Abstract
Virion host shutoff (vhs) is a 58-kDa protein encoded by the UL41 gene of herpes simplex virus (HSV). vhs resides within the tegument of HSV, enters the cell cytoplasm at infection, and destabilizes host cell and viral mRNA. Late in infection, vhs must be assembled into the tegument of progeny virions, a poorly understood process. Using an anti-vhs antiserum and Western blotting of total cell or cytoplasmic extracts, we found that vhs is largely insoluble in HSV-infected cells, even in the presence of high levels of salt and the detergent Triton X-100. Furthermore, a subpopulation of vhs appears to be associated with detergent-insoluble lipid rafts and this raft population is enriched in a cytoplasmic fraction which contains assembling and mature HSV particles. Our data raise the possibility that HSV tegument polypeptides associate with membrane rafts, in common with the matrix proteins of a number of other viruses.
Herpesviruses are complex particles consisting of an icosahedral capsid containing the double-stranded DNA genome, a lipid envelope which is derived from the host cell and which contains virally encoded membrane proteins, and, the tegument, an amorphous, electron-dense layer of proteins which lies between the capsid and the inner surface of the envelope (40). In the case of herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), more than 10 envelope proteins have been described previously and at least a dozen different polypeptides reside in the tegument (21, 39).
HSV capsids, like those of all herpesviruses, are assembled and packaged with DNA in the cell nucleus. They then bud into the inner nuclear membrane to generate enveloped, tegument-containing virions in the perinuclear space. Although subsequent events have been the subject of much controversy, it now appears that at least for the alphaherpesviruses, the envelopes of perinuclear virions fuse with the outer nuclear membrane, resulting in a nonenveloped cytoplasmic capsid. These capsids then bud into a cytoplasmic organelle, in the process acquiring their final envelopes and mature teguments (10, 33).
How much of the mature tegument is acquired at the initial envelopment step, how much may be lost at de-envelopment, and which tegument polypeptides are acquired only at the final envelopment step remain open questions, as does the precise identity of the organelles used for cytoplasmic envelopment (33). Harley et al. have recently shown that during a single synchronized wave of assembly and egress, HSV capsids travel to and accumulate within organelles which have the biochemical properties of the trans-Golgi network and endosomes (20), consistent with the findings of others (7, 57). Ultrastructural and biochemical examination of these organelles revealed them to contain enveloped capsids in their interiors as well as nonenveloped capsids and tegument-like electron-dense layers on the cytoplasmic face of their limiting membranes (20), consistent with the notion of at least some tegument addition in the cytoplasm. Several lines of evidence indicate that some alphaherpesvirus tegument polypeptides associate with cellular membranes via interaction with the cytoplasmic tails of envelope glycoproteins (2, 3, 15, 33).
In addition to the major polypeptides VP1/2, VP13/14, VP11/12, VP16, and VP22 (29, 39, 49, 59), the HSV tegument contains the less-abundant virion host shutoff protein vhs (13, 46). vhs is a 58-kDa phosphoprotein which is encoded by the HSV late gene UL41 (27, 28, 37, 46) and which during infection appears to form a complex with the cellular translation initiation factor eIF4H (12, 30). vhs or the vhs-eIF4H complex is an RNase (11) which suppresses host cell gene expression by accelerating the rate of turnover of cellular mRNAs (1, 24). vhs also stimulates the turnover of all kinetic classes of viral mRNA and is likely to be important in removing immediate-early and early viral transcripts to facilitate the switch to late gene expression (35). vhs is known to form a complex with VP16 (42, 47), and this may serve to dampen vhs activity late in infection. The function of vhs is critically important for viral pathogenesis; inactivation of UL41 results in a virus with greatly reduced ability to grow in trigeminal ganglia, brains, and corneas, a minimal ability to cause clinical disease, and reduced ability to establish and reactivate from latency (37, 50, 51, 52).
In the present study, we used vhs as a model tegument protein to further investigate the nature of tegument-membrane association. While subjecting infected cell lysates and cytoplasmic organelles to various extraction procedures, we were surprised to find that the majority of vhs is insoluble, even in the presence of high concentrations of salt or detergent. Furthermore, some of this insoluble vhs is stably membrane associated and, unexpectedly, partitions with Triton X-100 (TX-100)-insoluble glycolipid-enriched lipid complexes, also termed detergent-insoluble glycolipid complexes (DIGs) or lipid rafts (5, 6, 45). Although only a small proportion of total cellular vhs is raft associated, it nevertheless represents a large proportion of the vhs present in HSV-containing cytoplasmic organelles. We speculate that raft association may correlate with assembly of vhs into the tegument.
MATERIALS AND METHODS
Cells and viruses.
COS and Vero cells were grown in Dulbecco modified Eagle's medium supplemented with 1% penicillin-streptomycin and 10% fetal calf serum or 10% newborn calf serum, respectively (GIBCO Laboratories). HSV strains PAAR5 and Pvhs(−) (24) were grown and the titers of the virus were determined on Vero cells as previously described (8). HSV strain tsProt.A was grown and the titers of the virus were determined at permissive temperature as previously described (8). For expression of the lipid raft marker placental alkaline phosphatase (PLAP), COS cells were transfected with plasmid pGT60PLAP (Invivogen) by using Lipofectamine Plus reagent (Invitrogen). PLAP-expressing cells were then infected with HSV strain PAAR5 after a further 16 h and harvested 24 h postinfection.
Preparation of HSV-infected total cell lysates and PNS.
COS cells were infected at a multiplicity of infection (MOI) of 10 with HSV strain PAAR5 or tsProt.A for 1 h at 37°C and then overlaid with fresh prewarmed medium. PAAR5-infected cells were incubated at 37°C, and tsProt.A-infected cells were incubated at 31°C (permissive temperature) or 39°C (nonpermissive temperature), as appropriate, for 18 to 24 h. The cells were rinsed twice in ice-cold homogenization buffer (HBA buffer; 0.25 M sucrose, 2 mM MgCl2, 10 mM Tris-HCl [pH 7.6]), collected by scraping, resuspended in HBA buffer containing 500 μM phenylmethylsulfonyl fluoride and 25 μg of antipain/ml, and then broken by passage through a 25 5/8-gauge needle. To prepare postnuclear supernatants (PNS), these cell lysates were centrifuged at 2,000 × g for 10 min to remove nuclei.
DIG isolation.
Cell lysates or PNS were prepared as described above and then incubated with 1% TX-100 on ice for 0.5 h. They were then adjusted to 1.2 M sucrose, overlaid with 0.88 M sucrose in TNE buffer (150 mM NaCl, 5 mM EDTA, 25 mM Tris-HCl [pH 7.4]) and then with 0.15 M sucrose in TNE buffer, and centrifuged at 200,000 × g for 16 h. The gradient was then fractionated from the top, and any pelleted material at the bottom of the tube was recovered by resuspension in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer.
To disrupt lipid rafts, cells were overlaid with 10 mM methyl-β-cyclodextrin (MβCD) (Sigma) in serum-free medium for 2 h at 37°C to deplete cholesterol prior to lysate preparation. Alternatively, cell lysates were incubated with 1% TX-100 at 37°C or with 60 mM octyl glucoside on ice for 0.5 h.
Metabolic labeling of infected cell proteins.
COS cells were infected with HSV strain PAAR5 at an MOI of 10 in serum-free medium and, after 1 h, were washed with glycine-buffered saline (136 mM NaCl, 5 mM KCl, 100 mM glycine [pH 2.8]). They were then overlaid with Dulbecco modified Eagle's medium (lacking methionine and cysteine and containing 1% dialyzed fetal calf serum) and incubated at 37°C. After 2 h, the cell culture medium was supplemented with 10 μCi of 35S-radiolabeled methionine and cysteine (New England Nuclear) per ml and incubation was continued for a further 22 h before harvesting.
Western blotting and antibodies.
Extracts were boiled in Laemmli buffer and then subjected to denaturing SDS-PAGE with 10% resolving gels. Proteins were transferred to a polyvinylidene difluoride membrane at 100 V for 1 h with a Bio-Rad Trans-blot, and the membrane was blocked for 1 h at room temperature in Tris-buffered saline (150 mM NaCl, 10 mM Tris-HCl [pH 7.6]) supplemented with 0.5% Tween 20 and 5% nonfat dried milk. Membranes were then incubated overnight at 4°C with the appropriate primary antibody. The following mouse monoclonal antibodies were purchased: anti-human PLAP antibody (DAKO), anti-human transferrin receptor (TfR) antibody (Zymed Laboratories), anti-β-COP antibody (Sigma), anti-VP16 antibody (Santa Cruz Biotechnology), and anti-VP5 antibody (Advanced Biotechnologies). Polyclonal rabbit anti-glycoprotein H (gH) antisera and a monoclonal mouse anti-VP23 antibody were kind gifts from Anthony Minson and Jay Brown, respectively. Polyclonal rabbit anti-eIF5 antiserum was kindly provided by Uma Maitra. Secondary antibody detection used Western Lightning chemiluminescence reagent from Perkin-Elmer Life Sciences, Inc.
Purification of extracellular virus.
Cells were infected at an MOI of 0.025, and medium was collected when the complete cytopathic effect developed. Cell debris was removed by low-speed centrifugation and filtration. Virions were then pelleted by centrifugation at 80,000 × g for 1 h. The pellet was resuspended in HBA buffer (see above), sonicated, and layered onto a linear sucrose gradient (0.58 to 1.5 M in TNE buffer). The gradient was centrifuged at 200,000 × g for 2 h at 4°C, and the visible virus band at the center of the gradient was harvested.
Production of an anti-vhs rabbit antiserum.
A StyI-ScaI restriction fragment encoding vhs residues Lys 96 to Tyr 243 was filled in at the StyI site and cloned into the filled-in XhoI site of vector pGEX-KG (19), creating a translational fusion between residues 96 to 243 of vhs and the carboxy terminus of glutathione S-transferase (GST). Fusion protein expression was induced in a log phase culture by standard procedures (19). Since the fusion protein was found to be insoluble, it was partially purified by preparation of Escherichia coli inclusion bodies as follows. Induced cells were incubated for 0.5 h at 4°C in 0.67 M sucrose-10 mM Tris-HCl (pH 8.0)-1.5 mM EDTA-0.1 mg of lysozyme/ml and then sonicated, incubated with RNase I and DNase I (Sigma), and centrifuged at 35,000 × g for 1 h at 4°C, and the pellet was resuspended in 0.67 M sucrose-10 mM Tris-HCl (pH 8.0)-1.5 mM EDTA-0.05% TX-100. Following a second centrifugation, the pellet was washed twice by resuspension in water, overlaid with 8 M urea-20 mM Tris-HCl (pH 7.6), and incubated at room temperature. After being solubilized, the pellet was subjected to a clearing spin and urea was removed by dialysis against phosphate-buffered saline. The resulting material was electrophoresed on a preparative SDS-10% polyacrylamide gel, an acrylamide slice containing the GST-vhs fusion protein was excised from the gel, and the protein was electrophoresed from the gel slice into a dialysis bag containing 0.2 M Tris acetate (pH 7.0)-1% SDS-100 mM dithiothreitol. Eluted protein was dialyzed against 0.2 M NaHCO3-0.02% SDS and concentrated by lyophilization, and then SDS was depleted by passage through an Extracti-Gel D column (Pierce). The resulting sample contained a single polypeptide (as determined by Coomassie brilliant blue staining of an analytical SDS-PAGE gel), which ran at the expected molecular weight and reacted with anti-GST antibodies following Western blotting (data not shown). The antigen was used to immunize New Zealand White rabbits (Covance Research Products Inc.). Antiserum was tested for specificity by Western blotting.
RESULTS
Generation and characterization of an anti-vhs antiserum.
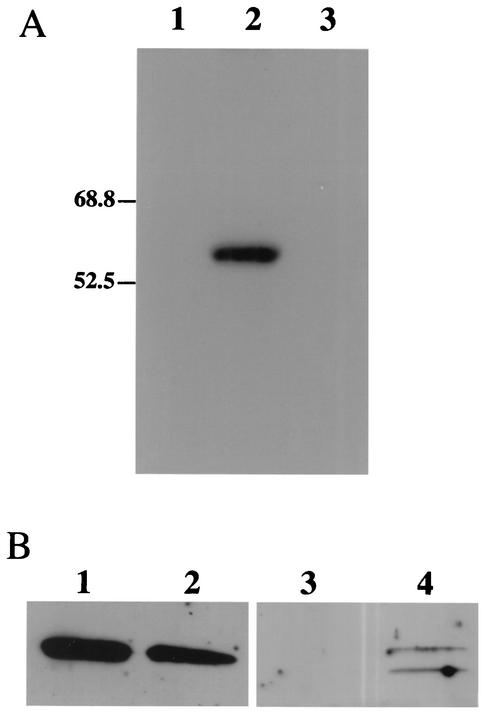
To prepare an anti-vhs antiserum, a fragment of the UL41 gene encoding vhs amino acids Lys 96 to Tyr 243 was fused to the open reading frame of GST and the resulting fusion protein was used to raise an antiserum in rabbits. In Western blots, the antiserum cross-reacted with a single polypeptide of the expected size in HSV-1-infected cells but not in mock-infected cells or cells infected with a vhs null virus (Fig. 1A). To examine the intracellular distribution of vhs, cell nuclei and a PNS were prepared from HSV-1-infected cells and subjected to Western blotting as described above. As shown in Fig. 1B, similar levels of vhs were present in the cell nuclei and the cytoplasm.
FIG. 1.
Specificity of anti-vhs antiserum and subcellular location of vhs. (A) COS cells were infected at an MOI of 20 with the vhs null virus Pvhs(−) (lane 3) or with its parental wild-type KOS-based strain PAAR5 (lane 2) or were mock infected (lane 1). Cells were harvested 20 h later and subjected to SDS-PAGE and Western blotting using the anti-vhs antiserum. Lanes 2 and 3 were loaded with 9.3 × 106 and 6.6 × 106 PFU of HSV, respectively. (B) Western blot similar to that shown in panel A, except that nuclear (lanes 1 and 3) and postnuclear (lanes 2 and 4) fractions were prepared and probed with either anti-vhs antiserum (lanes 1 and 2) or, as a fractionation control, with antiserum against the exclusively cytoplasmic translation factor eIF5 (18) (lanes 3 and 4).
vhs is insoluble in HSV-infected cells.
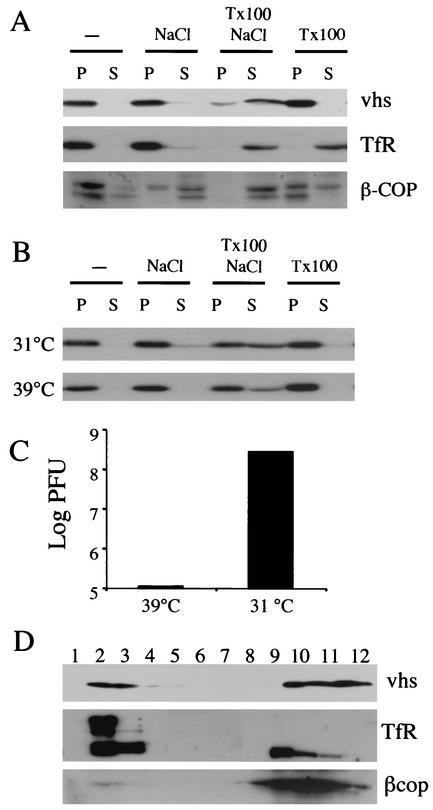
To study the intracellular properties of vhs during infection, COS cells were infected at an MOI of 10 with HSV-1 strain PAAR5 for 16 h at 37°C and a PNS was prepared. Insoluble material including cytoplasmic organelles was recovered by centrifugation at 18,600 × g, and the pellet and supernatant were subjected to SDS-PAGE and Western blotting. In Western blots, vhs was seen mostly in the insoluble fraction (Fig. 2A). To further characterize this insolubility, the PNS was incubated with 1% TX-100 and/or 0.5 M NaCl prior to centrifugation. Whereas the integral membrane protein TfR was solubilized by TX-100 and the peripheral membrane protein β-COP (44) was partially solubilized by either TX-100 or NaCl, vhs required incubation with both TX-100 and NaCl to become substantially solubilized (Fig. 2A).
FIG. 2.
vhs is largely insoluble in infected cells. (A) PNS were prepared from HSV PAAR5-infected cells at 16 h postinfection and incubated on ice for 0.5 h either alone (−) or in the presence of 0.5 M NaCl or 1% TX-100 or NaCl and TX-100 together as shown at the top of the figure. Following centrifugation at 18,500 × g, the resulting pellet (lanes P) and supernatant (lanes S) were subjected to SDS-PAGE and Western blotting and probed with antibodies against vhs, TfR, and β-COP, as indicated at the right side of the panel. (B) Cells were infected with HSV strain tsProt.A for 18 h at 31 or 39°C as indicated, and then PNS were collected and treated as described for panel A, followed by Western blotting with anti-vhs antibodies. (C) To confirm that capsid maturation had been blocked in the experiment described for panel B, samples of the PNS prepared as described for panel B were assayed for levels of PFU. (D) PNS from HSV PAAR5-infected cells was incubated with 100 mM Na2CO3 (pH 11.5) on ice for 0.5 h and loaded at the bottom of a sucrose flotation gradient (see Materials and Methods). After centrifugation and fractionation, an equal volume of each fraction was subjected to SDS-PAGE and Western blotting, as indicated at the right side of the panel. Gradient fractions are numbered from the top (lane 1) to the bottom (lane 12).
We considered the possibility that vhs may be pelleting under these conditions because of its incorporation into viral particles. To test this, we made use of the HSV strain tsProt.A, which is unable to assemble mature capsids and particles at 39°C (8, 17, 36) although L particles (which contain tegument and envelope proteins) can still form (38). COS cells were infected at an MOI of 10 with tsProt.A for 16 h at 31°C (permissive temperature) or 39°C (nonpermissive temperature), and a PNS was prepared and examined as described above. vhs was found to behave the same way at both temperatures (Fig. 2B) despite a decrease of greater than 3 log in the number of infectious particles formed at the nonpermissive temperature (Fig. 2C). This suggests that vhs insolubility under these conditions is not due to its association with capsids.
Insolubility may be due to the interaction of vhs with pelletable proteinaceous structures such as elements of the cytoskeleton or large protein complexes and aggregates. In addition, or as an alternative, it may reflect association of vhs with membranous organelles. These two possibilities can be distinguished by buoyant density gradient centrifugation. To test whether any of the pelletable vhs was stably associated with membranes, we incubated an infected-cell PNS with Na2CO3 at pH 11.5, which is a rigorous means of removing peripheral proteins from cellular membranes (16). The treated PNS was then adjusted to 1.4 M sucrose and loaded at the bottom of a 0.8 M-1.2 M-1.4 M sucrose density step gradient identical to that previously used to isolate HSV-containing organelles (20). Following centrifugation, lipid membranes float to the 0.8 M-1.2 M interface in gradient fractions 2 and 3. As can be seen in Fig. 2D, a substantial portion of the cellular vhs was (like the integral membrane protein TfR) stably membrane associated, even following alkaline extraction; however, the peripheral protein β-COP was substantially depleted from the membrane fraction. The presence and abundance of a higher-molecular-weight form of the TfR differed somewhat between experiments, but its size suggests that it corresponds to the intact TfR dimer, which is not always completely disrupted during SDS-PAGE (26).
vhs is associated with DIGs.
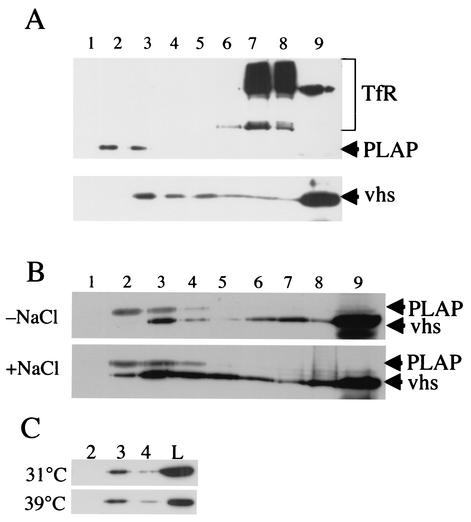
Since at least some of the cellular vhs appeared to be TX-100 insoluble (Fig. 2A) and membrane associated (Fig. 2D), we questioned whether vhs is associated with DIGs, also known as lipid rafts (5, 6, 45). To test this, we made use of membrane solubilization and centrifugation conditions that have been developed to specifically separate rafts from other lipids. HSV-infected cells were incubated with 1% TX-100 at 4°C and then adjusted to 1.2 M sucrose and overlaid with steps of 0.88 M and 0.15 M sucrose. Under these conditions, centrifugation leads to the flotation of DIGs to the upper portion of the gradient, whereas solubilized membranes remain at the bottom (14, 25, 43). Immunoblots of gradient fractions were then probed with antibodies raised against TfR (known not to reside in lipid rafts) (41) and the membrane raft marker PLAP (4, 41) (endogenous levels of PLAP were undetectable under these conditions, so these studies were conducted using COS cells transiently transfected to express elevated levels of this protein). As anticipated, PLAP migrated to the lower-density portion of this gradient, where rafts were expected to accumulate, whereas TfR stayed at the bottom (Fig. 3A). A small proportion of vhs was also seen to float to the low-density regions of the gradient, although the bulk of vhs remained at the bottom (Fig. 3A). Densitometric analysis of several independent Western blots indicated that between 3 and 5% of total cellular vhs was present in the raft region of these gradients (data not shown). However, the distributions of vhs and PLAP within these gradients do not completely overlap. This may be because PLAP (a plasma membrane protein) and vhs (presumably organelle associated) must reside in different populations of lipid rafts. This issue is further addressed in the Discussion section.
FIG. 3.
A subpopulation of vhs is localized to detergent-insoluble membranes. (A) COS cells were transfected to express PLAP and then infected with HSV PAAR5. After 24 h, cell lysates were prepared, incubated with 1% TX-100 for 0.5 h on ice, and then loaded at the bottom of a sucrose density gradient optimized for the separation of DIG fractions (see Materials and Methods). Fractions were collected from the top of the gradient (fraction 1) to the bottom (fraction 9, which also included any insoluble pelleted material) and subjected to SDS-PAGE and then Western blotting with antibodies, as indicated at the right side of the panel. (B) COS cells were infected with PAAR5 and cell lysates were treated as described for panel A, except that TX-100 solubilization was in the presence or absence of 0.5 M NaCl, as indicated at the left side of the panel. Gradient fractions were subjected to Western blotting for vhs or PLAP, as indicated at the right side of the panel. The locations of vhs and PLAP polypeptides are indicated at the right side of the panel. (C) COS cells were infected with tsProt.A at 39 or 31°C (as indicated at the left side of the panel), and cell lysates were prepared, incubated with 1% TX-100 at 4°C, and then gradient fractionated and subjected to Western blotting for vhs, as described for panel A. The results for the raft-containing fractions 2, 3, and 4 and 10% of the total amount of vhs loaded on the gradient (panel L) are shown.
Inspection of the vhs primary amino acid sequence reveals no amino-terminal signal sequence, no apparent membrane spanning domain, and no known motifs for fatty acid or GPI (glycosylphosphatidylinositol) anchor addition. Its apparent interaction with membranes in general (Fig. 2D) and DIG rafts in particular (Fig. 3A) is therefore surprising and unexpected. The simplest explanation for these observations is that vhs is associated with viral or cellular polypeptides which are themselves membrane and raft associated. To attempt to demonstrate this, PNS preparations were incubated with 0.5 M NaCl during membrane solubilization in TX-100 to disrupt any such possible protein-protein interaction. However, despite the fact that TX-100 and NaCl together did solubilize most of the cellular vhs (Fig. 2A), this treatment had no apparent effect on the association of vhs with rafts (Fig. 3B). Similarly, disrupting mature virus particle assembly by blocking capsid maturation had no effect on raft association, as shown by examining vhs from tsProt.A-infected cells following replication at permissive and nonpermissive temperatures (Fig. 3C).
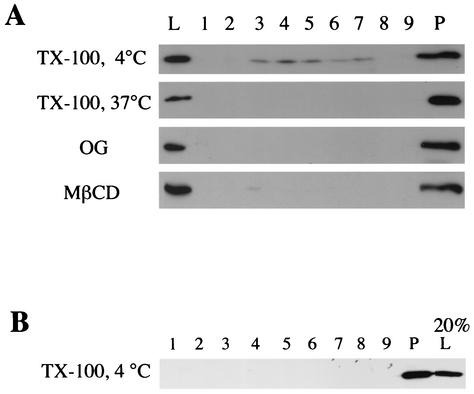
As a further means of testing whether the floating portion of vhs truly corresponds to the vhs present in lipid rafts, we examined whether this population of vhs was affected by conditions known to disrupt DIGs. Raft-associated proteins are known to be solubilized by incubation with 1% TX-100 at 37°C or by incubation with 60 mM octyl glucoside at 4°C (4). Indeed, either of these treatments prevented vhs from moving to the raft position of the density gradients (Fig. 4A). Rafts are also known to be disrupted following depletion of cholesterol from membranes by using agents such as MβCD (23). As can be seen in Fig. 4A, incubation of HSV-infected cells with 10 mM MβCD prior to TX-100 extraction also diminished the buoyancy of vhs. These results imply that a small subpopulation of cellular vhs is present as a raft-associated protein. Interestingly, vhs in purified virions did not appear to be raft associated to any detectable level (Fig. 4B), suggesting that the small raft-associated population of vhs plays no role in HSV infectivity but instead may serve a function earlier in the virus life cycle.
FIG. 4.
Flotation of vhs is inhibited by agents which disrupt lipid raft formation. (A) Cell lysates were prepared from HSV PAAR5-infected COS cells and incubated for 0.5 h with 1% TX-100 at 4°C, 1% TX-100 at 37°C, or 60 mM octyl glucoside (OG) at 4°C, as indicated at the left side of the panel. Alternatively, HSV PAAR5-infected COS cells were incubated with 10 mM MβCD for 2 h and then cell lysates were prepared and incubated with 1% TX-100 at 4°C as described above. Lysates were then subjected to sucrose gradient centrifugation, and fractions were prepared and subjected to Western blotting for vhs as described for Fig. 3A. (B) Extracellular virus was purified from PAAR5-infected COS cell medium, incubated with 1% TX-100 at 4°C, and processed exactly as described for panel A.
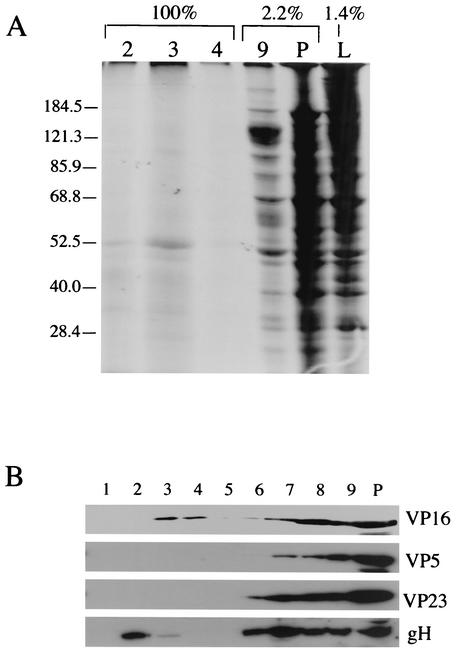
We were concerned that the floating population of vhs might represent nonspecific trapping of this protein by floating rafts. Although the failure of TfR to float under these conditions argues against this (Fig. 3A), we sought to determine whether there was any measurable nonspecific bulk trapping of proteins in DIG fractions. To investigate this issue, HSV-infected cells were metabolically labeled with [35S]methionine and cysteine. Cell lysates were then solubilized and centrifuged on raft gradients exactly as described above. All of the raft-containing gradient fractions (fractions 2, 3, and 4) (Fig. 5A, lanes 2, 3, and 4) and small proportions of fraction 9 (solubilized material; lane 9), of pelleted insoluble material (lane P), and of the load material (lane L) were then subjected to SDS-PAGE and autoradiography. Note that lanes 2, 3, and 4 of Fig. 5 represent the DIG-associated polypeptides present in 71 times the amount of PNS in lane L. It thus appears that little or no bulk trapping of general polypeptides into rafts occurs under these conditions.
FIG. 5.
The bulk of infected cell proteins do not associate with the DIG-containing fractions. (A) PAAR5-infected COS cells were metabolically labeled using [35S]methionine and cysteine. Cell lysates were prepared and then incubated with 1% TX-100 at 4°C and gradient fractionated as described for Fig. 3A. One hundred percent of fractions 2, 3, and 4, 2.2% of fraction 9 and of pelleted insoluble material (lane P), and 1.4% of the quantity of material originally loaded onto the sucrose gradient were then subjected to SDS-PAGE and autoradiography. Positions of molecular weight markers are indicated at the left side of the panel. (B) A raft gradient was prepared exactly as described for Fig. 3A and then subjected to Western blotting and probed with antibodies against VP16, VP5, VP23, and gH, as indicated at the right side of the panel. Lane P, pelleted material.
Using the raft separation gradient, we next analyzed the TX-100 insolubility of other viral proteins. Immunoblots of gradient fractions were probed with antibodies against the tegument protein VP16, capsid proteins VP5 and VP23, and gH (39, 48). Although a substantial proportion of these proteins were solubilized or pelleted under these conditions, a minor population of VP16 and gH (but not the capsid subunits) was visible in the raft portion of the gradient (Fig. 5B).
vhs is associated with lipid rafts in gradient fractions enriched for HSV-bearing cytoplasmic organelles.
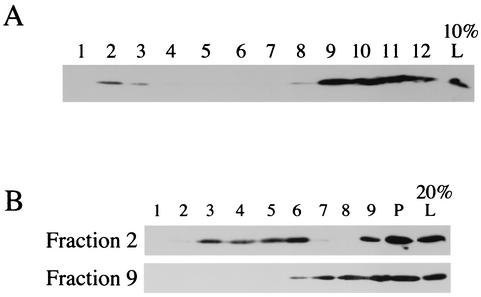
vhs serves a number of roles in the life cycle of HSV, including degradation of host and viral mRNA and interaction with VP16 and a role as a structural component of the viral tegument. The DIG-associated subpopulation of vhs may be involved in any of these roles or other functions that are as yet undefined. To attempt to clarify this issue, we made use of previous studies by Harley et al. in which HSV-containing organelles and HSV assembly intermediates from the cytoplasm of infected cells were partially purified and these structures were shown to have biochemical properties similar to those of endosomes and the trans-Golgi network (20). As expected (Fig. 6A), when an infected-cell PNS was fractionated in this way and then subjected to Western blotting for vhs, the protein separated into two populations: a dense population which, as we have shown, corresponds to viral particles released from broken microsomes and also contains lysosomes and cytoplasm (fractions 9 to 12), and a second population consisting of a low-density fraction which is known to contain HSV particles and membrane-associated capsids and tegument (fraction 2). Harley et al. have previously shown that the organelles in fraction 2 are the first and only cytoplasmic compartments which accumulate HSV particles during a single synchronized wave of assembly (20). Next, fraction 2 and fraction 9 from this gradient were incubated with TX-100 at 4°C and centrifuged on raft gradients. As can be seen in Fig. 6B, a substantial amount of the vhs in fraction 2 was raft associated, whereas there was no detectable raft-associated vhs in fraction 9.
FIG. 6.
Raft-associated vhs is enriched in organellar membrane fractions which contain virus assembly intermediates. (A) Cytoplasmic HSV-containing organelles were fractionated by sucrose equilibrium density flotation, and the fractions were subjected to SDS-PAGE and Western blotting for vhs. Gradient fractions are numbered from the top (lane 1) to the bottom (lane 12). The results for lane L represent 10% of the material loaded onto the gradient. (B) Fractions 2 and 9 (as indicated at the left side of the panel) from the gradient described for panel A were incubated with 1% TX-100 at 4°C for 0.5 h and then centrifuged in raft isolation gradients, and the fractions were subjected to Western blotting for vhs as described for Fig. 3A. For lane L, represent 20% of fraction 2 or fraction 9 material was loaded onto the raft isolation gradient.
DISCUSSION
In the present study, we found that a subpopulation of HSV-infected-cell vhs is associated with lipid rafts. Although only a small proportion of total vhs demonstrates this behavior (Fig. 3A), this small amount is a large proportion of the vhs present in low-density cytoplasmic organelles (Fig. 6). Since even known raft-associated polypeptides such as HSV-2 UL56p and caveolin 1 exhibit a 10 to 30% association with rafts in flotation gradients of the kind used here (25), we believe that it is reasonable to designate this vhs as raft associated. Although raft-associated vhs is enriched in gradient fractions thought to contain enveloping virions, we cannot exclude the possibility that this population of membrane-bound vhs plays another role, unrelated to assembly, during infection.
How might vhs interact with cell membranes and lipid rafts? It is becoming increasingly clear that some tegument proteins are capable of interaction with envelope protein tails (2, 3, 15, 33). Furthermore, it is quite reasonable to suppose that some of these envelope proteins may be raft associated, since the organelles thought to be used for alphaherpesvirus cytoplasmic envelopment are known to be rich in sphingolipids and cholesterol (7, 20, 55, 57). Moreover, at least one herpes simplex membrane protein, UL56p of HSV-2, has already been shown to partition into lipid rafts (25) and in this study we have demonstrated that some gH appears to be raft associated (Fig. 5B). We believe that the simplest explanation of our data, taken together, is that vhs binds to the cytoplasmic tail of one or more raft-localized membrane proteins. If so, vhs behaves similarly to the influenza virus M1 matrix protein in this regard. The influenza virus envelope glycoproteins hemagglutinin and neuraminidase are raft-associated polypeptides and together direct the soluble polypeptide M1 into rafts in a tail-dependent manner (58).
An alternative possibility is that vhs may itself have an intrinsic ability to associate with rafts. vhs has no known sequence motifs to suggest that it interacts with rafts or even membranes in general; however, this observation is not unprecedented. Influenza virus M1 is capable of membrane (although not raft) association even in the absence of hemagglutinin and neuraminidase (58), and the M matrix protein of measles virus is a soluble polypeptide which is able to target to rafts even when the measles virus envelope glycoproteins are absent (31, 56). However, although vhs becomes localized to rafts in cytoplasmic organelles, it seems not to be raft associated in mature, secreted HSV particles (Fig. 4B). This may imply that raft-associated vhs plays no role in HSV assembly, but it has also been suggested that rafts act only in certain steps of the viral assembly process (53). Indeed, even though many viral matrix proteins appear to be present in DIGs, only in the case of influenza virus has it been clearly shown that the mature viral envelope is raft-like (53).
Whatever the mechanism of vhs-raft interaction happens to be, it is resistant to disruption by 0.5 M NaCl (Fig. 3B) and is not dependent on the presence of mature capsids (Fig. 3C). This is consistent with the stability of the HSV tegument, which remains partially intact even when virions or L particles are treated with 1% NP-40 and 2 M NaCl (32, 54). This stability is presumably a consequence of multiple protein-protein interactions; for example, vhs is known to associate with VP16 (42, 47), which in turn may bind VP22 (9) and possibly also the tails of gH and gB (60).
Following raft gradient centrifugation, the levels of vhs and VP16 typically peaked in fractions 3 and 4, whereas the raft marker PLAP was most abundant in fractions 2 and 3 (Fig. 3 and 5). Furthermore, vhs commonly displayed a broader distribution across the gradient than PLAP, trailing down to relatively high-density fractions (Fig. 3). This might be a result of differences in the lipid composition, and thus density, of plasma membrane (PLAP-containing) rafts compared with those of vhs and VP16-containing rafts, which presumably derive from intracellular organelles. Consistent with this notion, gH, which is predominantly plasma membrane localized (22), is present within DIGs of the same density as PLAP rafts (Fig. 5B). Furthermore, it is interesting that other viral matrix proteins exhibit the same kind of raft fractionation behavior observed for vhs, even those which accumulate at the plasma membrane. HIV Pr55Gag and measles virus M protein are present in rafts with slightly higher density and broader distribution than control raft markers (31, 34). The significance of this is unclear.
Finally, not all cytoplasmic vhs is present in low-density organelles (Fig. 6A), yet essentially all of it is pelletable, even in the presence of 0.5 M NaCl or 1% TX-100 (Fig. 2). One possible explanation is that all of the vhs at the bottom of our sucrose float-up gradients is present in the non-organelle-associated HSV particles known to reside at this location (and which are derived from breakage of cytoplasmic organelles during their isolation [20]); however, it seems unlikely that all of the cytoplasmic vhs is part of the HSV particle. Another possibility is that this vhs is associated with large nonbuoyant structures, such as elements of the cytoskeleton. Alternatively, at these late times of infection, the cell may be accumulating excessive levels of viral structural proteins and this insoluble population may represent unneeded and possibly aggregated vhs. We are presently attempting to distinguish among these possibilities.
Acknowledgments
This work was supported by National Institutes of Health grants AI38265 and CA80723 to D.W.W., by the Howard Hughes Medical Institute Research Resources Program for Medical Schools, and by NIH training grant T32 GM07491 to G.A.C. Core support was provided by NIH Cancer Center grant P30-CA13330. HSV strains Pvhs(−) and PAAR5 were kind gifts from James Smiley.
We thank Lily Huang for technical assistance and Margaret Kielian and Carol Harley for helpful discussions.
REFERENCES
- 1.Becker, Y., E. Tavor, Y. Asher, C. Berkowitz, and M. Moyal. 1993. Effect of herpes simplex virus type-1 UL41 gene on the stability of mRNA from the cellular genes: beta-actin, fibronectin, glucose transporter-1, and docking protein, and on virus intraperitoneal pathogenicity to newborn mice. Virus Genes 7:133-143. [DOI] [PubMed] [Google Scholar]
- 2.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brack, A. R., B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, D., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D. A., and E. London. 1997. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 240:1-7. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti, C. R., K. S. Dingwell, C. Wale, F. L. Graham, and D. C. Johnson. 1998. Herpes simplex virus gD and virions accumulate in endosomes by mannose 6-phosphate-dependent and -independent mechanisms. J. Virol. 72:3330-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott, G., G. Mouzakitis, and P. O'Hare. 1995. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J. Virol. 69:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 11.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (vhs) protein of herpes simplex virus: genetic and biochemical evidence that vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenwick, M. L., and R. D. Everett. 1990. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J. Gen. Virol. 71:2961-2967. [DOI] [PubMed] [Google Scholar]
- 14.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1994. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 269:30745-30748. [PubMed] [Google Scholar]
- 15.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiki, Y., A. L. Hubbard, S. Fowler, and P. B. Lazarow. 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 93:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, M., L. Matusick-Kumar, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 68:3702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh, S., J. Chevesich, and U. Maitra. 1989. Further characterization of eukaryotic initiation factor 5 from rabbit reticulocytes. J. Biol. Chem. 264:5134-5140. [PubMed] [Google Scholar]
- 19.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 20.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: a role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heine, J. W., R. W. Honess, E. Cassai, and B. Roizman. 1974. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J. Virol. 14:640-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, F. E., C. A. Smibert, and J. R. Smiley. 1995. Mutational analysis of the herpes simplex virus virion host shutoff protein: evidence that vhs functions in the absence of other viral proteins. J. Virol. 69:4863-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshizuka, T., F. Goshima, H. Takakuwa, N. Nozawa, T. Daikoku, O. Koiwai, and Y. Nishiyama. 2002. Identification and characterization of the UL56 gene product of herpes simplex virus type 2. J. Virol. 76:6718-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong, A. D., and N. Frenkel. 1989. The herpes simplex virus virion host shutoff function. J. Virol. 63:4834-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie, J., F. J. Rixon, and J. McLauchlan. 1996. Overexpression of the herpes simplex virus type 1 tegument protein VP22 increases its incorporation into virus particles. Virology 220:60-68. [DOI] [PubMed] [Google Scholar]
- 30.Lu, P., F. E. Jones, H. A. Saffran, and J. R. Smiley. 2001. Herpes simplex virus virion host shutoff protein requires a mammalian factor for efficient in vitro endoribonuclease activity. J. Virol. 75:1172-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLauchlan, J., and F. J. Rixon. 1992. Characterization of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J. Gen. Virol. 73:269-276. [DOI] [PubMed] [Google Scholar]
- 33.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston, V. G., J. A. Coates, and F. J. Rixon. 1983. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 45:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rixon, F. J., C. Addison, and J. McLauchlan. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J. Gen. Virol. 73:277-284. [DOI] [PubMed] [Google Scholar]
- 39.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 40.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 41.Roper, K., D. Corbeil, and W. B. Huttner. 2000. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2:582-592. [DOI] [PubMed] [Google Scholar]
- 42.Schmelter, J., J. Knez, J. R. Smiley, and J. P. Capone. 1996. Identification and characterization of a small modular domain in the herpes simplex virus host shutoff protein sufficient for interaction with VP16. J. Virol. 70:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnitzer, J. E., D. P. McIntosh, A. M. Dvorak, J. Liu, and P. Oh. 1995. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science 269:1435-1439. [DOI] [PubMed] [Google Scholar]
- 44.Serafini, T., G. Stenbeck, A. Brecht, F. Lottspeich, L. Orci, J. E. Rothman, and F. T. Wieland. 1991. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature 349:215-220. [DOI] [PubMed] [Google Scholar]
- 45.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 46.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73:467-470. [DOI] [PubMed] [Google Scholar]
- 47.Smibert, C. A., B. Popova, P. Xiao, J. P. Capone, and J. R. Smiley. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J. Virol. 68:2339-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spear, P. G. 1994. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanisms. CRC Press, Boca Raton, Fla.
- 49.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strelow, L., T. Smith, and D. Leib. 1997. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology 231:28-34. [DOI] [PubMed] [Google Scholar]
- 51.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strelow, L. I., and D. A. Leib. 1996. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 70:5665-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suomalainen, M. 2002. Lipid rafts and assembly of enveloped viruses. Traffic 3:705-709. [DOI] [PubMed] [Google Scholar]
- 54.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72:661-668. [DOI] [PubMed] [Google Scholar]
- 55.van Genderen, I. L., R. Brandimarti, M. R. Torrisi, G. Campadelli, and G. van Meer. 1994. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology 200:831-836. [DOI] [PubMed] [Google Scholar]
- 56.Vincent, S., D. Gerlier, and S. N. Manie. 2000. Measles virus assembly within membrane rafts. J. Virol. 74:9911-9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, Y., and J. L. C. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 67:1482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, Q., and R. J. Courtney. 1994. Chemical cross-linking of virion envelope and tegument proteins of herpes simplex virus type 1. Virology 204:590-599. [DOI] [PubMed] [Google Scholar]