Abstract
Enzymes in the mitochondrial respiratory chain are involved in various physiological events in addition to their essential role in the production of ATP by oxidative phosphorylation. The use of specific and potent inhibitors of complex I (NADH-ubiquinone reductase) and complex III (ubiquinol-cytochrome c reductase), such as rotenone and antimycin, respectively, has allowed determination of the role of these enzymes in physiological processes. However, unlike complexes I, III, and IV (cytochrome c oxidase), there are few potent and specific inhibitors of complex II (succinate-ubiquinone reductase) that have been described. In this article, we report that atpenins potently and specifically inhibit the succinate-ubiquinone reductase activity of mitochondrial complex II. Therefore, atpenins may be useful tools for clarifying the biochemical and structural properties of complex II, as well as for determining its physiological roles in mammalian tissues.
The use of specific and potent inhibitors of respiration has enabled the investigation of how the respiratory enzymes function in physiological processes. However, unlike other enzyme complexes in the respiratory chain, there has been a lack of potent and specific inhibitors of complex II [succinate-ubiquinone reductase (SQR)]. Although carboxin (5,6-dihydro-2-methyl-N-phenyl-1,4-oxathiin-3-carboxamide), TTFA [4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione], and HQNO (2-heptyl-4-hydroxyquinoline N-oxide) have long been known as complex II inhibitors and have been used extensively to elucidate the structure-function relationships of complex II, rather higher concentration is required for the inhibition (1). This result has hampered the study of the structure-function relationship of the complex II enzyme, as well as its roles in physiological processes.
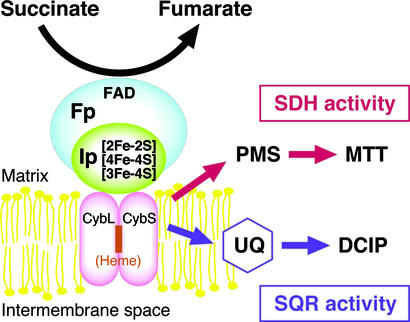
Complex II catalyzes the oxidation of succinate in the inner membrane of mitochondria and in the cytoplasmic membrane of bacteria (1–3). In addition to its function as a dehydrogenase in the respiratory system, complex II plays an important role in the tricarboxylic acid cycle. Mitochondrial complex II is an integral membrane protein consisting of four subunits (Fig. 1). The largest subunit is the 70-kDa, FAD-containing flavoprotein subunit (Fp). The dehydrogenase catalytic portion of complex II is formed by Fp and an ≈30-kDa iron-sulfur protein subunit (Ip) containing three different types of iron-sulfur clusters. The small hydrophobic subunits, SDHC or CybL (≈15 kDa) and SDHD or CybS (≈13 kDa), anchor the catalytic portion to the membrane and are also required for electron transfer to quinones. In contrast to mitochondrial complex IIs, some bacterial complex IIs contain only one larger hydrophobic polypeptide as a membrane anchor (see ref. 4 for reviews).
Figure 1.
Subunit structure and enzyme activities of complex II. SDH activity is determined by measuring the electron transfer from succinate to the water-soluble dyes, PMS and MTT, and is determined by monitoring the absorption change of MTT. SQR activity is determined as the electron transfer from succinate to ubiquinone. The formation of ubiquinol was measured by the ubiquinol-dependent reduction of DCIP. Fp, flavoprotein subunit; Ip, iron-sulfur protein subunit; CybL and CybS, large and small subunits of cytochrome b.
In addition to its essential role in energy production, various recent findings suggest that mutant variants of complex II are involved in causing diverse physiological disorders. For instance, a mutation in the CybL subunit in Caenorhabditis elegans (mev-1 mutant) results in a shortened life span of the organism, possibly due to an overproduction of superoxide (5, 6). An increasing number of reports also indicate a correlation between mutations in the CybS (SDHD) (7–12), CybL (SDHC) (13), and Ip (SDHB) (14) subunits of complex II and hereditary paraganglioma, a disorder of the carotid body, which is a chemoreceptive organ that senses oxygen levels in the blood. Mutations in these genes can apparently also cause tumor formation in familial pheochromocytomas (15). Based on these observations, it has been hypothesized that SDHB, -C, and -D are tumor suppressors and that complex II is a critical component of the oxygen-sensing system (16). Finally, the reverse function of complex II (fumarate reduction) is important in the recovery of mammalian tissues from ischemia-reperfusion (17).
In anaerobic parasitic eukaryotes, complex II acts as quinol-fumarate reductase (QFR) where it functions as a terminal oxidase in the NADH-fumarate pathway (18, 19). Thus, complex II is indispensable for the survival of anaerobic parasitic eukaryotes and, therefore, is regarded as a good chemotherapeutic target for novel antihelmintics. In the course of screening microbial broths for inhibitors of the NADH-fumarate pathway, we isolated a previously uncharacterized compound, nafuredin (20, 21). We determined that nafuredin is a selective inhibitor of helminth complex I (NADH-quinone oxidoreductase), and was an effective antihelmintic in animal trials (22). Further screening for inhibitors led to the isolation of harzianopyridone, an inhibitor of the NADH-fumarate reductase activity of adult Ascaris suum mitochondria.
In this paper, we show that harzianopyridone and the chemically related atpenins inhibit the QFR activity of complex II in the parasite mitochondria. Moreover, these compounds are also effective against SQR activity of mammalian complex II. The atpenins are specific to complex II and are much more potent than other known complex II inhibitors, such as TTFA, HQNO, and carboxin.
Materials and Methods
Fermentation and Purification of Harzianopyridone and Atpenins.
Trichoderma sp. FTD-0795 was cultured statically in 1-liter Loux flasks containing 200 ml of production medium (5.0% maltose/3.0% dry yeast/1.0% KBr/0.05% KH2PO4/0.05% MgSO4⋅7H2O, pH 6.0) at 27°C for 5 days. The cultured broth was first extracted with ethanol and then with ethyl acetate. The ethyl acetate layer was concentrated to dryness and applied on a silica gel chromatography column that was eluted with n-hexane and ethyl acetate (stepwise elution). The fractions that showed inhibitory activity of NADH-fumarate reductase were concentrated to dryness, and a white powder was obtained by precipitation in n-hexane. Its physico-chemical properties were identical to harzianopyridone (23, 24). Atpenins were isolated from a cultured broth of the atpenin-producing strain Penicillium sp. FO-125 (25). This strain was cultured in 500-ml Erlenmeyer flasks containing 100 ml of a production medium (1.0% glucose/0.5% tryptone/0.3% yeast extract/0.3% malt extract/0.1% agar, pH 6.0) on a rotary shaker (210 rpm) at 27°C for 6 days. The broth supernatant obtained after centrifugation was extracted with ethyl acetate. The mycelium pellet was first extracted with acetone and then with ethyl acetate. Both ethyl acetate layers were concentrated to dryness and subjected to chromatography on a silica gel column that was eluted with n-hexane and ethyl acetate (stepwise elution). The fractions that showed the inhibition were concentrated and applied on a Sephadex LH-20 (Pharmacia) column eluted with chloroform–methanol (1:2) to yield pure atpenins A4 and A5.
Enzyme Assays.
A. suum mitochondria were prepared from adult worms as described (26). Mammalian mitochondria were prepared as described (27). Succinate dehydrogenase (SDH) activity was measured by monitoring the absorbance change of 2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide (MTT) at 570 nm in the presence of phenazine methosulfate (PMS), by using the extinction coefficient of 17 mM−1⋅cm−1 for MTT. SQR activity was measured in the presence of 2,3-dimethoxy-5-methyl-6-geranyl-1,4-benzoquinone (UQ2) and 2,6-dichlorophenolindophenol (DCIP) (28). Rhodoquinol-fumarate reductase and NADH-fumarate reductase activities were measured under anaerobic conditions (22). NADH-cytochrome c reductase activity was measured as described (29).
Chemicals.
TTFA, HQNO, and UQ2 were purchased from Sigma. Carboxin was purchased from Wako (Tokyo).
Results and Discussion
The Inhibition of Complex II by Atpenins.
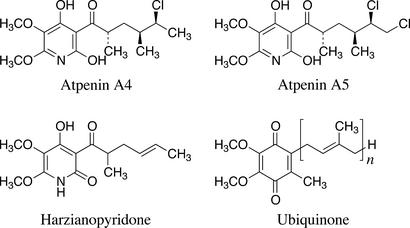
In the course of screening for inhibitors of the NADH-fumarate reductase of A. suum mitochondria, we obtained harzianopyridone from the cultured broth of Trichoderma sp. FTD-0795, a fungus isolated from soil samples. Harzianopyridone was originally isolated from Trichoderma harzianum and showed antifungal, antibacterial, and herbicidal activities (23, 24). Its structure was suggested to be a tautomer of 2-pyridone and 2-pyridinol (Fig. 2). Because we previously have isolated similar compounds, atpenins A4, A5, and B, as antifungal antibiotics (25, 30) and because atpenin B may inhibit the ATP-generating system (31), we examined the effect of these compounds on enzymatic activities of complex I and complex II from A. suum. As shown in Table 1, we found that the inhibition by atpenins and harzianopyridone is specific to QFR and SQR (complex II), but that they do not inhibit complex I. Furthermore, the atpenins were more effective than harzianopyridone in the inhibition of complex II.
Figure 2.
Structures of atpenins, harzianopyridone, and ubiquinone. The n in UQ indicates the isoprenoid side chain length.
Table 1.
Atpenin inhibition of respiratory enzymes in nematode and mammalian mitochondria
| Species | Activities | IC50, μM
|
||
|---|---|---|---|---|
| Atpenin A4 | Atpenin A5 | Harzianopyridone | ||
| A. suum adult muscle | Complex I + II* | 0.11 | 0.014 | 1.6 |
| Complex I† | >100 | >100 | >100 | |
| Complex II (QFR)‡ | 0.22 | 0.012 | 0.36 | |
| Complex II (SQR)§ | 0.22 | 0.032 | 2 | |
| Bovine heart | Complex I + III¶ | 140 | 82 | 420 |
| Complex II§ | 0.011 | 0.0036 | 0.017 | |
| Rat liver | Complex I + III¶ | >500 | >500 | >500 |
| Complex II§ | 0.024 | 0.0037 | 0.2 | |
Measured by NADH-fumarate reductase activity.
Measured by NADH-quinone reductase activity. UQ1, UQ2, or decylrhodoquinone was used as electron acceptor.
Measured by rhodoquinol-fumarate reductase activity.
Measured by succinate-UQ reductase activity.
Measured by NADH-cytochrome c reductase activity.
We further characterized the effects of atpenins on mammalian enzymes. Interestingly, atpenins were even more potent inhibitors of SQR activity of bovine heart mitochondria than of A. suum mitochondria (Table 1). Moreover, based on NADH-cytochrome c reductase activity measurements, atpenins had little effect on the activity of complexes I and III. These characteristics were also observed with mitochondria from rat liver (Table 1). These results show that atpenins selectively inhibit mitochondrial complex II irrespective of the enzyme source. In addition, it is likely that the antifungal effects of atpenins (25) are due to their potent inhibition of complex II.
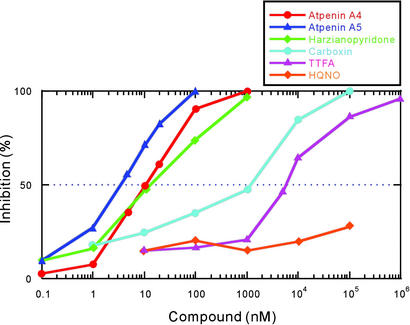
Next, we compared the potency of the atpenins and the known complex II inhibitors TTFA, HQNO, and carboxin. Fig. 3 clearly shows that atpenins are significantly more potent than the other complex II inhibitors. For instance, the IC50 value of atpenin A5 is 300-fold lower than that for carboxin (IC50 = 1.1 μM) and 1,600-fold lower than that for TTFA (IC50 = 5.8 μM). Moreover, complete inhibition of complex II by atpenins was achieved at 0.1–1 μM, a concentration 2–3 orders of magnitude lower than that for carboxin and TTFA. Much higher concentrations of atpenins (IC50 = 15 μM for atpenin A4, 5 μM for atpenin A5, and 10 μM for harzianopyridone) were required to inhibit the activity of purified Escherichia coli SQR (data not shown). This finding is consistent with the ineffectiveness of atpenins A4 and A5 against Gram-positive and Gram-negative bacteria, although they are effective antifungal agents (25).
Figure 3.
Inhibition of SQR activity of bovine heart mitochondria by atpenins and known complex II inhibitors. The assay was performed at 25°C in the presence of 50 mM potassium phosphate buffer (pH 7.5), 90 μM UQ2, 74 μM DCIP, potassium succinate (10 mM), potassium cyanide (1 mM), and 30 μg of protein of bovine heart mitochondria (111 and 159 nmol/min/mg of SQR and SDH activities, respectively). SQR activity was determined by using the millimolar extinction coefficient for DCIP (21 mM−1⋅cm−1 at 600 nm). The IC50 values for atpenins and harzianopyridone are presented in Table 1. The IC50 values for other inhibitors are as follows: carboxin, 1.1 μM; TTFA, 5.8 μM; and HQNO, >100 μM.
Mechanism of Complex II Inhibition by Atpenins.
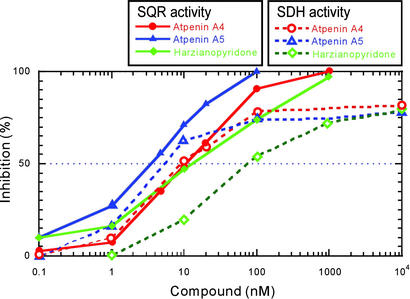
The mechanism of inhibition by atpenins was further studied in bovine heart mitochondria because the mammalian complex II is more sensitive to these compounds than the complex II from A. suum. We first examined the effect of atpenins on SDH activity. SDH activity is a partial reaction of SQR catalyzed by the electron transfer from succinate to the water-soluble electron acceptor PMS (Fig. 1). This activity does not require the presence of membrane anchor subunits, which are essential components for electron transfer to UQ (1, 2). Interestingly, atpenins inhibited the SDH activity of bovine heart complex II (Fig. 4). However, complete inhibition of SDH activity was not achieved even at 10 μM (Fig. 4). This finding indicates that atpenins are not capable of fully blocking electron transfer from the iron-sulfur centers in the Ip subunit to water-soluble dyes, a process considered to occur via a nonphysiological electron transfer pathway.
Figure 4.
Inhibition of SQR and SDH activities by atpenins. The inhibition of SQR and SDH activities by atpenins was examined by using bovine heart mitochondria as described in Fig. 3. Solid lines indicate the inhibition of SQR activity and dashed lines indicate the inhibition of SDH activity. The IC50 values for SQR activity are indicated in Table 1. The IC50 values for SDH activity are as follows: atpenin A4, 9.2 nM; atpenin A5, 5.5 nM; and harzianopyridone, 80 nM.
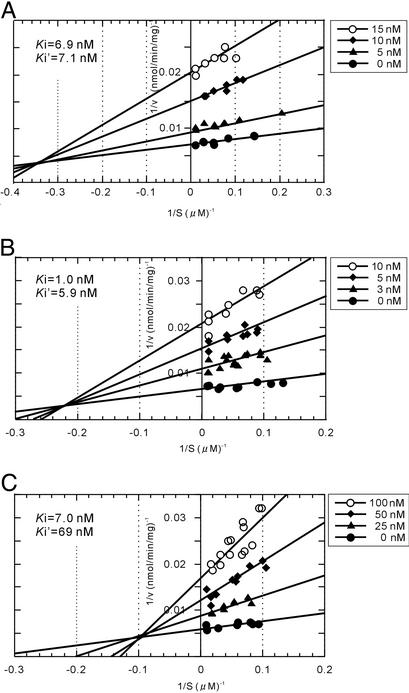
Double-reciprocal plots (Fig. 5) showed mixed inhibition of atpenins with UQ2. This finding indicates that atpenins may block the electron transfer between the enzyme and ubiquinone (UQ) by binding to a region that partly overlaps with the physiological UQ-binding site. This result is not surprising given the structural similarity between atpenins and UQ (Fig. 2). A similar result was obtained when the inhibition was analyzed by using purified bovine complex II (A.O., unpublished results).
Figure 5.
Double-reciprocal plots of atpenins inhibition of SQR activity. (A) Atpenin A4. (B) Atpenin A5. (C) Harzianopyridone. Inhibition of SQR activity of bovine heart mitochondria by atpenins was measured in the presence of various concentrations of UQ2. The assay conditions were the same as described for Fig. 2. Ki and Ki′ values of each compound are indicated in the graphs.
The kinetic analyses also show that the Ki values for atpenins A4 and A5 are in the low nanomolar range (Fig. 5). Atpenin A5 is the most potent inhibitor among the various atpenins tested and harzianopyridone (Table 1). This finding implies that, in addition to its aromatic ring structure, the structure of the alkyl side chain of the atpenins is important for their potency. The fact that atpenin A5 was the most effective compound against complex II from all of the species tested further suggests that the spatial arrangement in the UQ-binding site and the surrounding area of complex II are relatively conserved. Also, the highly specific inhibition of complex II by atpenins (Table 1) coincides well with a report by Tan et al. (32), suggesting that the structure of the quinone-binding site in complex II is not closely related to those in complexes I and III.
Unlike bacterial QFR (33, 34), the UQ-binding site in SQR has yet to be identified, although initial crystallographic analyses of E. coli SQR have recently been published (35). However, studies of carboxin resistant strains of Paracoccus denitrificans and fungi indicate that amino acid residues close to the [3Fe-4S] center in SDHB as well as a residue in SDHD, located in the cytoplasmic loop between transmembrane segments, are important for the binding of carboxin (36–40) and possibly for the recognition of UQ. Thus, it is predicted that UQ binds to complex II at the interface between Ip and the membrane anchor domain (36). This hypothesis can explain the observation that atpenins affected also SDH activity of bovine heart complex II. We found that atpenins inhibited SDH activity, but less efficiently, and complete inhibition was not achieved even at high concentrations. These findings and mixed type of inhibition of SQR activity are very similar to those reported with carboxin.
Respiratory enzyme complexes are thought to play various physiological roles in addition to their main task in energy production. For instance, complex I seems to participate in permeability transition pore (PTP)-induced cell death (41), and it may contribute to the pathogenesis of Parkinson's disease (42). Complex III, on the other hand, is thought to be involved in the generation of superoxide anion (43). These findings have been obtained due to the use of rotenone and antimycin A, well-known specific inhibitors of complex I and complex III, respectively. Accumulating evidence suggests that complex II dysfunction is coupled to various disorders, such as paraganglioma (7–17) and N-methyl-d-aspartate receptor-mediated excitotoxic neuronal death (44). However, the lack of specific and potent inhibitors of complex II has hampered the detailed physiological analysis of complex II function. Because of their potency and specificity, atpenins, especially atpenin A5, may be ideal tools to further study both the role of complex II in various cellular and physiological processes and the mechanisms of electron transfer in mitochondrial complex II.
Acknowledgments
We thank Drs. Achim Harder and Heinz Kölbl, Bayer AG, for useful discussions. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Culture and Sport, Japan (Grants 13226015 and 13854011, to K.K.), a grant for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health and Welfare (to K.K.), a grant from the Iwadare Foundation (to H.M.), a Grant-in-Aid for Scientific Research (Grant 14593006, to K.S.) from the Japan Society for the Promotion of Science, a Grant-in-Aid for Encouragement of Young Scientists (Grant 12771373, to H.U.) from the Japan Society for the Promotion of Science, a grant from the hoh-ansha foundation (to K.S.), and a grant from the Japan Keirin Association.
Abbreviations
- Fp
flavoprotein subunit
- Ip
iron-sulfur protein subunit
- SQR
succinate-ubiquinone reductase
- QFR
quinol-fumarate reductase
- SDH
succinate dehydrogenase
- TTFA
4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione
- HQNO
2-heptyl-4-hydroxyquinoline N-oxide
- MTT
2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide
- PMS
phenazine methosulfate
- DCIP
2,6-dichlorophenolindophenol
- UQ
ubiquinone
- UQ2
2,3-dimethoxy-5-methyl-6-geranyl-1,4-benzoquinone
References
- 1.Hägerhäll C. Biochim Biophys Acta. 1997;1320:107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 2.Ackrell B A C, Johnson M K, Gunsalus R P, Cecchini G. In: Chemistry and Biochemistry of Flavoenzymes. Muller F, editor. III. London: CRC; 1992. pp. 229–297. [Google Scholar]
- 3.Ohnishi T, Moser C C, Page C C, Dutton P L, Yano T. Struct Fold Dis. 2000;8:R23–R32. doi: 10.1016/s0969-2126(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 4. Lancaster, C. R. D., ed. (2002) Biochim. Biophys. Acta1553. [DOI] [PubMed]
- 5.Ishii N, Fujii M, Hartman P S, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- 6.Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman P S, Ishii N. J Biol Chem. 2001;276:41553–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- 7.Baysal B E, Rubinsterin W S, Taschner P E M. J Mol Med. 2001;79:495–503. doi: 10.1007/s001090100267. [DOI] [PubMed] [Google Scholar]
- 8.Astuti D, Douglas F, Lennard T W, Aligianis I A, Woodward E R, Evans D G R, Eng C, Latif F, Maher E R. Lancet. 2001;357:1181–1182. doi: 10.1016/S0140-6736(00)04378-6. [DOI] [PubMed] [Google Scholar]
- 9.Baysal B E, Ferrell R E, Willett-Brozick J E, Lawrence E C, Myssiorek D, Bosch A, van der Mey A, Taschner P E M, Rubinstein W S, Myers E N, et al. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 10.Gimenez-Ruqueplo A-P, Favier J, Rustin P, Mourad J-J, Plouin P-F, Corvol P, Rötig A, Jeunemaitre X. Am J Hum Genet. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taschner P E, Jansen J C, Baysal B E, Rosenberg E H, Brocker-Vriends A H, van Der Mey A G, van Ommen G J, Cornelisse C J, Devilee P. Genes Chromosomes Cancer. 2001;31:274–281. doi: 10.1002/gcc.1144. [DOI] [PubMed] [Google Scholar]
- 12.Badenhop R F, Cherian S, Lord R S, Baysal B E, Taschner P E, Schofield P R. Genes Chromosomes Cancer. 2001;31:255–263. doi: 10.1002/gcc.1142. [DOI] [PubMed] [Google Scholar]
- 13.Niemann S, Müller U. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 14.Astuti D, Larif F, Dallol A, Dahia P L M, Douglas F, George E, Sköldberg F, Husebye E S, Eng C, Maher E R. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimm O, Armanios M, Dziema H, Neumann H P H, Eng C. Cancer Res. 2000;60:6822–6825. [PubMed] [Google Scholar]
- 16.Rustin P, Rötig A. Biochim Biophys Acta. 2002;1553:117–122. doi: 10.1016/s0005-2728(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg J M, Venkatachalam M A, Roeser N F, Nissim I. Proc Natl Acad Sci USA. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tielens A G M, Van Hellemond J J. Biochim Biophys Acta. 1998;1365:71–78. doi: 10.1016/s0005-2728(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 19.Kita K, Hirawake H, Miyadera H, Amino H, Takeo S. Biochim Biophys Acta. 2002;1553:123–139. doi: 10.1016/s0005-2728(01)00237-7. [DOI] [PubMed] [Google Scholar]
- 20.Ui H, Shiomi K, Yamaguchi Y, Masuma R, Nagamitsu T, Takano D, Sunazuka T, Namikoshi M, Ōmura S. J Antibiot. 2001;54:234–238. doi: 10.7164/antibiotics.54.234. [DOI] [PubMed] [Google Scholar]
- 21.Takano D, Nagamitsu T, Ui H, Shiomi K, Yamaguchi Y, Masuma R, Kuwajima I, Ōmura S. Organ Lett. 2001;3:2289–2291. doi: 10.1021/ol010089t. [DOI] [PubMed] [Google Scholar]
- 22.Ōmura S, Miyadera H, Ui H, Shiomi K, Yamaguchi Y, Masuma R, Nagamitsu T, Takano D, Sunazuka T, Harder A, et al. Proc Natl Acad Sci USA. 2001;98:60–62. doi: 10.1073/pnas.011524698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickinson, J. M., Hanson, J. R., Hitchcock, P. B. & Claydon, N. (1989) J. Chem. Soc. Perkin Trans. 1, 1885–1887.
- 24.Cutler H G, Jacyno J M. Agric Biol Chem. 1991;55:2629–2631. [Google Scholar]
- 25.Ōmura S, Tomoda H, Kimura K, Zhen D-Z, Kumagai H, Igarashi K, Imamura N, Takahashi Y, Tanaka Y, Iwai Y. J Antibiot. 1988;41:1769–1773. doi: 10.7164/antibiotics.41.1769. [DOI] [PubMed] [Google Scholar]
- 26.Saruta F, Kuramochi T, Nakamura K, Takamiya S, Yu Y, Aoki T, Sekimizu K, Kojima S, Kita K. J Biol Chem. 1995;270:928–932. doi: 10.1074/jbc.270.2.928. [DOI] [PubMed] [Google Scholar]
- 27.Kita K, Takamiya S, Furushima R, Ma Y C, Suzuki H, Ozawa T, Oya H. Biochim Biophys Acta. 1988;935:130–140. doi: 10.1016/0005-2728(88)90210-1. [DOI] [PubMed] [Google Scholar]
- 28.Kita K, Vibat C R T, Meinhardt S, Guest J R, Gennis R B. J Biol Chem. 1989;264:2672–2677. [PubMed] [Google Scholar]
- 29.Miyadera H, Amino H, Hiraishi A, Taka H, Murayama K, Miyoshi H, Sakamoto K, Ishii N, Hekimi S, Kita K. J Biol Chem. 2001;276:7713–7716. doi: 10.1074/jbc.C000889200. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai H, Nishida H, Imamura N, Tomoda H, Ōmura S. J Antibiot. 1990;43:1553–1558. doi: 10.7164/antibiotics.43.1553. [DOI] [PubMed] [Google Scholar]
- 31.Oshino K, Kumagai H, Tomoda H, Ōmura S. J Antibiot. 1990;43:1064–1068. doi: 10.7164/antibiotics.43.1064. [DOI] [PubMed] [Google Scholar]
- 32.Tan A K, Ramsay R R, Singer T P, Miyoshi H. J Biol Chem. 1993;268:19328–19333. [PubMed] [Google Scholar]
- 33.Iverson T M, Luna-Chavez C, Croal L R, Cecchini G, Rees D C. J Biol Chem. 2002;277:16124–16130. doi: 10.1074/jbc.M200815200. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster C R D, Kröger A, Auer M, Michel H. Nature. 1999;402:377–385. doi: 10.1038/46483. [DOI] [PubMed] [Google Scholar]
- 35.Törnroth S, Yankovskaya V, Cecchini G, Iwata S. Biochim Biophys Acta. 2002;1553:171–176. doi: 10.1016/s0005-2728(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 36.Matsson M, Hederstedt L. J Bioenerg Biomembr. 2001;33:99–105. doi: 10.1023/a:1010744330092. [DOI] [PubMed] [Google Scholar]
- 37.Matsson M, Ackrell B A C, Cochran B, Hederstedt L. Arch Microbiol. 1998;170:27–37. doi: 10.1007/s002030050611. [DOI] [PubMed] [Google Scholar]
- 38.Keon J P R, White G A, Hargreaves J A. Curr Genet. 1991;19:475–481. doi: 10.1007/BF00312739. [DOI] [PubMed] [Google Scholar]
- 39.Broomfield P L E, Hargreaves J A. Curr Genet. 1992;22:112–117. doi: 10.1007/BF00351470. [DOI] [PubMed] [Google Scholar]
- 40.Skinner W, Bailey A, Renwick A, Keon J, Gurr S, Hargreaves J. Curr Genet. 1998;34:393–398. doi: 10.1007/s002940050412. [DOI] [PubMed] [Google Scholar]
- 41.Chauvin C, De Oliveira F, Ronot X, Mousseau M, Leverve X, Fontaine E. J Biol Chem. 2001;276:41394–41398. doi: 10.1074/jbc.M106417200. [DOI] [PubMed] [Google Scholar]
- 42.Betarbet R, Sherer T B, MacKenzie G, Garcia-Osuna M, Panov A V, Greenamyre J T. Nat Neurosci. 2001;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 43.Gille L, Nohl H. Arch Biochem Biophys. 2001;388:34–38. doi: 10.1006/abbi.2000.2257. [DOI] [PubMed] [Google Scholar]
- 44.Greene J G, Porter R H P, Eller R V, Greenamyre T J. J Neurochem. 1993;61:1151–1154. doi: 10.1111/j.1471-4159.1993.tb03634.x. [DOI] [PubMed] [Google Scholar]