Abstract
Canine parvovirus (CPV) is a host range variant of a feline virus that acquired the ability to infect dogs through changes in its capsid protein. Canine and feline viruses both use the feline transferrin receptor (TfR) to infect feline cells, and here we show that CPV infects canine cells through its ability to specifically bind the canine TfR. Receptor binding on host cells at 37°C only partially correlated with the host ranges of the viruses, and an intermediate virus strain (CPV type 2) bound to higher levels on cells than did either the feline panleukopenia virus or a later strain of CPV. During the process of adaptation to dogs the later variant strain of CPV gained the ability to more efficiently use the canine TfR for infection and also showed reduced binding to feline and canine cells compared to CPV type 2. Differences on the top and the side of the threefold spike of the capsid surface controlled specific TfR binding and the efficiency of binding to feline and canine cells, and these differences also determined the cell infection properties of the viruses.
Canine parvovirus (CPV) emerged in 1978 as the cause of new enteric and myocardial diseases in dogs. The new virus spread globally in a pandemic of disease during 1978 and has since remained endemic in dogs throughout the world (27, 43). The 1978 strain of CPV (termed CPV type 2) was a new virus infecting dogs since there is no serological or other evidence for infection of dogs by a related virus prior to the mid-1970s (27). Phylogenetic analysis shows that all CPV isolates were descended from a single ancestor which emerged during the mid-1970s, which was closely related to the long-known feline panleukopenia virus (FPV) which infects cats, mink, and raccoons but not dogs or cultured dog cells (43). FPV and CPV isolates differ by as little as 0.5% in DNA sequence, and the characteristic properties of CPV type 2 are controlled by a small number of changes in the capsid surface. Two differences between FPV and CPV changed VP2 residues 93 from Lys to Asn and 323 from Asp to Asn, and those changes alone could introduce the canine host range, a CPV-specific antigenic epitope, and a difference in the pH dependence of hemagglutination into FPV (9, 14). Despite the close relationship to FPV, CPV type 2 isolates did not replicate in cats (42, 44), and this host range was determined at least in part by VP2 residues 80, 564, and 568 which are in close proximity in the capsid structure (41). Other mutations in the same structural region of CPV type 2 were selected by passage in cat cells (VP2 residue 300 from Ala to Asp), and these reduced the infection of canine cells, as did closely adjacent changes in in vitro prepared mutants (VP2 residue 299 Gly to Glu) (18, 26).
Host range-controlling residues are located on a raised region of the capsid that surrounds the threefold axis (the threefold spike) (9, 46). VP2 residues 93 and 323 are found near the top of that structure, whereas residues 299 and 300, and changes controlling feline host range, are all on a ridge on the side (the shoulder) (18, 26).
During 1979 a CPV variant (CPV type 2a) emerged that spread worldwide within 1 year and replaced the CPV type 2 strain. CPV type 2a contained five substitutions in the capsid sequence compared to CPV type 2, including changes of VP2 residues 87 from Met to Leu, 300 from Ala to Gly, and 305 from Asp to Tyr (16, 29, 42). CPV type 2a isolates were antigenically variant from CPV type 2 and also infected and caused disease in cats (29, 30, 42). An antigenic variant of CPV type 2a (CPV type 2b) was recognized in 1984, and it differed in an antigenic epitope as a result of the substitution of VP2 residue 426 from Asn to Asp (29).
CPV and FPV are autonomous parvoviruses with single-stranded DNA genomes of ca. 5,120 bases. The genomes encode two genes which each form two proteins by alternative mRNA splicing (10, 49). The 28-nm-diameter nonenveloped capsid is assembled from 60 copies of a combination of the overlapping capsid proteins VP1 and VP2 (46). The three sites on the capsid that can affect canine host range on the threefold spike are separated from each other by 25 to 30 Å, and all affect the folding or flexibility of loops within the capsid structure, suggesting roles in virus-receptor interactions or in capsid uncoating (1, 18, 36).
CPV type 2 and FPV capsids bind the human or feline transferrin receptors (TfRs) and use those receptors to infect normally resistant Chinese hamster ovary (CHO) cells (25). The capsids normally enter cells by clathrin-mediated endocytosis, colocalize with transferrin (Tf) in perinuclear endosomes, and then slowly leave the endosome and enter the cytoplasm prior to the DNA gaining access to the nucleus for replication (24, 47, 48, 51).
Here we show that CPV infection of dog cells was associated with its specific ability to bind the canine TfR and that resistance of canine cells to FPV could be overcome by expression of the feline TfR in those cells. CPV type 2 and type 2b isolates bound the canine TfR expressed on CHO-derived cells, whereas FPV or a CPV host range mutant did not. Canine TfR-expressing CHO cells were infected more efficiently by the CPV type 2b isolate tested compared to CPV type 2. CPV type 2 bound feline and canine cells to 10- to 20-fold-higher levels than did either FPV or CPV type 2b, and that additional binding was controlled by residues within the shoulder region of the capsid.
MATERIALS AND METHODS
Cells and viruses.
Crandell feline kidney (CRFK) and canine Cf2Th cells were grown in a 50% mixture of McCoy's 5A and Leibovitz L15 media with 5% fetal calf serum. Chinese hamster ovary (CHO) cell-derived TRVb cells which lack the hamster TfR (19) were grown in Ham's F-12 with 5 or 10% fetal calf serum. Viruses or mutants have been previously described (4, 26, 28). Wild-type CPV-d (CPV type 2), FPV (FPV-b), and CPV-39 (CPV type 2b) were derived from infectious plasmid clones. A mutant of CPV type 2 with VP2 residue 299 changed from Gly to Glu (CPV type 2-G299E) shows a reduced infection of dog cells (26), whereas a mutant of CPV type 2 with VP2 residue 377 changed from Arg to Lys (CPV type 2-R377K) was nonhemagglutinating and did not bind sialic acid on erythrocytes or feline cells (4, 39). CPV type 2 with Asp305 changed to Tyr (CPV type 2-D305Y) was prepared by site-directed mutagenesis, and the triple mutant of CPV type 2 with changes of Ala300 to Gly, Asp305 to Tyr, and Asn375 to Asp was a recombinant between CPV type 2 and CPV type 2b sequences. Viruses were prepared by transfection of plasmids into NLFK cells and passaged fewer than six times. Capsids were prepared by growing virus in NLFK cells and concentrated with polyethylene glycol precipitation or by centrifugation, and then full and empty capsids were separated in sucrose gradients and dialyzed against phosphate-buffered saline (PBS) or 20 mM Tris-HCl (pH 7.5) (1, 36).
Viruses were titrated by using a 50% tissue culture infectious dose (TCID50) assay in NLFK cells. Cells in 96 wells were inoculated with dilutions of virus and then incubated for 2 days. The cells were fixed with 2.5% paraformaldehyde (PFA) in PBS for 10 min and then stained for the presence of viral capsid protein antigens with rabbit anti-CPV serum as described previously (54). Cell infection was also detected by immunostaining in PBS and 0.5% Triton X-100 with a Texas Red- or Cy2-conjugated monoclonal antibody (CE10) against the viral NS1 protein (53).
Virus and Tf labeling.
Capsids at 1 mg/ml were dialyzed overnight at 4°C against 0.1 M carbonate buffer (pH 9.0) and then incubated for 10 min with Cy2 or Cy3.5 (Pharmacia-Amersham, Piscataway, N.J.) at 1/10 the recommended concentration. Labeled capsids were separated from the free dye in a Sephadex G25 column in PBS and then stored in the dark at 4°C. Labeling levels were 8 to 13 dye molecules per virion. Canine Tf (Sigma, St. Louis, Mo.) was iron loaded by using a modification of a protocol described elsewhere (5, 6). Tf (5 mg/ml) was incubated for 30 min at 37°C with a mixture of 240 nM FeCl3 and 2 mM nitrilotriacetic acid in 250 mM Tris-HCl (pH 8.0) and 10 μM NaHCO3 and then separated from the free iron in a PD-10 column in PBS. Then, 2 mg of iron-loaded Tf was dialyzed against 50 mM borate buffer (pH 9.0), incubated with 0.1 mg of Texas Red sulfonyl chloride (Molecular Probes, Eugene, Oreg.), and incubated for 1 h at 4°C. The labeled Tf was separated from free dye in a Sephadex G25 column in PBS.
Antibody microinjection.
Cf2Th cells were microinjected with antibody H65.4 against an epitope in the TfR cytoplasmic tail (Zymed, South San Francisco, Calif.) (40) or with isotype-matched mouse immunoglobulin G (IgG; MOPC-21; Sigma). Antibodies were dialyzed against PBS and concentrated to 2 to 3 mg/ml, and between 0.1 and 0.5 pl of IgG injected per cell; then the culture was incubated with 2 TCID50 of CPV per cell for 1 h at 37°C. The cells were then washed and incubated with growth medium containing a 1:500 dilution of a neutralizing rabbit antiserum against CPV to prevent the secondary spread of the virus. After 42 h of incubation the cells were washed, fixed, and stained for the injected mouse IgG by using a Cy2-conjugated goat anti-mouse IgG, and then with Texas Red-labeled anti-NS1 antibody. The percentage of cells containing IgG that became infected was compared to the percentage of infected cells among the noninjected cells in the same culture.
Cloning the canine TfR gene cDNA.
Canine mRNA was isolated from cultured Walter Reed canine cells (12). The cDNA was prepared and amplified as two fragments by using the Access RT-PCR System (Promega, Madison, Wis.) with specific primers as described previously (25) and then cloned into the plasmid pGEM-T Easy (Promega). The canine TfR sequence was determined by using automated sequencing and then submitted to GenBank (accession no. AF297626). The translated sequence of the canine TfR was aligned with those of the feline and human TfRs, and differences were mapped onto the human TfR structure (7, 17). The intact cDNA of the canine TfR gene was prepared and cloned into the vector pcDNA3.1(−) (Invitrogen, Carlsbad, Calif.) for expression.
Receptor expression from plasmids.
TRVb or Cf2Th cells seeded at 2 × 104 per cm2 in 25-cm2 flasks were transfected with 5 μg of plasmid clones containing the feline or canine TfR cDNAs. The DNA was mixed with 15 μl of Lipofectamine (Invitrogen) and added to the cells according to the manufacturer's directions. Cells were incubated at 37°C for 2 to 4 days, and then binding and uptake of capsids and Tf was determined by microscopy and flow cytometry. For fluorescence microscopy, cells were incubated at 37°C for 1 h with labeled Tf or with labeled or unlabeled virus capsids at 37°C for 1 h, and then they were washed and fixed with 4% PFA in PBS. For antibody staining, the cells were permeabilized with PBS containing 0.1% Triton X-100 and 0.5% bovine serum albumin, incubated with Cy2-labeled MAb8 to detect virus, and then examined with a UV microscope.
For flow cytometry the cells were removed from the plastic with 1 mM EDTA in Hanks buffered saline without Ca2+ and Mg2+, fixed with 4% PFA, and stained with Cy2-labeled MAb8 as described above. Cells were then analyzed with a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.).
The role of sialic acid in virus binding was examined by neuraminidase treatment of cells. CRFK cells were incubated for 1 h at 37°C with 0.5 U of neuraminidase (Clostridium perfringens, type X; Sigma)/ml and then incubated for 1 h with either fluorescein isothiocyanate-labeled peanut agglutinin or with 10 μg of capsids/ml, and virus binding was assayed by flow cytometry as described above.
Infection assays.
Cells transfected with plasmids expressing canine or feline TfR or with the empty vector were incubated for 4 days. Cells were inoculated with 10 TCID50 of either CPV type 2, CPV type 2b, FPV, or CPV type 2-G299E per cell for 1 h at 37°C and then incubated for 24 or 48 h at 37°C. The cells were then incubated with serum-free medium for 30 min, followed by the addition of Texas Red-labeled canine Tf for 60 min at 37°C. After a washing step and fixation with 4% PFA, the infected cells were stained with Cy2-labeled anti-NS1, and the percentage of cells that became infected was determined. To detect the relative infection by CPV type 2 and CPV type 2b of the TRVb cells expressing canine TfR, the cells were inoculated with a 9:1 TCID50 ratio of CPV type 2 and CPV type 2b for 1 h at 37°C and then washed and incubated for 5 days. Samples were removed from the culture on days 0, 2, and 5, and the viral DNA amplified by PCR between nucleotides 2948 and 4014. The PCR products were sequenced, and the proportion of each viral sequence was determined from the profile.
RESULTS
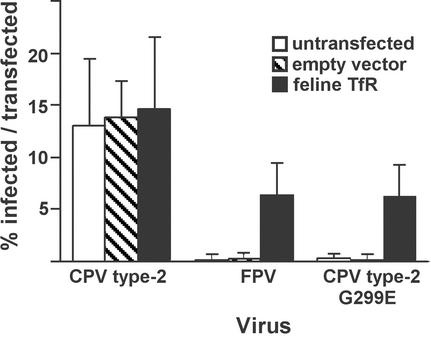
Expressing the feline TfR in Cf2Th cells made them susceptible to infection by both FPV and the CPV type 2-G299E host range mutant (26) (Fig. 1). The percentage of transfected cells infected was determined by comparison with the proportion of cells transfected in parallel with a plasmid expressing green fluorescent protein. A total of 6 to 9% of the feline TfR-expressing canine cells became infected by either FPV or CPV type 2-G299E (Fig. 1). A total of 10 to 20% of the Cf2Th cells were infected by wild-type CPV type 2 that could potentially infect all of the cells, a finding which is typical for these viruses in susceptible nonsynchronized cells, since cellular S phase is needed for virus DNA replication.
FIG. 1.
Expression of the feline TfR in canine Cf2Th cells makes them susceptible to FPV and CPV type 2-G299E infection. Cf2Th cells were transfected with a plasmid expressing the feline TfR or with the empty plasmid vector, incubated for 4 days, and then inoculated with 10 TCID50 per cell of CPV type 2, FPV, or CPV type 2-G299E. After 24 h more incubation, the cells were fixed and infection was detected with an antibody against the NS1 protein. Bars show one standard deviation of the data from three separate experiments.
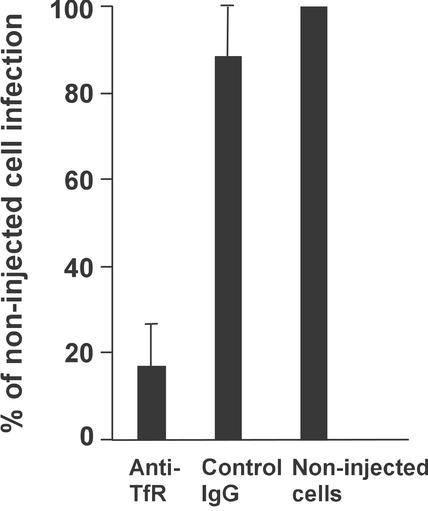
The canine TfR was required for infection of Cf2Th cells by CPV type 2. When we injected an antibody against the TfR cytoplasmic tail into these cells before virus inoculation, there was an 80 to 100% reduction in virus infection, whereas no significant effect was seen after injection of a control IgG (Fig. 2).
FIG. 2.
Effect of a microinjected antibody against the TfR cytoplasmic tail on CPV infection of Cf2Th cells. Cells were injected with anti-TfR IgG or with a control IgG, inoculated with 2 TCID50 of CPV type 2 per cell, and then incubated for 48 h. Injected cells were identified by staining for the IgG, and infected cells were identified by staining for the viral NS1 protein. The proportion of antibody-injected cells that became infected was compared to the proportion of noninjected cells infected in the same culture. Bars show one standard deviation of the mean for four experiments with the anti-TfR and for two experiments with the control IgG.
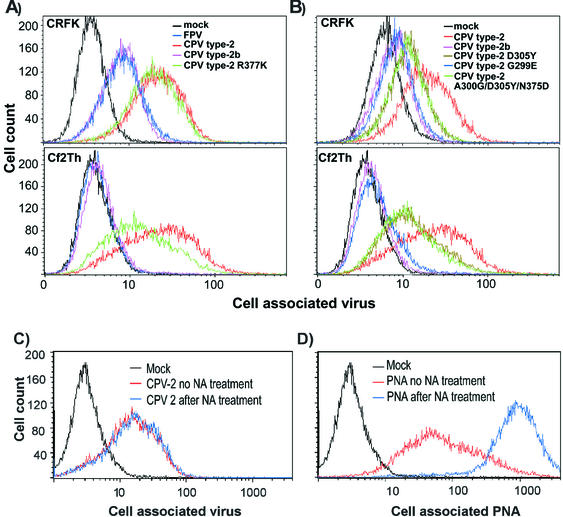
There was only a partial correlation between binding of the viruses to canine and feline cells at 37°C and their ability to infect these cells. All viruses tested bound and were taken up into feline CRFK cells efficiently (Fig. 3). However, between 5- and 20-fold more capsids of the wild-type CPV type 2 and CPV type 2-R377K became cell associated than did those of FPV or CPV type 2b (Fig. 3A). When incubated with canine Cf2Th cells, CPV type 2 and CPV type 2-R377K bound and were taken up at high levels, CPV type 2b capsids was taken up at 10- to 20-fold-lower levels, and FPV capsids showed no detectable cell association (Fig. 3A). Although these viruses can bind sialic acids on erythrocytes and cells at lower pHs and temperatures (4, 36, 39), this does not appear to be a factor in the binding to the cells examined here. The CPV type 2-R377K does not bind sialic acid, but it bound feline cells to levels similar to those of the wild-type CPV type 2 (Fig. 3A). In addition, pretreatment of cells with neuraminidase prior to incubation with CPV type 2 capsids did not reduce virus binding (Fig. 3C), although increased binding of peanut agglutinin to the galactosyl(β-1,3)-N-acetylgalactosamine exposed on the treated cells indicated that the sialic acids had been removed (Fig. 3D).
FIG. 3.
(A) Binding and uptake of FPV (blue), CPV type 2 (red), CPV type 2b (purple), or CPV type 2-R377K (green) capsids incubated with feline CRFK cells or canine Cf2Th cells. Cells were incubated with 20 μg of capsid/ml for 1 h at 37°C and then detached with EDTA, fixed, and permeabilized; the cell-associated virus was quantified with a Cy2-labeled anti-capsid monoclonal antibody, followed by analysis by flow cytometry. (B) Binding and uptake of CPV type 2 (red), CPV type 2b (purple), CPV type 2 D305Y (dark green), CPV type 2 G299E (blue), and CPV type 2 A300G/D305Y/N375D (light green) capsids into CRFK or Cf2Th cells as described above. (C and D) Binding of CPV type 2 capsids to CRFK cells that were either mock treated (red) or incubated with neuraminidase (blue). Cells were incubated with CPV type 2 (C) or fluorescein isothiocyanate-labeled peanut agglutinin (PNA) (D).
The increased binding of CPV type 2 to the host cells was affected by mutations replacing residues within the shoulder region of the capsid. CPV type 2-G299E showed binding similar to that of CPV type 2b (Fig. 3B). Capsids of CPV type 2 containing changes derived from the CPV type 2a and type 2b sequence (D305Y or A300G/D305Y/N375D) bound to both cell types at levels intermediate between those seen for CPV type 2 and CPV type 2b capsids, indicating a partial role for the 305Y change (Fig. 3B).
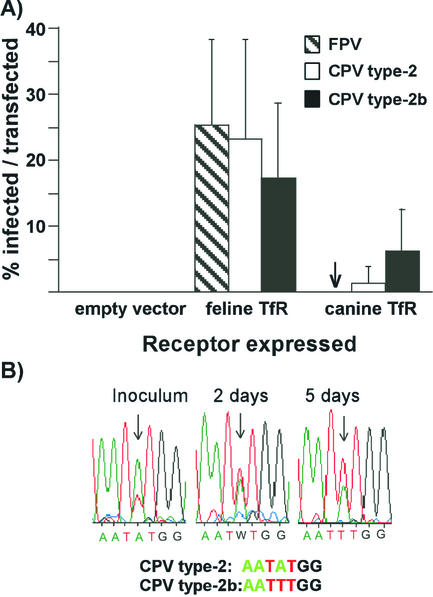
To directly determine the role of the canine TfR in specific viral binding and infection, we prepared the canine TfR cDNA by reverse transcription-PCR and expressed that receptor in the TfR-deficient CHO-derived TRVb cells (19). Cells were then incubated with FPV, CPV type 2, CPV type 2-G299E, or CPV type 2b capsids for 1 h at 37°C. All four viruses bound and were taken up into TRVb cells expressing the feline TfR, whereas only CPV type 2 or CPV type 2b capsids bound efficiently to cells expressing the canine TfR (Fig. 4). No binding above background was seen to cells transfected with the control plasmid (results not shown). The susceptibility of TRVb cells expressing the feline or canine TfRs to virus infection generally paralleled the natural host ranges of these viruses (Fig. 5). Cells expressing the feline TfR were highly susceptible to FPV, CPV type 2, or CPV type 2b. TRVb cells expressing the canine TfR completely resisted infection by FPV, whereas 0.5 to 1% of these cells were infected by CPV type 2 and 7 to 10% were infected by CPV type 2b (Fig. 5A). When the TRVb cells expressing the canine TfR were inoculated with a 9:1 TCID50 ratio of CPV type 2 and CPV type 2b and the cells were incubated for 5 days, the CPV type 2b sequence became dominant in the culture, as seen in the DNA sequence profile (Fig. 5B).
FIG. 4.
Virus binding to TRVb cells expressing feline or canine TfRs. TRVb cells were transfected with plasmids expressing the feline or canine TfR and then incubated for 4 days. The cells were incubated at 37°C for 1 h with Cy5-labeled canine Tf and with 10 μg of FPV, CPV type 2, CPV type 2b, or CPV type 2-G299E capsids/ml and then washed, suspended by EDTA treatment, fixed, and permeabilized. Capsids were detected with Cy2-labeled antibody. Cell associated Tf is shown on the y axis, and virus capsids are shown on the x axis.
FIG. 5.
(A) Virus susceptibility of TRVb cells expressing feline or canine TfRs. Cells transfected with plasmids expressing the feline or canine TfRs were inoculated with CPV type 2, FPV, or CPV type 2b and then incubated for 24 h before incubation for 30 min with Texas Red-labeled canine Tf. After fixation and permeabilization virus infection detected by staining for the viral NS1 protein. The bars represent one standard deviation of the mean of the percentage of Tf-binding cells that became infected in six separate experiments. (B) The replacement of CPV type 2 by CPV type 2a when the two viruses were grown together in TRVb cells expressing the canine TfR for 5 days. The inoculum contained nine times more TCID50 of CPV type 2 than CPV type 2b, which was reflected in the double sequencing profile at position 3046. Samples of virus were collected from the culture inoculum and from the culture at days 2 and 5 after inoculation. The viral DNA was amplified by PCR and sequenced, and the profiles of sequences from nucleotides 3043 to 3049 are shown.
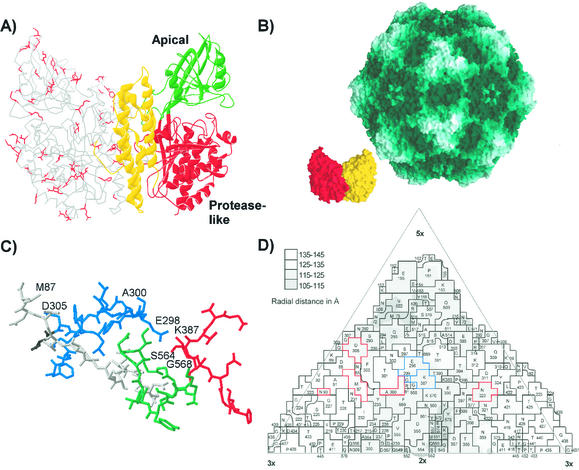
The translated sequences of the feline and canine TfRs differed by 13%, and both differed from the human TfR sequence by ca. 22% (Fig. 6). Differences between the feline and canine TfR sequences were found in all three domains defined for the human TfR structure, and most were of surface-exposed residues (Fig. 7A) (7, 17). Although a detailed model of the docking of the virus and TfRs is not yet possible, it is clear that changes of VP2 residues 93 or 299 on the top or the shoulder of the threefold spike of the capsid can affect binding to the canine TfR (Fig. 7C and D).
FIG. 6.
Aligned sequences of the human, feline, and canine TfRs. The sequence differences that were uniquely seen between the feline and canine TfR sequences are indicated in red. Predicted N-linked glycosylation sites (Asn-X-Ser/Thr) in the different sequences are indicated by shading. The domains of the ectodomain of the receptor determined from the structure of the human TfR are underlined with red (protease-like domain), green (apical domain), and yellow (helical domain) lines.
FIG. 7.
(A) Differences between the feline and canine TfR sequences mapped within the structure of the human TfR. One monomer of the ectodomain of the human TfR is shown as a ribbon diagram, while the other monomer is shown as an α-carbon tracing. The side chains of residues in the human TfR model that are unique differences between the feline and canine TfR sequences are indicated in red on one monomer. (B) Models showing the surface of the capsid of CPV (46) and of a dimer of the human TfR ectodomain (17) at the same scale. The monomers of the TfR dimer are shown in red and yellow. (C) The structure of the CPV type 2 capsid in the region that controls host range and canine TfR binding. The polypeptide chains contributed by the four VP2 monomers that make up this region of the capsid are colored differently, and the residues that are discussed in the text as controlling host range or receptor binding are labeled. (D) A road map determined by the method of Rossmann and Palmenberg (32) showing the surface exposure of VP2 residues in one asymmetric unit of the CPV type 2 capsid. The region shown is comprised of several symmetry-related VP2 subunits, and the residues are given, along with the positions in the VP2 sequence. Residues that affect receptor binding or host range and which differ naturally between FPV and CPV strains are outlined in red, whereas residues that were experimentally identified as affecting the feline or canine host ranges of the viruses are indicated in blue.
DISCUSSION
The emergence of CPV as a new pathogen of dogs presents a unique opportunity for understanding the adaptation of a virus to a new host, since we can examine the ancestral viruses, as well as the variants derived during the process of host adaptation. The viral controls of host range involve only a small number of changes on or near the viral capsid surface, and here we show that some of those changes control a critical molecular interaction with the host cell: the specific binding by CPV capsids to the canine TfR. In addition, a second step in the adaptation of CPV to dogs lead to the natural global replacement of CPV type 2 by the CPV type 2a variant, and the CPV type 2b strain later emerged as a variant of the CPV type 2a through an additional point mutation. We show that a CPV type 2b isolate was more efficient in its use of the canine TfR for infection of TRVb cells and, by comparison with CPV type 2, show that it bound canine and feline cells to low levels, perhaps indicating that it had lost the ability to bind an additional receptor on the host cells.
The host range for canine cells was controlled by virus-specific binding to the canine TfR.
FPV did not bind to the canine cells at 37°C (Fig. 3), indicating that the block to FPV infection of canine cells involved the absence of a functional surface receptor for that virus. However, the expression of the feline TfR in these cells made them susceptible to virus infection (Fig. 1). This presents a simpler model of host range control than has been proposed in previous studies, in which radioactively labeled capsids of the closely related mink enteritis virus or of CPV host range mutants bound to canine cells when incubated at 4°C; these studies had suggested that the infection was blocked at a stage after cell entry (15, 26). The lack of binding and uptake of FPV in dog cells at 37°C seen here suggests that at 4°C labeled capsids can associate with cells that they cannot infect. That the canine TfR is a major determinant of the infection of dog cells by CPV was confirmed by the block to infection seen after microinjection of Cf2Th cells with an antibody against the TfR cytoplasmic tail (Fig. 2).
Expression of the feline and canine TfRs in TRVb cells yielded cells that acted as surrogates for the original host cells; these cells expressing the feline TfR bound and were infected by all of the viruses that infected feline cells, whereas those expressing the canine TfR bound and were infected by CPV type 2 and CPV type 2b but not by FPV (Fig. 4 and 5). An interesting finding was that the two natural variants of CPV differed in their ability to efficiently infect the canine TfR-expressing TRVb cells, with CPV type 2 infecting the cells only to very low levels, whereas the CPV type 2b isolate infected the cells at levels similar to those seen for TRVb cells expressing the feline TfR (Fig. 5). The CPV type 2 strain was the cause of the original pandemic in 1978, while the CPV type 2a later spread worldwide and replaced CPV type 2. This suggests that several biological differences can explain the rapid rise and dominance of the CPV type 2a strain, since those viruses differ antigenically, in their ability to infect cats, and in their ability to use the canine TfR as an efficient receptor when it is present alone on cells.
Sequences in the shoulder region of the CPV type 2 capsid control increased binding to feline and canine cells.
CPV type 2 capsids bound to 5- to 20-fold-higher levels on feline and canine cells than did either CPV type 2b or FPV capsids (Fig. 3A). That additional binding of CPV type 2 was partially or completely reduced by mutations that introduced changes adjacent to VP2 residue 300 in the shoulder region of the capsid. The CPV type 2b-derived change of 305 D-Y reduced binding by about fivefold, whereas the host range mutant CPV type 2-G299E was reduced in binding to feline cells to levels similar to those seen for CPV type 2b (Fig. 3 and 7C). FPV bound to fivefold-lower levels to feline cells compared to CPV type 2, and these viruses differ in residues 80, 564, and 568, which are also within the structure of the shoulder region (Fig. 7C and D). The region of the receptor that determines the specific virus binding of the canine TfR has not yet been defined, and feline-canine variant sequences were found throughout the receptor structure (Fig. 7A). However, the large size of the receptor relative to the capsid suggests that the interacting surface would be near the apical domain of the TfR structure (Fig. 7B).
One of the mysteries of the evolution of CPV was the inability of CPV type 2 to infect cats despite its efficient infection of feline cells in culture (44), and the subsequent reacquisition of feline host range by CPV type 2a (16, 42). Recombinants between CPV type 2 and FPV showed that cat infection required VP2 residue 80, along with residues 564 and/or 568, to be the FPV sequence (41). It is therefore possible that the same changes that control the high binding levels of CPV type 2 to cat and dog cells also affect the in vivo replication of that virus in cats through an unknown mechanism.
Host range and changes in receptor usage in the evolution of CPV.
These data suggest a model whereby CPV type 2 emerged as a variant of an FPV-like virus through acquisition of up to six changes that gave it the ability to bind the canine TfR and to efficiently infect both canine cells and dogs. That initial group of mutations also resulted in increased virus binding to feline cells compared to FPV or to the later CPV type 2a/b strain of virus. That additional binding activity of the CPV type 2 is not due to sialic acid, a potential ligand for the virus (4, 39), since the CPV type 2-R377K nonhemagglutinating mutant bound feline cell, as well as the wild-type virus, and neuraminidase pretreatment of the cells did not reduce the binding. Potential explanations for this result would be that CPV type 2 capsids bind both the TfR and a second receptor on the feline or canine cells or, alternatively, that those viruses bind only to the canine or feline TfRs, but they bind with higher affinity or avidity when the receptors are expressed on the natural host cells. That increased binding of CPV type 2 to the canine cells presumably allows the efficient infection to occur. The use of multiple receptors by viruses or variation in receptor usage is seen with other viruses (8, 13, 23, 37, 38, 52).
Subsequent changes in CPV type 2a (which were also retained in CPV type 2b) allowed the virus to use the canine TfR more efficiently for infection, and these viruses therefore no longer needed the additional or coreceptor binding. A number of possible mechanisms remain to be investigated. It is possible that CPV type 2a/b capsids have a higher affinity or avidity of binding to the canine TfR, which permits the more efficient use for cell binding and infection, or that the changes in the CPV type 2a/b capsid structure may facilitate later uncoating or membrane penetration steps in infection with the canine TfR. Many different viruses undergo receptor-induced changes in viral structural proteins that are required for successful infection of cells either directly or through second steps involving low-pH exposure (21, 22, 33, 35, 45).
Host range or tissue tropism controlled by capsid sequences has been defined for several different parvoviruses. The tissue specificities of the fibrotropic and lymphotropic strains of minute virus of mice [MVM(p) and MVM(i), respectively] are also determined by a small number of changes in the surface of the capsid in a position similar to VP2 residue 323 in CPV which controls the host range for dogs (2, 3). The infection of mink by the Aleutian mink disease parvovirus is also controlled by a small group of residues that are probably on the surface of the capsid (20).
Host range switching by viruses.
Natural host range switches by viruses are rare events, but when they occur the results can be severe since the viruses may then spread widely through immunologically naive and nonadapted host populations. Examining the ancestors and descendents of CPV allows us to define the important cellular and viral determinants involved. It is clear that the emergence of CPV was a multistep process, where small numbers of mutations in the capsid protein gene allowed it to efficiently infect and spread within a new host order. Even the partially adapted CPV type 2 was very successful, spreading worldwide in only a few months. The subsequent replacement of that strain by CPV type 2a was also completed within 1 or 2 years in the face of immunity to the original virus.
These results show that under the right circumstances even a genetically stable DNA virus can gain the mutations required to adapt to a new host. This type of multistep adaptation is seen in the emergence of a new influenza viruses in humans or other animals (31, 50) and possibly is seen in the adaptation from other primates of human immunodeficiency virus type 1 to humans (11, 34). Although each system has distinct features, they all show that specific sets of mutations need to occur for the virus to become a successful pathogen of the new host, that they are likely selected in a specific order, and that they are favored where viable intermediate viruses can be selected. Although these types of host range jumps are likely to remain very rare events, understanding the mechanisms involved may allow us to anticipate and perhaps prevent the emergence of new viruses in the future.
Acknowledgments
Gail Sullivan provided expert technical assistance.
This work was supported by grants AI28385 and AI33468 from the National Institutes of Health to C.R.P. J.S.L.P. was supported by National Research Service award F32 AI10134.
REFERENCES
- 1.Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. [DOI] [PubMed] [Google Scholar]
- 2.Agbandje-McKenna, M., A. L. Llamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 6:1369-1381. [DOI] [PubMed] [Google Scholar]
- 3.Ball-Goodrich, L. J., and P. Tattersall. 1992. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J. Virol. 66:3415-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbis, D. P., S.-F. Chang, and C. R. Parrish. 1992. Mutations adjacent to the dimple of canine parvovirus capsid structure affect sialic acid binding. Virology 191:301-308. [DOI] [PubMed] [Google Scholar]
- 5.Bates, G. W., and M. R. Schlabach. 1973. The reaction of ferric salts with transferrin. J. Biol. Chem. 248:3228-3232. [PubMed] [Google Scholar]
- 6.Bates, G. W., and J. Wernicke. 1971. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J. Biol. Chem. 246:3679-3685. [PubMed] [Google Scholar]
- 7.Bennett, M. J., J. A. Lebron, and P. J. Bjorkman. 2000. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature 403:46-53. [DOI] [PubMed] [Google Scholar]
- 8.Carson, S. D. 2001. Receptor for the group B coxsackieviruses and adenoviruses: CAR. Rev. Med. Virol. 11:219-226. [DOI] [PubMed] [Google Scholar]
- 9.Chang, S. F., J. Y. Sgro, and C. R. Parrish. 1992. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J. Virol. 66:6858-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotmore, S. F., and P. Tattersall. 1987. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 33:91-174. [DOI] [PubMed] [Google Scholar]
- 11.Hahn, B. H., G. M. Shaw, K. M. De Cock, and P. M. Sharp. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287:607-614. [DOI] [PubMed] [Google Scholar]
- 12.Harrison, L. R., E. L. Styer, A. R. Pursell, L. E. Carmichael, and J. C. Nietfeld. 1992. Fatal disease in nursing puppies associated with minute virus of canines. J. Vet. Diagn. Investig. 4:19-22. [DOI] [PubMed] [Google Scholar]
- 13.Hensley, L. E., K. V. Holmes, N. Beauchemin, and R. S. Baric. 1998. Virus-receptor interactions and interspecies transfer of a mouse hepatitis virus. Adv. Exp. Med. Biol. 440:33-41. [DOI] [PubMed] [Google Scholar]
- 14.Horiuchi, M., H. Goto, N. Ishiguro, and M. Shinagawa. 1994. Mapping of determinants of the host range for canine cells in the genome of canine parvovirus using canine parvovirus/mink enteritis virus chimeric viruses. J. Gen. Virol. 75:1319-1328. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi, M., N. Ishiguro, H. Goto, and M. Shinagawa. 1992. Characterization of the stage(s) in the virus replication cycle at which the host-cell specificity of the feline parvovirus subgroup is regulated in canine cells. Virology 189:600-608. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, Y., M. Mochizuki, R. Naito, K. Nakamura, T. Miyazawa, T. Mikami, and E. Takahashi. 2000. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology 278:13-19. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence, C. M., S. Ray, M. Babyonyshev, R. Galluser, D. W. Borhani, and S. C. Harrison. 1999. Crystal structure of the ectodomain of human transferrin receptor. Science 286:779-782. [DOI] [PubMed] [Google Scholar]
- 18.Llamas-Saiz, A. L., M. Agbandje-McKenna, J. S. L. Parker, A. T. M. Wahid, C. R. Parrish, and M. G. Rossmann. 1996. Structural analysis of a mutation in canine parvovirus which controls antigenicity and host range. Virology 225:65-71. [DOI] [PubMed] [Google Scholar]
- 19.McGraw, T. E., L. Greenfield, and F. R. Maxfield. 1987. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J. Cell Biol. 105:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna, R., N. H. Olson, P. R. Chipman, T. S. Baker, T. F. Booth, J. Christensen, B. Aasted, J. M. Fox, M. E. Bloom, J. B. Wolfinbarger, and M. Agbandje-McKenna. 1999. Three-dimensional structure of Aleutian mink disease parvovirus: implications for disease pathogenicity. J. Virol. 73:6882-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 22.Nemerow, G. R. 2000. Cell receptors involved in adenovirus entry. Virology 274:1-4. [DOI] [PubMed] [Google Scholar]
- 23.Overbaugh, J., A. D. Miller, and M. V. Eiden. 2001. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 65:371-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker, J. S. L., W. J. Murphy, D. Wang, S. J. O'Brien, and C. R. Parrish. 2001. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 75:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker, J. S. L., and C. R. Parrish. 1997. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J. Virol. 71:9214-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish, C. R. 1990. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv. Virus Res. 38:403-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrish, C. R. 1991. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology 183:195-205. [DOI] [PubMed] [Google Scholar]
- 29.Parrish, C. R., C. Aquadro, M. L. Strassheim, J. F. Evermann, J.-Y. Sgro, and H. Mohammed. 1991. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 65:6544-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrish, C. R., P. Have, W. J. Foreyt, J. F. Evermann, M. Senda, and L. E. Carmichael. 1988. The global spread and replacement of canine parvovirus strains. J. Gen. Virol. 69:1111-1116. [DOI] [PubMed] [Google Scholar]
- 31.Reid, A. H., J. K. Taubenberger, and T. G. Fanning. 2001. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 3:81-87. [DOI] [PubMed] [Google Scholar]
- 32.Rossmann, M. G., and A. C. Palmenberg. 1988. Conservation of the putative receptor attachment site in picornaviruses. Virology 164:373-382. [DOI] [PubMed] [Google Scholar]
- 33.Sattentau, Q. J. 1998. HIV gp120: double lock strategy foils host defences. Structure 6:945-949. [DOI] [PubMed] [Google Scholar]
- 34.Sharp, P. M., E. Bailes, R. R. Chaudhuri, C. M. Rodenburg, M. O. Santiago, and B. H. Hahn. 2001. The origins of acquired immune deficiency syndrome viruses: where and when? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 356:867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons, G., J. D. Reeves, S. Hibbitts, J. T. Stine, P. W. Gray, A. E. Proudfoot, and P. R. Clapham. 2000. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 177:112-126. [DOI] [PubMed] [Google Scholar]
- 36.Simpson, A. A., V. Chandrasekar, B. Hebert, G. M. Sullivan, M. G. Rossmann, and C. R. Parrish. 2000. Host range and variability of calcium binding by surface loops in the capsids of canine and feline parvoviruses. J. Mol. Biol. 300:597-610. [DOI] [PubMed] [Google Scholar]
- 37.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, Y., T. Ito, T. Suzuki, R. E. Holland, Jr., T. M. Chambers, M. Kiso, H. Ishida, and Y. Kawaoka. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74:11825-11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tresnan, D. B., L. Southard, W. Weichert, J. Y. Sgro, and C. R. Parrish. 1995. Analysis of the cell and erythrocyte binding activities of the dimple and canyon regions of the canine parvovirus capsid. Virology 211:123-132. [DOI] [PubMed] [Google Scholar]
- 40.Trowbridge, I. S., J. F. Lesley, D. Domingo, R. Schulte, C. Sauvage, and H. G. Rammensee. 1987. Monoclonal antibodies to transferrin receptor and assay of their biological effects. Methods Enzymol. 147:265-279. [DOI] [PubMed] [Google Scholar]
- 41.Truyen, U., M. Agbandje, and C. R. Parrish. 1994. Characterization of the feline host range and a specific epitope of feline panleukopenia virus. Virology 200:494-503. [DOI] [PubMed] [Google Scholar]
- 42.Truyen, U., J. F. Evermann, E. Vieler, and C. R. Parrish. 1996. Evolution of canine parvovirus involved loss and gain of feline host range. Virology 215:186-189. [DOI] [PubMed] [Google Scholar]
- 43.Truyen, U., A. Gruenberg, S. F. Chang, B. Obermaier, P. Veijalainen, and C. R. Parrish. 1995. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J. Virol. 69:4702-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Truyen, U., and C. R. Parrish. 1992. Canine and feline host ranges of canine parvovirus and feline panleukopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. J. Virol. 66:5399-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsang, S. K., B. M. McDermott, V. R. Racaniello, and J. M. Hogle. 2001. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. J. Virol. 75:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao, J., M. S. Chapman, M. Agbandje, W. Keller, K. Smith, H. Wu, M. Luo, T. J. Smith, M. G. Rossmann, R. W. Compans, and C. R. Parrish. 1991. The three-dimensional structure of canine parvovirus and its functional implications. Science 251:1456-1464. [DOI] [PubMed] [Google Scholar]
- 47.Vihinen-Ranta, M., D. Wang, W. S. Weichert, and C. R. Parrish. 2002. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J. Virol. 76:1884-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vihinen-Ranta, M., W. Yuan, and C. R. Parrish. 2000. Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport. J. Virol. 74:4853-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, D., W. Yuan, I. Davis, and C. R. Parrish. 1998. Nonstructural protein-2 and the replication of canine parvovirus. Virology 240:273-281. [DOI] [PubMed] [Google Scholar]
- 50.Webby, R. J., and R. G. Webster. 2001. Emergence of influenza A viruses. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 356:1817-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weichert, W. S., J. S. Parker, A. T. M. Wahid, S. F. Chang, E. Meier, and C. R. Parrish. 1998. Assaying for structural variation in the parvovirus capsid and its role in infection. Virology 250:106-117. [DOI] [PubMed] [Google Scholar]
- 52.Wentworth, D. E., and K. V. Holmes. 2001. Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): influence of N-linked glycosylation. J. Virol. 75:9741-9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeung, D. E., G. W. Brown, P. Tam, R. H. Russnak, G. Wilson, I. Clark-Lewis, and C. R. Astell. 1991. Monoclonal antibodies to major nonstructural nuclear protein of minute virus of mice. Virology 181:35-45. [DOI] [PubMed] [Google Scholar]
- 54.Yuan, W., and C. R. Parrish. 2000. Comparison of two single-chain antibodies that neutralize canine parvovirus: analysis of an antibody-combining site and mechanisms of neutralization. Virology 269:471-480. [DOI] [PubMed] [Google Scholar]