The ion channel field has benefited enormously from a plethora of natural toxins that target specific ion channels (1–13), and those toxins continue to be key molecular tools in the postgenomics era. The ion transporter field has not been so lucky, in general. Its arsenal of specific toxins is much more limited, perhaps because it's not so easy to bring one's prey, or biological enemy, quickly to its knees by inhibiting an ion transporter or an ion pump. However, the Na/K pump, which establishes the normal sodium and potassium gradients across the outer membranes of most animal cells, is an exception. The Na/K pump is the specific target of two types of natural toxins, heart glycosides (14, 15) and palytoxin (16–18). Although heart glycosides will be well known to the great majority of readers, palytoxin is likely to be obscure. Long deserving of more careful attention, a careful functional analysis of palytoxin actions presented in this issue of PNAS by Artigas and Gadsby (19) takes an important step to pick up the slack.
The two classes of Na/K pump toxins could hardly be more different in their chemical structures (see Fig. 1) or mechanisms of action. Heart glycosides, such as ouabain and strophantidin, are atypical steroids produced by numerous plants, such as foxglove (Digitalis pupurea) (15), presumably to discourage those who would eat them. Na/K pumps are the only known targets for heart glycosides, although all Na/K pumps are not inhibited equally. Potency is both species- and isoform-dependent (20–22). Specifically, heart glycosides bind to the pump when its sodium binding sites are open to the extracellular side (23), thereby locking two sodium ions into the pump (24). Elucidation of this mechanism consolidated in many respects our understanding of the Na/K pump cycle. Heart glycosides continue to be important therapeutic agents in the treatment of cardiac insufficiency (15, 25), and a lively debate continues as to whether heart glycosides really increase cardiac contraction by decreasing the transmembrane sodium gradient (26–28). Potentially, this debate will lead to new insights into cardiac cell signaling and the physiology of endogenous ouabain-like compounds (29, 30). But it would be very surprising if present concepts about the molecular action of ouabain on the Na/K pump would need major correction.
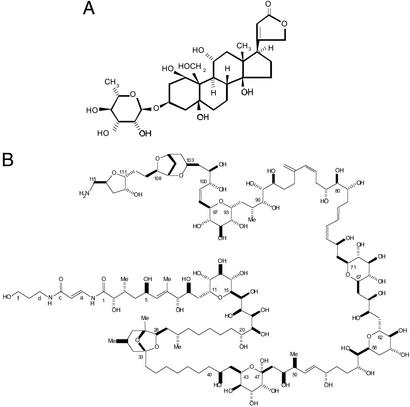
Figure 1.
Structures of ouabain (A) and palytoxin (B). [B modified with permission from ref. 32 (Copyright 1982, American Chemical Society).]
Palytoxin, on the other hand, is a marine toxin (16). It kills mice with an impressive LD50 of 15 ng/kg (16, 31). It is a large nonpeptide molecule with a molecular weight of ≈3,000, containing a contiguous chain of >100 carbon atoms with 64 stereogenic centers (see Fig. 1) (32). Isolated >30 years ago from Palythoa toxica corals on the coast of Hawaii (16) and identified more recently in other sea animals (33), its chemical synthesis was a major technical feat (32), and its mechanism of action is no less outstanding.
Although palytoxin was suggested to have multiple sites of action (31), recent work suggests specificity for Na/K pumps (34, 35) and possibly a closely related H/K pump (36). The results of Artigas and Gadsby (19) provide very strong new support for specific actions at the Na/K pump in cardiac cells. Entirely unique among toxins characterized to date, palytoxin bridges the worlds of ion channels and transporters by converting the Na/K pump from an ion pump into a nonspecific ion channel (17, 34). The mechanism is devious, because in so doing palytoxin short-circuits the membrane function of cells that rely on Na/K pumps to generate ion gradients (37) and finally can cause cell lysis (38). The mechanism is fundamental, because it touches on long-standing questions about the commonalities of ion channels and pumps, as well as their molecular evolution. Artigas and Gadsby (19) are providing important insights into this molecular conversion by demonstrating that partial reactions of the Na/K pump cycle become gating reactions for the palytoxin-induced channel activity.
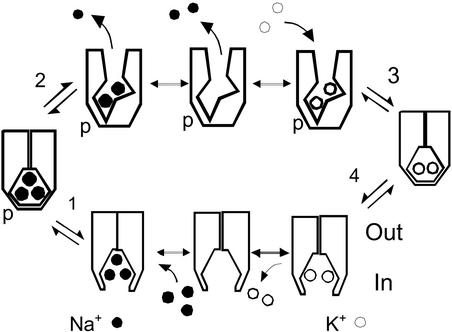
Most physiologists appreciate that ion channels and ion pumps are somehow related in their function and structure. But the devil still lurks in the details! Most ion channels undergo multiple types of gating reactions that open and close their conduction pathway (1). As sketched by Artigas and Gadsby (19), the partial reactions of the Na/K pump (39) seem tantalizingly homologous to the gating reactions of ion channels. Two gating reactions must occur in the pump, one that opens ion binding sites to the cytoplasmic side and one that opens binding sites to the extracellular side. In contrast to an ion channel, one gate must remain closed at all times. With these non-negotiable specifications, the Na/K pump cycle occurs via four “gating” reactions as illustrated in Fig. 2: When the cytoplasmic gate is open (Fig. 2 Lower), the binding of sodium ions turns a molecular switch that activates ATP hydrolysis and closes the cytoplasmic gate. With the cytoplasmic gate closed, the extracellular gate can open, allowing sodium to leave and potassium ions to bind on the extracellular side (Fig. 2 Upper). The binding of potassium ions turns a molecular switch to dephosphorylate the pump and close the extracellular gate (40, 41). Finally, the cytoplasmic gate can open and release potassium ions to the cytoplasmic side.
Figure 2.
The Na/K pump cycle. Each cartoon of the pump is drawn with the cytoplasmic side facing downward. When binding sites are open to the cytoplasmic side, the pump has a high affinity for sodium. When binding sites are open to the extracellular side, the pump has a high affinity for potassium. From the cytoplasmic side, three sodium ions can bind when binding sites are not occupied by potassium, and the pump then autophosphorylates (39) and closes its cytoplasmic gate (reaction 1). Therewith, the extracellular gate can open (reaction 2), releasing one sodium ion, and the last two sodium ions can dissociate and be replaced by potassium ions. When two potassium ions are bound, the extracellular gate can close and the pump dephosphorylates (reaction 3). Therewith, the cytoplasmic gate can open and potassium ions can be replaced by sodium ions on the cytoplasmic side (reaction 4).
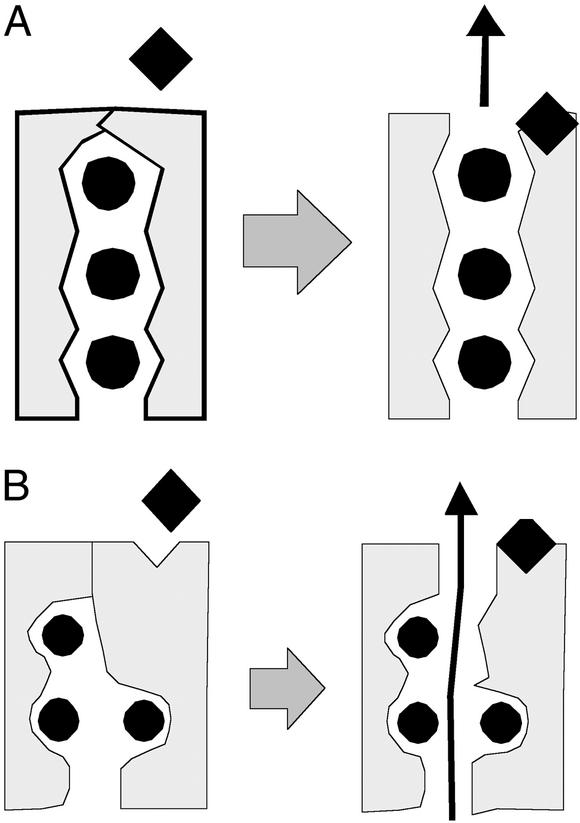
Now comes palytoxin, which allows, according to Artigas and Gadsby (19), both gates of the Na/K pump to be open at the same time. What can be learned by careful analysis of the channel activity? The first eye-opener is that the channel formed is nonspecific and of relatively low conductance (≈10 pS). For both sodium and potassium channels, a number of toxins and drugs are known to act by preventing channel closure or inactivation (see Fig. 3A). In the case of veratradine (9), for example, sodium channels remain open for very long times and thereby prolong action potentials and load cells with sodium. In the case of the KATP potassium channel opener, pinacidil, the closure of channels induced by cytoplasmic ATP can be largely prevented (42, 43). In no case is it reported that the selectivity or single channel conductance is changed by an “ion channel opener.” As seen in Fig. 3A, the selectivity and high through-put of ion channels relies on a single-file pathway of the ions through multiple binding sites. If selectivity of the Na/K pump occurred in a similar single-file pathway, the opening of pump's gates would generate a selective channel conductance. That is not the case. The second eye-opener is that all of the ligands known to act on the Na/K pump can be demonstrated to modulate the gating of palytoxin-induced ion channel activity. Furthermore, the actions of ions and ATP on channel gating can be accounted for reasonably by knowledge of the pump cycle, sketched in Fig. 2. This is strong evidence that the palytoxin-induced channels are indeed generated by Na/K pumps, not by some associated ion channel. More importantly, the results open entirely new possibilities for studying conformational changes of the Na/K pump and their control by pump ligands at the level of single pumps.
Figure 3.
Cartoons of the permeation pathways of an ion channel (A) and the Na/K pump (B) when their gates are opened by a toxin. The ion diffusion pathway of the channel is selective, whereas that of the Na/K pump is not.
How are the molecular mechanisms of ion pumps and channels really similar and different? Artigas and Gadsby (19) make an eloquent case for studying the Na/K pump as a modified ion channel with two gates and a central pore in which ions bind. At the same time, fundamental differences between ion pumps and channels are also coming into focus. After all, very different evolutionary pressures must have existed to generate efficient ion channels and pumps from a hypothetical common ancestor. The pores of ion channels clearly evolved to maximize selectivity and throughput, and single filing of ions through sequential binding sites is fundamental to these ends (44). Ion pumps have no need of single filing, because their turnover rates are determined by slow conformational changes. Indeed, the two calcium ions transported by the calcium pump of the sarcoplasmic reticulum are bound side by side (45), not in a single file, and ligand binding reactions also are suggested to take place in parallel sites in other transporters (e.g, ref. 46). There are certainly exceptions, but the gates of ion channels in general work rather independently from the binding of ions in the permeation pathway (47). That is why gating currents could be measured with high resolution in voltage-gated ion channels in the absence of permeating ions (48). The gates of transporters and pumps, on the other hand, must be controlled with high selectivity and reliability by the binding and unbinding of ions. For the Na/K pump, at least four different gating reactions must be controlled reliably. Given these requirements, very different from those of any ion channel, it would not be surprising if ion binding and coordination reactions were rather different from in a channel. Slower reactions with substantially higher interaction energies would certainly not be a surprise. For sodium channels, it has been suggested that a change of ion selectivity can be used as a physiological signaling mechanism to bring calcium into cells (49, 50). However, those claims remain unproved (51, 52). For the Na/K pump, a change of ion binding selectivity is the bread and butter of every transport cycle. The task of understanding these exquisitely controlled molecular reactions is daunting, and major strides in the structural arena are, of course, a prerequisite. However, new approaches to tease apart and analyze the individual reactions of the pump are also a prerequisite, and palytoxin in the right hands clearly provides an important handle to do so.
Acknowledgments
The chemical structure of palytoxin was kindly provided by Dr. Yoshito Kishi (Harvard, Boston).
Footnotes
See companion article on page 501.
References
- 1.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 2.Rash L D, Hodgson W C. Toxicon. 2002;40:225–254. doi: 10.1016/s0041-0101(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 3.Garcia M L, Gao Y, McManus O B, Kaczorowski G J. Toxicon. 2001;39:739–748. doi: 10.1016/s0041-0101(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 4.Escoubas P, Diochot S, Corzo G. Biochimie. 2000;82:893–907. doi: 10.1016/s0300-9084(00)01166-4. [DOI] [PubMed] [Google Scholar]
- 5.Cestele S, Catterall W A. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 6.Lewis R J. Ther Drug Monit. 2000;22:61–64. doi: 10.1097/00007691-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Benoit E. C R Seances Soc Biol Fil. 1998;192:409–436. [PubMed] [Google Scholar]
- 8.Scheiner-Bobis G. Naunyn-Schmiedebergs Arch Pharmacol. 1998;357:477–482. doi: 10.1007/pl00005196. [DOI] [PubMed] [Google Scholar]
- 9.Ulbricht W. Rev Physiol Biochem Pharmacol. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- 10.Mathie A, Wooltorton J R, Watkins C S. Gen Pharmacol. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 11.Uchitel O D. Toxicon. 1997;35:1161–1191. doi: 10.1016/s0041-0101(96)00210-3. [DOI] [PubMed] [Google Scholar]
- 12.Ohizumi Y. Jpn J Pharmacol. 1997;73:263–289. doi: 10.1254/jjp.73.263. [DOI] [PubMed] [Google Scholar]
- 13.Oliveraa B M, Cruzab L J. Toxicon. 2001;39:7–14. [Google Scholar]
- 14.Schwartz A, Adams R J. Circ Res. 1980;46:I154–I160. [PubMed] [Google Scholar]
- 15.Eichhorn E J, Gheorghiade M. Prog Cardiovasc Dis. 2002;44:251–266. doi: 10.1053/pcad.2002.31591. [DOI] [PubMed] [Google Scholar]
- 16.Moore R E, Scheuer P J. Science. 1971;172:495–498. doi: 10.1126/science.172.3982.495. [DOI] [PubMed] [Google Scholar]
- 17.Habermann E. Toxicon. 1989;27:1171–1187. doi: 10.1016/0041-0101(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki H, Nagase H, Urakawa N. Eur J Biochem. 1985;152:475–480. doi: 10.1111/j.1432-1033.1985.tb09221.x. [DOI] [PubMed] [Google Scholar]
- 19.Artigas P, Gadsby D C. Proc Natl Acad Sci USA. 2003;100:501–505. doi: 10.1073/pnas.0135849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta R S, Chopra A, Stetsko D K. J Cell Physiol. 1986;127:197–206. doi: 10.1002/jcp.1041270202. [DOI] [PubMed] [Google Scholar]
- 21.Kent R B, Emanuel J R, Ben Neriah Y, Levenson R, Housman D E. Science. 1987;237:901–903. doi: 10.1126/science.3039660. [DOI] [PubMed] [Google Scholar]
- 22.Blanco G, Mercer R W. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 23.Shainskaya A, Schneeberger A, Apell H J, Karlish S J. J Biol Chem. 2000;275:2019–2028. doi: 10.1074/jbc.275.3.2019. [DOI] [PubMed] [Google Scholar]
- 24.Sturmer W, Apell H J. FEBS Lett. 1992;300:1–4. doi: 10.1016/0014-5793(92)80151-6. [DOI] [PubMed] [Google Scholar]
- 25.Erdmann E. Basic Res Cardiol. 2000;95, Suppl. 1:I3–I7. doi: 10.1007/s003950070002. [DOI] [PubMed] [Google Scholar]
- 26.Xie Z. Cell Mol Biol. 2001;47:383–390. [PubMed] [Google Scholar]
- 27.Nishio M, Ruch S W, Wasserstrom J A. Am J Physiol. 2002;283:H2045–H2053. doi: 10.1152/ajpheart.00203.2002. [DOI] [PubMed] [Google Scholar]
- 28.Reuter H, Henderson S A, Han T, Ross R S, Goldhaber J I, Philipson K D. Circ Res. 2002;90:305–308. doi: 10.1161/hh0302.104562. [DOI] [PubMed] [Google Scholar]
- 29.Schoner W. Eur J Biochem. 2002;269:2440–2448. doi: 10.1046/j.1432-1033.2002.02911.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferrandi M, Manunta P, Rivera R, Bianchi G, Ferrari P. Clin Exp Hypertens. 1998;20:629–639. doi: 10.3109/10641969809053241. [DOI] [PubMed] [Google Scholar]
- 31.Frelin C, Van Renterghem C. Gen Pharmacol. 1995;26:33–37. doi: 10.1016/0306-3623(94)00133-8. [DOI] [PubMed] [Google Scholar]
- 32.Cha J K, Christ W J, Finan J M, Fujioka H, Kishi Y. J Am Chem Soc. 1982;104:7369–7371. [Google Scholar]
- 33.Hokama Y. Food Addit Contam. 1993;10:71–82. doi: 10.1080/02652039309374131. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Horisberger J D. FEBS Lett. 1997;409:391–395. doi: 10.1016/s0014-5793(97)00559-0. [DOI] [PubMed] [Google Scholar]
- 35.Guennoun S, Horisberger J D. FEBS Lett. 2000;482:144–148. doi: 10.1016/s0014-5793(00)02050-0. [DOI] [PubMed] [Google Scholar]
- 36.Scheiner-Bobis G, Hubschle T, Diener M. Eur J Biochem. 2002;269:3905–3911. doi: 10.1046/j.1432-1033.2002.03056.x. [DOI] [PubMed] [Google Scholar]
- 37.Tosteson M T, Halperin J A, Kishi Y, Tosteson D C. J Gen Physiol. 1991;98:969–985. doi: 10.1085/jgp.98.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bignami G S. Toxicon. 1993;31:817–820. doi: 10.1016/0041-0101(93)90389-z. [DOI] [PubMed] [Google Scholar]
- 39.Post R L. Annu Rev Physiol. 1989;51:1–15. doi: 10.1146/annurev.ph.51.030189.000245. [DOI] [PubMed] [Google Scholar]
- 40.Hobbs A S, Albers R W, Froehlich J P. J Biol Chem. 1980;255:3395–3402. [PubMed] [Google Scholar]
- 41.Swann A C, Albers R W. J Biol Chem. 1979;254:4540–4544. [PubMed] [Google Scholar]
- 42.Fujita A, Kurachi Y. Pharmacol Ther. 2000;85:39–53. doi: 10.1016/s0163-7258(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 43.Fan Z, Nakayama K, Hiraoka M. J Physiol (London) 1990;430:273–295. doi: 10.1113/jphysiol.1990.sp018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle D A, Morais C J, Pfuetzner R A, Kuo A, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 45.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 46.Hilgemann D W, Lu C C. J Gen Physiol. 1999;114:459–475. doi: 10.1085/jgp.114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bezanilla F. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong C M, Bezanilla F. J Gen Physiol. 1974;63:533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorbera L A, Morad M. Science. 1990;247:969–973. doi: 10.1126/science.2154853. [DOI] [PubMed] [Google Scholar]
- 50.Santana L F, Gomez A M, Lederer W J. Science. 1998;279:1027–1033. doi: 10.1126/science.279.5353.1027. [DOI] [PubMed] [Google Scholar]
- 51.Piacentino V, III, Gaughan J P, Houser S R. Circ Res. 2002;90:435–442. doi: 10.1161/hh0402.105666. [DOI] [PubMed] [Google Scholar]
- 52.Hirano Y, Hiraoka M. Jpn J Physiol. 2001;51:679–685. doi: 10.2170/jjphysiol.51.679. [DOI] [PubMed] [Google Scholar]