ball device, which represents the first generation of the dissecting sealer.28 In contrast, the RCT by Takayama et al revealed comparable transection times in patients undergoing liver resection with clamp crushing technique or CUSA.19 However, the study population of this trial included patients with normal (48%) and chronic diseased (52%) livers, and all patients underwent routine inflow occlusion, therefore not comparable with our population. The duration of surgery is an increasingly important cost factor, and the use of the operation room is charged per minute in many countries. In addition, more procedures per day could be possible with the shorter duration of surgery in each case.
The forces of cost control on health care raise the question of how costs can be saved without reducing the quality of treatment. This issue has also special implication for the operation equipment in our study since costs of purchase, maintenance, and disposal material are largely different for the 4 transection devices investigated. Therefore, a comparative cost analysis was included into the study to estimate the appropriateness of each device in terms of costs and efficacy. The clamp crushing technique proved to be the most cost-efficient device. The cost advantage of the clamp crushing technique is not only attributed to the very low equipment costs but is also related to the shorter resection time, lower blood loss with less need for blood transfusion. The equipment costs of CUSA and Hydrojet depend strongly on the number of cases per year since both devices require a large initial purchase with lower costs for disposal material. Thus, the costs of these devices decrease by increasing the case load. However, CUSA and Hydrojet were, respectively, 3- to 6-fold and 2- to 4-fold more expensive compared with the clamp crushing technique. In contrast, the dissecting sealer caused only costs of disposal material without the need to purchase a machine, but costs were also 3 times higher than the clamp crushing technique. These data demonstrate that the clamp crushing technique has the highest cost-savings potential compared with the other devices. Even if the advantageous results of the clamp crushing technique such as shorter resection time and lesser blood loss are ignored, the cost of the equipment itself would still significantly favor the clamp crushing technique.
CONCLUSION
Liver resection with the clamp crushing technique was associated with a shorter resection time, less blood loss, and a lower frequency of blood transfusion compared with CUSA, Hydrojet, and dissecting sealer. Furthermore, the clamp crushing technique proved to be the most cost-efficient device with the highest cost-saving potential among all 4 tested devices. Based on these findings, we propose that the clamp crushing technique should be considered as standard technique in noncirrhotic and noncholestatic patients undergoing elective liver resection. The role of CUSA, Hydrojet, and dissecting sealer in patients with diseased liver still needs to be evaluated.
ACKNOWLEDGMENTS
The authors thank Tyco Healthcare (Mansfield, MA), Erbe (Tubingen, Germany), and TissueLink (Dover, NH) for their sponsorship; as well as the anesthesiologist team for its assistance in the management of the patients; the study coordinators, especially Cornelia Ortlieb, for their excellent job in collecting data; and Valentin Rousson for his assistance in the statistical analysis.
Discussions
Dr. Bismuth: Face to the number of techniques of liver transection, the search for the best one is very attractive. This justifies the work of the Clavien's group, well done, I must say, in terms of methodology. I have, however, some criticism on the choice of the techniques studied. As for the modality of clamping in the group of the clamp crushing, why do you use the continuous clamping instead of the intermittent one, which appears now to be superior in terms of tolerance for the liver parenchyma? Indeed, in your paper, your maximum ischemic time was 47 minutes, but we may need longer transection time.
My main remark is in the application of the Pringle maneuver: 100% in the clamp crushing group compared with around 30% in the other groups. It seems to me that as the blood loss is one primary endpoint of your study, this group has evidently an advantage in the comparison. I would have like to have the same use of portal clamping for all groups, which would have reinforce the value of the comparison. Paradoxically, if I follow your conclusion, the high technology tools may be overcome by a very simple tool as a clamp. Personally, I am pleased of this statement for it could be that I was the first in the early 1980s to introduce this way of liver transection. At that time, the method for liver transection was the finger fracture (digitoclasy) introduced by the Chinese surgeons. I found when I started using this technique that the occidental fingers were too large and I preferred to use a clamp, the Kelly clamp (kellyclasy).
Dr. Clavien: Thank you, Professor Bismuth, for the insightful comments and questions. The first question relates to the method of inflow occlusion. Should we have used intermittent rather than continuous inflow occlusion, as some studies have suggested better tolerance against ischemia with the intermittent inflow occlusion strategy? Of note, these studies have also shown significant bleeding during the successive reperfusion periods, which of course do not occur with continuous inflow occlusion. As the Kelly clamp technique enables a relatively quick resection, the clamping time never exceeded 50 minutes in our study with a mean of 26 minutes, we felt that the standard, still widely used, continuous inflow occlusion was preferable for the purpose of this study. The data also convincingly show that this strategy is safe, as we failed to detect increased hepatic injury compared with the 3 other techniques, where inflow occlusion was used only when needed. Your question is also very timely. As a follow-up of this study, assuming now that the Kelly technique with inflow occlusion is superior to the other strategies, we just initiated a trial testing intermittent clamping versus ischemic preconditioning using the Kelly technique for major liver resection. The second question relates to a possible bias in routinely using inflow occlusion only in the Kelly clamp group, while inflow occlusion was an endpoint in the 3 other groups. Here we need to go back to the study design and the question we wanted to address. We did not test a specific device but rather a strategy of liver resection. We and others have used the Kelly clamp technique with routine inflow occlusion for many years. Over the past 2 decades, several novel and often quite expensive devices have been marketed with the claim that they were safer and can be used without the need of inflow occlusion. Thus, we tested this hypothesis and found that for each endpoint evaluated, including cost, the Kelly clamp strategy was superior. Whether the routine use of inflow occlusion in each group would have still favored the Kelly clamp technique is not addressed by this study. However, even if similar bleeding or duration of resection would have been achieved, which I doubt, the Kelly clamp would still be the winner in terms of cost-effectiveness, particularly in low-volume centers, as shown in the paper. Thanks, Professor Bismuth, for the historical insight into the “kellyclasy” and, of course, for your many contributions to liver surgery, which have paved the way for most of us.
Dr. Belghiti: I would like to congratulate the authors because we need such studies. The surgical community needs such data to be more flexible and, according to your results, it means that we can use any kind of transection with equal effectiveness. This is a very important observation.
My first question would be that, although you are very flexible in the transection technique, why so rigid in using pedicle clamping during crush clamping alone and why not in other methods of transection?
The second point I would like to mention is that you should be using the transected liver surface area of each group to compare the results. What is your opinion regarding this?
Third, I am curious to have your interpretation on why the postoperative transaminase levels are not significantly elevated in the clamped group compared with others?
Dr. Clavien: Thank you, Prof. Belghiti, for the kind comment and questions. The first question about why we were not so flexible with the use of inflow occlusion relates to the study design. We tested the hypothesis that the “so-called” novel bloodless liver resection techniques, ie, obviating the need for routine inflow occlusion, are superior to the “classic” clamp crushing technique with inflow occlusion. Thus, we applied inflow occlusion in the 3 “bloodless” techniques only based on strict criteria. Of interest, periods of inflow occlusion were needed in about a third of patients with the “blood less” devices. Regarding your second question, we indeed standardized the data regarding resection time and blood loss to the calculated surface of resection. This is described in detail in the methodology of the paper. Finally, your third question focuses on the lack of difference in postoperative AST levels among the groups. We were also somewhat surprised with this finding, and I may suggest several explanations for this observation. First, clamping time was less than 30 minutes in half of the patients, a timing that has been previously shown to be associated with minimal detectable injury in normal liver. Second, the putative injury associated with the routine use of inflow occlusion in the Kelly group may have been counterbalanced by injury related to increased resection times and blood loss in the other groups. Of note, based on the current data, we calculated that we would need 250 patients to show any differences in postoperative AST levels.
Dr. Heberer: This is an excellent study with regard to the design, the sample size calculation, and the randomization. We sometimes have difficulties with patients to accept the randomization, particularly when a placebo arm is included. Therefore, my question is: what number of patients accepted and did not accept the randomization.
Dr. Clavien: Four patients refused to sign the study protocol, and in another 6 cases liver resection was not performed due to extrahepatic disease or unresectable tumor at the time of surgery; thus, we considered 110 patients to include 100 patients.
Dr. Höckerstedt: I want to ask 3 short questions: were the same surgeons doing these 100 resections? Since you have 4 deaths here in patients with noncirrhotic livers, I would like to know if these patients had received adjuvant therapy or anything else that could have predisposed them to some degree of liver dysfunction. And the final question is: in the next series of liver resection in patients if they are not in a randomized study, what technique would you use?
Dr. Clavien: Prof. Höckerstedt, thank you for these questions. To address your first question, I was involved in each case in this study. Regarding the second point, a dismal outcome occurred in 4 patients between 10 and 28 days after surgery, and these cases are presented in detail in the manuscript. These patients underwent an extended right hemi-hepatectomy for a cholangiocarcinoma in 2 patients and a gallbladder cancer in 2 others. One died of a mesenteric infarction of unclear etiology in an otherwise uneventful course, the 3 other patients from multiorgan failure. None had received chemotherapy, and I believe it is fair to state, based on the intraoperative data, that the dismal outcome was not related to the resection strategy. Of note, more than two thirds of patients in this series underwent major surgeries, and this mortality rate compares favorably with other expert centers. Your final question is very important, and phrased differently could sound “do you believe your data?” Yes, we do. Our current strategy in patients with normal liver, outside of a study protocol, is inflow occlusion with the Kelly clamp crushing technique.
Footnotes
Supported in an equivalent amount by Erbe (Tubingen, Germany), Tissuelink (Dover, NH), and Tyco Healthcare (Mansfield, MA). Dr. Selzner and Dr. Petrowsky are the recipients of the Novartis fellowship in HPB surgery and liver transplantation.
Reprints: Pierre-Alain Clavien, MD, PhD, Department of Visceral and Transplant Surgery, University Hospital of Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland. E-mail: clavien@chir.unizh.ch.
REFERENCES
- 1.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406–407. [DOI] [PMC free article] [PubMed]
- 3.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708–710. [DOI] [PMC free article] [PubMed]
- 4.Clavien PA. Malignant Liver Tumor: Current and Emerging Therapies. Malden, MA: Blackwell Science, 1999. [Google Scholar]
- 5.Cunningham JD, Fong Y, Shriver C, et al. One hundred consecutive hepatic resections: blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–1056. [DOI] [PubMed] [Google Scholar]
- 6.Sitzmann JV, Greene PS. Perioperative predictors of morbidity following hepatic resection for neoplasm: a multivariate analysis of a single surgeon experience with 105 patients. Ann Surg. 1994;219:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869–870. [DOI] [PMC free article] [PubMed]
- 8.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 9.Tsao JI, Loftus JP, Nagorney DM, et al. Trends in morbidity and mortality of hepatic resection for malignancy: a matched comparative analysis. Ann Surg. 1994;220:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pringle J. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huguet C, Gavelli A, Chieco PA, et al. Liver ischemia for hepatic resection: where is the limit? Surgery. 1992;111:251–259. [PubMed] [Google Scholar]
- 12.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–458. [PubMed] [Google Scholar]
- 13.Kimura F, Miyazaki M, Suwa T, et al. Evaluation of total hepatic vascular exclusion and Pringle maneuver in liver resection. Hepatogastroenterology. 2002;49:225–230. [PubMed] [Google Scholar]
- 14.Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am. 2004;84:563–585. [DOI] [PubMed] [Google Scholar]
- 15.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya S, Jackson DJ, Beard CI, et al. Central venous pressure and its effects on blood loss during liver resection. Br J Surg. 1999;86:282–283. [DOI] [PubMed] [Google Scholar]
- 17.Lin T, Tsu K, Mien C, et al. Study on lobectomy of the liver. J Formosa Med Assoc. 1958;57:742–759. [Google Scholar]
- 18.Lin TY. A simplified technique for hepatic resection: the crush method. Ann Surg. 1974;180:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama T, Makuuchi M, Kubota K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922–928. [DOI] [PubMed] [Google Scholar]
- 20.Rau HG, Wichmann MW, Schinkel S, et al. Surgical techniques in hepatic resections: ultrasonic aspirator versus Jet-Cutter. A prospective randomized clinical trial. Zentralbl Chir. 2001;126:586–590. [DOI] [PubMed] [Google Scholar]
- 21.Papachristou DN, Barters R. Resection of the liver with a water jet. Br J Surg. 1982;69:93–94. [DOI] [PubMed] [Google Scholar]
- 22.Schröder T, Hasselgren P, Brackett K. Techniques of liver resection: comparison of suction knife, ultrasonic dissector and contact neodymium-YAG laser. Arch Surg. 1987;122:1166–1171. [DOI] [PubMed] [Google Scholar]
- 23.Rau HG, Schardey HM, Buttler E, et al. A comparison of different techniques for liver resection: blunt dissection, ultrasonic aspirator and jet-cutter. Eur J Surg Oncol. 1995;21:183–187. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Ikai I, Kume M, et al. New simple technique for hepatic parenchymal resection using a Cavitron Ultrasonic Surgical Aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23:1032–1037. [DOI] [PubMed] [Google Scholar]
- 25.Taniai N, Onda M, Tajiri T, et al. Hepatic parenchymal resection using an ultrasonic surgical aspirator with electrosurgical coagulation. Hepatogastroenterology. 2002;49:1649–1651. [PubMed] [Google Scholar]
- 26.Schmidbauer S, Hallfeldt KK, Sitzmann G, et al. Experience with ultrasound scissors and blades (UltraCision) in open and laparoscopic liver resection. Ann Surg. 2002;235:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto Y, Yamamoto J, Kokudo N, et al. Bloodless liver resection using the monopolar floating ball plus ligature diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166–172. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama H, Masuda H, Shibata M, et al. Incidence of bile leakage after three types of hepatic parenchymal transection. Hepatogastroenterology. 2003;50:1517–1520. [PubMed] [Google Scholar]
- 30.Poon RT, Fan ST, Wong J. Liver resection using a saline-linked radiofrequency dissecting sealer for transection of the liver. J Am Coll Surg. 2005;200:308–313. [DOI] [PubMed] [Google Scholar]
- 31.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850; discussion 851–852. [DOI] [PMC free article] [PubMed]
- 32.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka S, Hirohashi K, Tanaka H, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484–489. [DOI] [PubMed] [Google Scholar]
- 35.Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695–698. [DOI] [PubMed] [Google Scholar]