Abstract
In a 3D model of breast morphogenesis, CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) plays an essential role in lumen formation in a subline of the nonmalignant human breast cell line (MCF10A). We show that mammary carcinoma cells (MCF7), which do not express CEACAM1 or form lumena when grown in Matrigel, are restored to a normal morphogenic program when transfected with CEACAM1-4S, the short cytoplasmic isoform of CEACAM1 that predominates in breast epithelia. During the time course of lumen formation, CEACAM1-4S was found initially between the cells, and in mature acini, it was found exclusively in an apical location, identical to its expression pattern in normal breast. Lumena were formed by apoptosis as opposed to necrosis of the central cells within the alveolar structures, and apoptotic cells within the lumena expressed CEACAM1-4S. Dying cells exhibited classical hallmarks of apoptosis, including nuclear condensation, membrane blebbing, caspase activation, and DNA laddering. Apoptosis was mediated by Bax translocation to the mitochondria and release of cytochrome c into the cytoplasm, and was partially inhibited by culturing cells with caspase inhibitors. The dynamic changes in CEACAM1 expression during morphogenesis, together with studies implicating extracellular matrix and integrin signaling, suggest that a morphogenic program integrates cell–cell and cell–extracellular matrix signaling to produce the lumena in mammary glands. This report reveals a function of CEACAM1-4S relevant to cellular physiology that distinguishes it from its related long cytoplasmic domain isoform.
Keywords: mammary morphogenesis‖lumen formation
CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) is a cell–cell adhesion molecule expressed in a variety of different cell types, such as granulocytes and epithelial cells found in liver, colon, bladder, prostate, and breast tissues (1, 2). CEACAM1 is a member of the CEA (carcinoembryonic antigen) gene family and has a number of alternatively spliced isoforms with either three or four Ig-like extracellular domains and a long (CEACAM1-3L and -4L) or a short (CEACAM1-3S and -4S) cytoplasmic tail (3–5). CEACAM1 has been found to be down-regulated in premalignant adenomas (6), ≈30% of breast carcinomas (7, 8), and 90% of colon tumors (9). Forced expression of CEACAM1 in prostate and bladder carcinomas has been shown to markedly reduce the tumorigenic phenotype in both cell culture and animal models (10–13). Recently, we have shown that the cell–cell adhesion molecule CEACAM1 is also important in lumen formation in a model of breast morphogenesis (14).
The morphology of normal mammary glands is characterized by an inner layer of polarized epithelial cells with secretory surfaces facing a central lumen and basal surfaces surrounded by an outer layer of myoepithelial cells. When normal mammary epithelial cells are cultured ex vivo in Matrigel, a source of extracellular matrix (ECM), they form alveolar structures resembling their in vivo morphology (15). On contact with either Matrigel or basement membrane proteins such as laminin and collagen IV, normal mammary epithelial cells become polarized, form spherical acini or branched tubules, and in the presence of prolactin initiate β-casein secretion (16). In contrast to normal cells, tumor mammary epithelial cells form large, nonpolarized, undifferentiated colonies without lumena when grown in Matrigel (17). Blocking antibodies that inhibit expression of β1-integrin, epidermal growth factor receptor (EGFR), or mitogen-activated protein kinase (MAPK) in tumor cells grown in Matrigel have been shown to induce down-regulation of β1-integrin and EGFR, causing growth arrest and reversion to normal morphogenesis, as indicated by the formation of acinus-like structures (18, 19). In addition, there is evidence that cell–cell adhesion factors such as E-cadherin play a role in mammary differentiation (20).
We have previously shown that blocking CEACAM1 in a spontaneously immortalized nonmalignant human breast cell line (MCF10F, a subclone of MCF10A) by antibodies or an antisense gene blocks lumen formation in Matrigel cultures (14). Here we show that mammary tumor (MCF7) cells, which do not express CEACAM1 or make lumen containing acini when cultured in Matrigel, revert to normal phenotype marked by the formation of acini structures with central lumena when transfected with CEACAM1-4S. Unlike CEACAM1-4L-transfected MCF7 cells, which uniformly die in Matrigel, CEACAM1-4S transfection mediates lumen formation in MCF7 cells via apoptosis of central cells, as determined by caspase activation, Bax translocation to mitochondria, and cytochrome c release into the cytosol. These findings show that mammary morphogenesis relies on coordinated signaling not only of the various growth factor receptors, and integrins, as previously shown, but also of cell–cell adhesion molecules, such as CEACAM1-4S. In the case of the MCF7 mammary carcinoma cell line, restoration of cell–cell signaling via CEACAM1-4S alone is sufficient to reestablish normal morphology.
Materials and Methods
Materials.
All cells were obtained from American Type Culture Collection and grown as described (14). Matrigel and MatriSperse were purchased from Collaborative Biomedical Products (Bedford, MA). The anti-CEACAM1 mAb 4D1C2 (1 μg/ml) was a kind gift from C. Wagener (21); mAb M30 (diluted 1:50) was obtained from Roche Molecular Biochemicals, mAb to cytochrome c (diluted 1:20) from CLONTECH, and anti-Bax, Bcl-2, and Bcl-xL mAb (diluted 1:250) from PharMingen. SuperArray Human Apoptosis GEAarray Q series was obtained from SuperArray (Bethesda). ApoAlert Cell Fractionation Kit and MitoSensor (JC-1) cationic dye were purchased from CLONTECH. Annexin V-FITC and caspase inhibitors were from PharMingen.
Matrigel Culture.
Cells (2.5 × 105) were plated in a thick layer (1 mm) of Matrigel in two-well chamber slides (Nunc). Solidified Matrigel was overlaid with mammary epithelial cell growth medium (MEGM) supplemented with SingleQuots (Clonetics, San Diego). To harvest cells, gels were incubated in MatriSperse solution for 1 h at 4°C. Cells grown in Matrigel were scored for the presence or absence of lumena. Statistical analysis was done by Student's t test or χ2 test.
CEACAM1-4S-ectoGFP.
GFP was inserted between the transmembrane and the A2 domain of CEACAM1-4S. For detailed cloning procedure, see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Microscopy.
Transmission electron microscopy (TEM) was performed as described (14) on cells grown in Matrigel and collected by treatment with MatriSperse. Confocal microscopy was performed on a Zeiss Model 310 confocal microscope on cells grown in Matrigel and stained with MitoSensor (JC-1) dye or with annexin V-FITC per the manufacturer's instructions. Immunohistochemical staining was performed as described (14).
Results
Time Course of Apoptosis During Lumen Formation in MCF10F Cells.
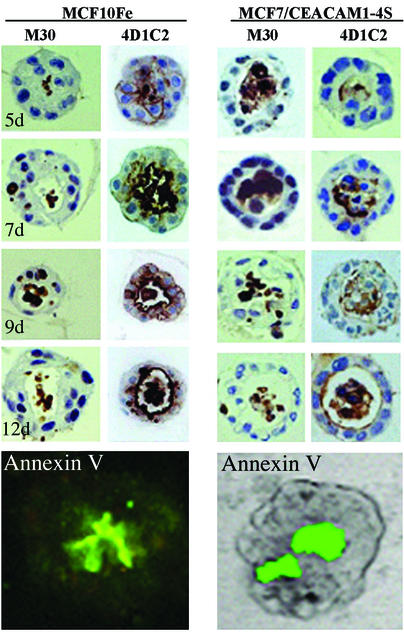
Previous studies from our laboratory provided strong evidence that CEACAM1 expression played an essential role in lumen formation (14). Nonmalignant mammary epithelial cells (MCF10F) grown in Matrigel formed spherical acini with central lumena and expressed CEACAM1 on the luminal surface. Blocking CEACAM1 expression with an antisense gene or antibodies blocked lumen formation (14). We also showed that the lumena were formed by apoptosis of the central cells. To further define the apoptotic process in nonmalignant cells, we performed a time course analysis and followed CEACAM1 expression by staining with mAb 4D1C2 (21) and apoptosis with monoclonal antibody M30, which detects caspase cleaved cytokeratin 18 (22, 23). CEACAM1 staining of MCF10F cells revealed the initiation of lumen formation at day 5, with 17% of colonies having lumena with a gradual increase in lumen formation until day 12 when 61% of colonies had lumena (Fig. 1 and Table 1). Cells in the center of the colonies exhibited signs of apoptosis, as demonstrated by nuclear and cytoplasmic condensation along with positive M30 staining (Fig. 1). To complete the lumen formation process, cells must clear the apoptotic bodies within the lumena. Phosphatidylserine (PS) translocation to the outside of the plasma membrane constitutes a phagocytic signal for the surrounding cells to clear the dead debris (24) and can be detected by staining with annexin V. As revealed by fluorescent annexin V staining, PS was exposed on the cells in the center of the developing acini (Fig. 1). These results not only mimic the apoptotic nature of lumen formation in normal breast, they also reveal that the apoptotic cells can be cleared by surrounding cells, even in the absence of professional phagocytes.
Figure 1.
Time course of apoptosis and lumen formation in normal and carcinoma cells. MCF10F and MCF7/CEACAM1-4S cells (2.5 × 105 per well) were grown in Matrigel for the indicated number of days, fixed, embedded in paraffin, and stained with caspase-cleaved cytokeratin 18 detecting mAb (M30) and anti-CEACAM1 mAb (4D1C2). For annexin V expression, cells were grown in Matrigel for 8 days, stained with annexin V-FITC, and visualized by confocal microscopy. For MCF7/CEACAM1-4S cells, the annexin V-FITC staining image was superimposed on the transmission image for easier visualization. (Magnification, ×600.)
Table 1.
Time course of acini formation in MCF10F cells
| Days in Matrigel | N | % Acini |
|---|---|---|
| 5 | 299 | 17 |
| 7 | 151 | 23 |
| 9 | 212 | 41 |
| 12 | 218 | 61 |
MCF10F cells (2.5 × 105 per well) were grown in Matrigel for the indicated number of days, fixed, embedded in paraffin, and stained with anti-CEACAM1 mAb (4D1C2). Acini were scored for lumen formation. N, number of colonies scored.
CEACAM1-4S Reverts MCF7 Cells to Normal Morphological Differentiation via Apoptosis of the Central Cells Within Acini.
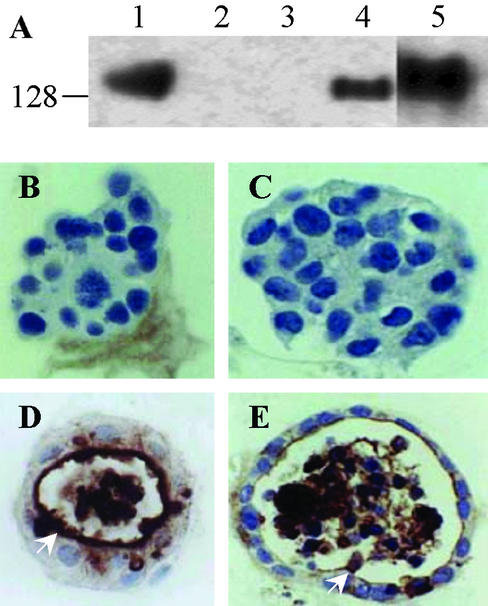
In contrast to nonmalignant MCF10F cells, MCF7 cells lack CEACAM1 expression and fail to form acini with lumena when cultured in Matrigel. To further test the hypothesis that CEACAM1 is necessary and sufficient for lumen formation, we transfected MCF7 cells with either CEACAM1-4L or -4S and grew the CEACAM1-expressing cells in Matrigel. Because the short cytoplasmic isoform, CEACAM1-4S, but not the long cytoplasmic isoform, is the predominant isoform in normal breast (8), it was important to determine their differential effects on acinar morphogenesis. Stable cell lines were produced, and in both cases, CEACAM1-specific isoform expression was confirmed by Western blotting (Fig. 2A). When grown in Matrigel for 12 days, MCF7 and vector-transfected control cells developed into solid spheres without lumena, and immunohistochemical staining of these colonies revealed no CEACAM1 expression (Fig. 2 B and C). However, MCF7 cells transfected with CEACAM1-4S formed lumena and exhibited acinar differentiation and staining patterns similar to the nonmalignant control cells (Fig. 2 E vs. D). In contrast, MCF7 cells transfected with CEACAM1-4L, which had comparable growth rates to control cells when grown in tissue culture on plastic, died in Matrigel culture before lumen formation could be assessed (Table 2). Therefore, expression of CEACAM1-4S, but not CEACAM1-4L, mediates the reversion of MCF7 cells from tumor to normal morphology in a pattern similar to that seen in nonmalignant breast epithelial cells.
Figure 2.
CEACAM1-4S mediated reversion of MCF7 cells to normal phenotype. (A) Cell lysate preparations (60 μg of total protein) from cells grown on plastic were separated by 8% reducing SDS/PAGE gel and immunoblotted with anti-CEACAM1 mAb (4D1C2). Lane 1, positive control HT29 colon carcinoma cells; lane 2, parental MCF7 cells; lane 3, MCF7/pHβ (vector); lane 4, MCF7/CEACAM1-4S; lane 5, MCF7/CEACAM1-4L. (B–E) Cells (2.5 × 105 per well) were grown in Matrigel for 12 days, fixed, and embedded in paraffin, and sections were stained with anti-CEACAM1 mAb (4D1C2). (B) MCF7 cells exhibiting no lumen formation. (C) Vector-transfected MCF7 cells exhibiting no lumen formation. (D) Nonmalignant mammary epithelial cells MCF10F exhibiting lumen formation. (E) MCF7/CEACAM1-4S-transfected cells exhibiting lumen formation. (Magnification, ×600.)
Table 2.
Effect of CEACAM1 isoforms on acini viability
| Cells | % Dead colonies | N | P value |
|---|---|---|---|
| Untransfected | 24.79 | 216 | — |
| Vector | 36.28 | 395 | — |
| CEACAM1-4S | 45.81 | 345 | >0.05 |
| CEACAM1-4L | 79.53 | 641 | <0.001 |
Wild-type and transfected MCF7 cells (2.5 × 105 per well) were grown in Matrigel for 12 days, fixed, embedded in paraffin, stained with anti-CEACAM1 mAb (4D1C2), and scored for the dead colonies. N, number of acini scored. P values were calculated by Student's t test.
Similar to MCF10F cells, MCF7 cells transfected with CEACAM1-4S form lumena in a time-dependent manner with the central cells exhibiting apoptosis (Fig. 1). In contrast to the parental cells and vector transfected controls, which showed no CEACAM1 or M30 staining, CEACAM1-4S-transfected MCF7 cells exhibited progressively higher percentages of lumen formation beginning at day 5 and culminating at day 12, with 63% percent of lumen-containing colonies having lumena (Table 3). The final level of lumen formation reached by CEACAM1-4S-transfected MCF7 cells (63%) was similar to that seen in MCF10F cells (61%) (Table 1 vs. Table 3). As expected, CEACAM1-4S-transfected MCF7 cells were found also to have flipped their PS molecules to the outer leaflet of the plasma membrane, as indicated by the positive annexin V-FITC staining of the developing acinus (Fig. 1).
Table 3.
Time course for acini formation in MCF7/CEACAM1-4S cells
| Cells | Days in Matrigel | N | % Acini | P value |
|---|---|---|---|---|
| Untransfected | 12 | 168 | 19 | — |
| Vector | 12 | 247 | 17 | — |
| CEACAM1-4S | 4 | 268 | 21 | >0.05 |
| CEACAM1-4S | 5 | 250 | 46 | <0.001 |
| CEACAM1-4S | 7 | 365 | 54 | <0.001 |
| CEACAM1-4S | 9 | 308 | 51 | <0.001 |
| CEACAM1-4S | 12 | 180 | 63 | <0.001 |
Wild-type and transfected MCF7 cells (2.5 × 105 per well) were grown in Matrigel for the indicated number of days, fixed, embedded in paraffin, and stained with anti-CEACAM1 mAb (4D1C2). Acini were scored for lumen formation. N, number of live colonies scored. P values were calculated by the χ2 method compared to vector control.
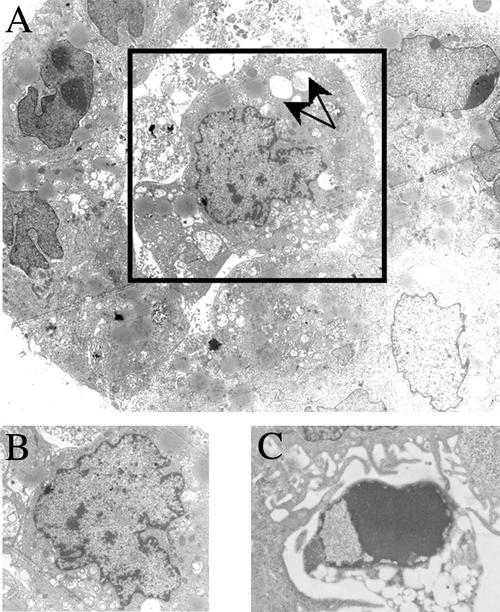
To further document the morphologic events of lumen formation, transmission electron microscopy was performed. Apoptosis in the central cells of the developing acinus began with nuclear condensation at day 5 [Fig. 3 A (box) and B] and proceeded to the formation of an apoptotic body by day 12 (Fig. 3C). Consistent with the nuclear changes, DNA laddering was observed in the apoptotic cells (data not shown). In addition, cytoplasmic vacuolization was observed in the cells undergoing apoptosis (Fig. 3A, arrows).
Figure 3.
Morphologic analysis of apoptotic cells during lumen formation. MCF7/CEACAM1-4S cells (2.5 × 105 per well) were grown in Matrigel for 5 (A and B) or 12 (C) days, and transmission electron microscopy was performed. (A) An acinus undergoing apoptosis of the central cell (box). (Magnification, ×5,500.) (B) Nuclear condensation occurring during apoptosis of the central cell seen in A. (Magnification, ×7,375.) (C) An apoptotic body in the lumen of the acinus. (Magnification, ×16,525.) Arrows point to the vacuoles seen in the cytoplasm of the apoptotic cell.
Because caspase activation is another hallmark of apoptosis, we treated the developing acini with cell-permeable, irreversible caspase inhibitors. Fresh caspase inhibitor-supplemented medium was added to the cultures every other day. All inhibitors partially prevented lumen formation at 50 μM concentration, with the caspase 8 inhibitor showing the strongest effect (Table 4). Because MCF7 cells do not express caspase 3 (25), the results obtained by Z-DEVD-fmk treatment were attributed to caspase 7 inhibition. Moreover, the lack of caspase 3 in MCF7 cells indicates that cytokeratin 18 cleavage, as measured by M30 staining, can be attributed to the activation of caspases 6 and/or 7.
Table 4.
Effect of caspase inhibitors on lumen formation
| Treatment | Caspase | Amount, μM | % Lumen | P value |
|---|---|---|---|---|
| Z-VAD-fmk | Pan | 10 | 86 | <0.001 |
| 50 | 81 | <0.001 | ||
| Z-DEVD-fmk | 3, 7 | 10 | 95 | >0.05 |
| 50 | 77 | <0.001 | ||
| Z-VEID-fmk | 6 | 10 | 99 | >0.05 |
| 50 | 86 | <0.001 | ||
| Z-IEDT-fmk | 8 | 10 | 89 | <0.005 |
| 50 | 75 | <0.001 | ||
| 100 | 70 | <0.001 | ||
| Z-LEDH-fmk | 9 | 10 | 94 | <0.05 |
| 50 | 80 | <0.001 | ||
| 100 | 87 | <0.001 |
MCF7/CEACAM1-4S cells (2.5 × 105 per well) were grown in Matrigel for 12 days in the presence of indicated amounts of caspase inhibitors (replenished every other day). Acini were scored for lumen formation based on the average of two to four repeated experiments with N ≥ 100 acini scored. Lumen formation was calculated as % lumen formed in the presence of caspase inhibitors compared to the 0.1% DMSO-treated controls. P values were calculated by the χ2 method, compared to the 0.1% DMSO-treated controls. Because limited amounts of caspase inhibitors were available, not all treatments were carried out to the 100-μM levels.
Mitochondrial Involvement in Lumen Formation.
To further characterize the mechanism of apoptosis during lumen formation, we assessed mitochondrial involvement in the death of central cells within the acini. Dying cells exhibit a drop in mitochondrial potential from the opening of the mitochondrial transition pore, resulting in release of cytochrome c. The membrane-permeable MitoSensor (JC-1 cationic dye) aggregates in the mitochondria of healthy cells resulting in red fluorescent staining of mitochondria with intact potential and green fluorescent staining in the cytosol of cells with disrupted mitochondrial potential. In this study, we were able to directly stain cells grown in Matrigel with JC-1 dye without any disruption to the 3D culture. Vector transfected MCF7 cells grown in Matrigel for 12 days exhibited no signs of cell death, as shown by the red fluorescence (Fig. 4A). However, CEACAM1-4S-transfected MCF7 cells cultured under identical conditions exhibited loss of mitochondrial potential restricted to the central cells as shown by the green fluorescence (Fig. 4B). We conclude that the cells that have been shown to die during the course of lumen formation are the same cells that lose their mitochondrial potential under the same conditions.
Figure 4.
Mitochondrial potential and CEACAM1-4S expression in apoptotic cells. Cells (2.5 × 105 per well) were grown in Matrigel for the indicated number of days, stained with JC-1 dye, and visualized by confocal microscopy. (A) MCF7/pHβ (vector) cells stained with JC-1 dye. (B) MCF7/CEACAM1-4S cells stained with JC-1 dye. (C) MCF7/CEACAM1-4S-ectoGFP cells. (D–F) MCF7/CEACAM1-4S-ectoGFP cells stained with JC-1 dye. (Magnification, ×600.)
Previous studies demonstrated that CEACAM1 functions as a homotypic cell–cell adhesion molecule (26–28), and yet staining of mature mammary glands revealed its luminal expression (14). We therefore analyzed the cellular localization of CEACAM1 during the course of lumen formation by using a CEACAM1-4S-ectoGFP fusion protein where GFP was inserted just before the transmembrane domain of CEACAM1-4S to avoid interference with either cell–cell adhesion (N domain) or signaling (cytoplasmic domain). The CEACAM1-4S-ectoGFP construct was transfected into MCF7 cells and the location of CEACAM1-4S was directly visualized by confocal microscopy (Fig. 4C). In addition, these cells were stained with JC-1 dye to determine whether CEACAM1-4S-ectoGFP-positive cells within the lumena exhibit the loss of mitochondrial potential (Fig. 4 D–F). As acinus development progressed, CEACAM1 expression was redistributed from a cell–cell adhesion pattern to the luminal surface of the acinus (Fig. 4). At day 3, the acini were in the initial stages of formation with four cells per colony. These cells were healthy as shown by the red staining of mitochondria, and CEACAM1-4S-ectoGFP was localized between the cells (Fig. 4D). At day 7, a larger colony was seen with a few cells expressing CEACAM1-4S-ectoGFP between the cells and the central lumen staining green either because of accumulation of CEACAM1-4S-ectoGFP or apoptotic cells (staining with JC-1), or both (Fig. 4E). The cells of the colony that were in contact with ECM had healthy mitochondria, as indicated by the red fluorescence of the JC-1 dye. By day 12, lumen formation was complete, with CEACAM1-4S-ectoGFP exclusively expressed on the luminal surface (Fig. 4F). As described above, cells in contact with ECM remain healthy, as displayed by the red staining of mitochondria with JC-1 dye. Based on these results, we would expect the central cells to have lost their mitochondrial potential and stain green with JC-1 dye similar to Fig. 4B, where the cells were transfected with the non-GFP version of CEACAM1-4S. However in Fig. 4F, these are the same cells that express CEACAM1-4S-ectoGFP. If the central cells were not apoptotic, then the lumen of the 12-day acinus would have appeared yellow because of JC-1 staining of healthy (red) mitochondria and CEACAM1-4S-ectoGFP (green) expression. Because the lumen of the12 day acinus stained green, we conclude that both events have occurred: CEACAM1-4S-ectoGFP expression was exclusively luminal and the central cells underwent apoptosis (green only).
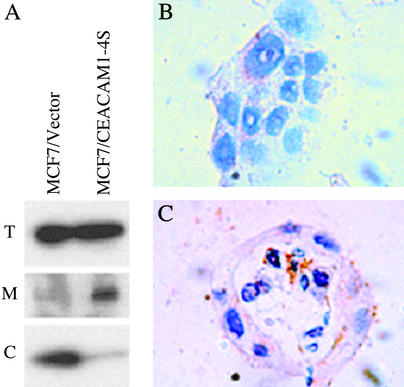
The opening of the mitochondrial transition pore is governed by a set of pro- and antiapoptotic members of the Bcl-2 family of proteins. Because the above results clearly show that the opening of the mitochondrial transition pore occurs in the apoptotic cells within the acinus, we examined the expression and localization of these proteins. When the levels of mRNA expression of the Bcl-2 family members were analyzed by the GEArray, no differences in mRNA expression were observed (data not shown). Also, there were no differences in expression levels of the antiapoptotic Bcl-2 or Bcl-xL proteins, as measured by immunoblotting (data not shown). However, translocation of proapoptotic members of the Bcl-2 family, such as Bax, to the mitochondria causes apoptosis by creating a pore that leads to the release of cytochrome c and subsequent caspase activation. To examine this possibility, vector- and CEACAM1-4S-transfected MCF7 cells grown in Matrigel were fractionated into mitochondrial and cytosolic fractions, and Bax levels were determined by Western blotting. Although both showed similar levels of total Bax expression, Bax was found to accumulate in the mitochondrial fraction of CEACAM1-4S-transfected cells and in the cytosolic fraction of controls (Fig. 5A). Because the opening of the mitochondrial pores lead to the release of cytochrome c, we stained cells for cytochrome c (Fig. 5 B and C). Punctate mitochondrial cytochrome c staining was seen in vector-transfected controls and in the healthy cells of the MCF7/CEACAM1-4S colonies (Fig. 5 B and C); however, the central apoptotic cells inside the CEACAM1-4S-transfected acinus exhibited high levels of diffuse cytoplasmic staining because of cytochrome c release into the cytosol (Fig. 5C). We conclude that the central cells involved in lumen formation exhibit all of the hallmarks of the mitochondrial pathway of apoptosis.
Figure 5.
Bax expression and cytochrome c release during lumen formation. (A) MCF7/pHβ (vector) or MCF7/CEACAM1-4S cells (2.5 × 105 per well) were grown in Matrigel for 5 days, lysed, and fractionated into mitochondrial (M) and cytosolic (C) fractions. Total lysates (T) and the fractions of vector and CEACAM1-4S-transfected MCF7 cells were separated on an SDS/PAGE gel and immunoblotted with anti-Bax antibody. (B and C) Cells (2.5 × 105 per well) were grown in Matrigel for 12 days, fixed, and embedded in paraffin, and sections were stained with anti-cytochrome c antibody. (B) MCF7/pHβ (vector). (C) MCF7/CEACAM1-4S. (Magnification, ×600.)
Discussion
This study extends previous work where we showed that CEACAM1 is essential for lumen formation in nonmalignant cells expressing the gene by demonstrating that transfection of malignant MCF7 cells with CEACAM1-4S causes reversion to a morphologic phenotype similar to normal breast acini or nonmalignant cells grown in Matrigel. Furthermore, the effect is isoform specific, in that the short cytoplasmic isoform, but not the long cytoplasmic isoform, is responsible for the phenotypically correct reversion. Given that the short cytoplasmic isoform is the physiologically predominant form, the results mimic the picture in normal breast. Because low amounts of the long cytoplasmic isoform are found in normal breast epithelial cells and transfection of MCF7 cells with CEACAM1-4L caused massive cell death when grown in Matrigel, it is likely that CEACAM1-4L may play a role in reinforcing the apoptotic signal in normal breast epithelia. To test this possibility, cells could be transfected with both isoforms, mimicking the ratio found in normal breast epithelia. It should be noted that the mammary gland is unusual in that most tissues have an excess of the long form or have equal expression of long and short forms (2). Furthermore, studies in mouse colon cells where the isoform ratio was varied suggest that the long isoform is more tumor suppressive than the short form (29, 30), in agreement with our finding that it is a stronger inducer of cell death than the short form.
Our data clearly demonstrate that the central cells undergo apoptosis. Formation of hollow tubes by apoptosis of the central cells has also been shown in the early mouse embryo (31). The central apoptotic cells exhibit strong staining for cytokeratin 18 cleaved by caspase 3, 6, or 7. Because MCF7 cells lack caspase 3 (25), caspases 6 and 7 are likely to mediate this event. In agreement with this possibility, both caspase 6 and 7 inhibitors were able to partially inhibit lumen formation. However, none of the inhibitors tested caused >25% inhibition of lumen formation, suggesting that either the inhibitors were largely ineffective or that other apoptotic processes were operative. Considering the latter possibility, the mitochondrial pathway is implicated by three lines of evidence: translocation of Bax from the cytoplasm to the mitochondria, drop in mitochondrial potential, and release of cytochrome c. Caspase inhibitors would be expected to be more effective when caspase activation occurs upstream rather than downstream in the apoptotic pathway. For example, ligation of TNF-receptor family receptors activates caspase early, but their expression was undetectable at either the protein or mRNA level in our transfected cells (data not shown). In fact, using gene arrays for a variety of death receptors, we detected only TNFR2, a receptor associated with protection from apoptosis. Thus, the mitochondrial pathway, initiated by Bax translocation, is the primary downstream event leading to apoptosis of the central cells, and this process is not very sensitive to caspase inhibitors.
To study the events upstream of the mitochondria, we are making mutations in the cytoplasmic domain of CEACAM1-4S. Although the cytoplasmic domain of CEACAM1-4S is only 10–14 aa long, it has been shown to bind actin, tropomyosin, and calmodulin (32). In addition, Edlund et al. (33) have proposed that serine and threonine residues in the short cytoplasmic domain may be phosphorylated by PKC. Preliminary results show that null mutations of the serine and threonine residues block lumen formation, suggesting that the short cytoplasmic domain directly participates in the upstream signaling events. Previously, little attention was paid to CEACAM1-4S, reasoning that a 10- to 14-aa cytoplasmic domain would not have a signaling function. However, our results demonstrate that the CEACAM1-4S isoform can mediate biological effects in the absence of the long cytoplasmic isoform.
Although CEACAM1 has been proposed to be a homotypic cell–cell adhesion molecule (34), its luminal expression pattern in mature tissues such as the breast (14), colon (35), and liver (36) would argue otherwise. Our studies with the CEACAM1-4S-ectoGFP fusion protein allowed direct visualization of the fusion protein in colonies in Matrigel by confocal microscopy. These studies demonstrated that CEACAM1-4S-ectoGFP was found between the cells through day 5, suggesting that it functions as a cell–cell adhesion molecule during the early stages of colony formation. From days 5–12, the expression pattern of CEACAM1-4S becomes exclusively apical, demonstrating that it exhibits a dynamic shift in expression pattern during the course of acinar morphogenesis. This change is characteristic of other molecules in tissue-specific differentiation. For example, once mammary epithelial cells differentiate, they vectorially transport proteins to the lumen that are essential for proper function of the mammary gland (16). The high level of CEACAM1 accumulation in lumena is especially evident from the direct observation of the GFP fusion protein in mature acini (Fig. 4C) and suggests a biological function in the luminal space.
The findings that blocking CEACAM1 expression in nonmalignant cells blocks lumen formation while the reintroduction of CEACAM1 into malignant cells induces lumen formation are complementary to the findings that reintroduction of the proper integrin subunits into malignant cells restores lumen formation (37). In fact ECM has been shown to protect mammary epithelial cells from apoptosis (38). Because integrin–ECM signaling is critical for lumen formation, and cells that no longer contact ECM are prone to death by anoikis, the combined signaling of the two pathways, cell–cell and cell–ECM, allows developing colonies to sense their neighboring cells and the ECM environment, determining which cells are required to die to form a lumen. Recently, it has been shown that CEACAM1-4L directly associates with the beta-3 integrin subunit in neutrophils (39). Although no similar association has been found for CEACAM1-4S, there is the possibility of an indirect association with integrin via the cytoskeleton. It is also possible that growth factor receptors are coordinated with cell–cell and cell–ECM signaling. In this respect, Erb2, but not Erb1, ligation can cause repopulation of the lumen in mammary acini (40).
In summary, the results presented here demonstrate that CEACAM1-4S is capable of reverting MCF7 mammary carcinoma cells to a normal acinar phenotype, similar to the pattern seen with nonmalignant cells that endogenously express CEACAM1-4S. Furthermore, CEACAM1-4S mediates lumen formation via an apoptotic mechanism with both caspase and mitochondrial involvement. We hypothesize that the central cells are triggered to apoptose because of an apoptotic signal from CEACAM1-4S and the lack of an antiapoptotic signal from integrin–ECM interactions. Apoptosis was shown to proceed primarily via a mitochondrial pathway involving translocation of Bax from the cytosol to the mitochondria. These findings emphasize that mammary morphogenesis relies on coordinated signaling, not just among the various growth factor receptors and integrins as previously found, but also on cell–cell adhesion molecules, such as CEACAM1-4S. In the case of the MCF7 mammary carcinoma cell line, restoration of cell–cell signaling via CEACAM1-4S alone is sufficient to reestablish normal morphology.
Supplementary Material
Acknowledgments
We acknowledge Sophia Loera for performing immunohistochemistry staining and John Hardy for electron microscopy. This research was supported by National Cancer Institute Grant CA84202. J.K. was supported by Department of the Army DAMD 17-00-1-02-3. Facilities for electron microscopy were supported by National Science Foundation Grant BNS-8415920.
Abbreviation
- ECM
extracellular matrix
References
- 1.Svenberg T, Hammarstrom S, Hedin A. Mol Immunol. 1979;16:245–252. doi: 10.1016/0161-5890(79)90063-4. [DOI] [PubMed] [Google Scholar]
- 2.Obrink B. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinoda Y, Neumaier M, Hefta S A, Drzeniek Z, Wagener C, Shively L, Hefta L J, Shively J E, Paxton R J. Proc Natl Acad Sci USA. 1988;85:6959–6963. doi: 10.1073/pnas.85.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett T R, Kretschmer A, Austen D A, Goebel S J, Hart J T, Elting J J, Kamarck M E. J Cell Biol. 1989;108:267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett T R, Drake L, Pickle W D. Mol Cell Biol. 1993;13:1273–1282. doi: 10.1128/mcb.13.2.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmuller F, Wagener C, Neumaier M. Cancer Res. 1997;57:2354–2357. [PubMed] [Google Scholar]
- 7.Riethdorf L, Lisboa B W, Henkel U, Naumann M, Wagener C, Loning T. J Histochem Cytochem. 1997;45:957–963. doi: 10.1177/002215549704500705. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Simpson J F, Glackin C, Riethorf L, Wagener C, Shively J E. Anticancer Res. 1998;18:3203–3212. [PubMed] [Google Scholar]
- 9.Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C. Proc Natl Acad Sci USA. 1993;90:10744–10748. doi: 10.1073/pnas.90.22.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh J T, Luo W, Song W, Wang Y, Kleinerman D I, Van N T, Lin S H. Cancer Res. 1995;55:190–197. [PubMed] [Google Scholar]
- 11.Lin S H, Pu Y S. Semin Oncol. 1999;26:227–233. [PubMed] [Google Scholar]
- 12.Estrera V T, Luo W, Phan D, Earley K, Hixson D C, Lin S H. Biochem Biophys Res Commun. 1999;263:797–803. doi: 10.1006/bbrc.1999.1443. [DOI] [PubMed] [Google Scholar]
- 13.Pu Y S, Luo W, Lu H H, Greenberg N M, Lin S H, Gingrich J R. J Urol. 1999;162:892–896. doi: 10.1097/00005392-199909010-00085. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Hardy J D, Sun Y, Shively J E. J Cell Sci. 1999;112:4193–4205. doi: 10.1242/jcs.112.23.4193. [DOI] [PubMed] [Google Scholar]
- 15.Gomm J J, Coope R C, Browne P J, Coombes R C. J Cell Physiol. 1997;171:11–19. doi: 10.1002/(SICI)1097-4652(199704)171:1<11::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Muschler J, Lochter A, Roskelley C D, Yurchenco P, Bissell M J. Mol Biol Cell. 1999;10:2817–2828. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen O W, Ronnov-Jessen L, Howlett A R, Bissell M J. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Weaver V M, Petersen O W, Larabell C A, Dedhar S, Briand P, Lupu R, Bissell M J. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver V M, Petersen O W, Wang F, Larabell C A, Briand P, Damsky C, Bissell M J. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgeirsson K S, Jonasson J G, Tryggvadottir L, Olafsdottir K, Sigurgeirsdottir J R, Ingvarsson S, Ogmundsdottir H M. Eur J Cancer. 2000;36:1098–1106. doi: 10.1016/s0959-8049(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 21.Drzeniek Z, Lamerz R, Fenger U, Wagener C, Haubeck H D. Cancer Lett. 1991;56:173–179. doi: 10.1016/0304-3835(91)90093-w. [DOI] [PubMed] [Google Scholar]
- 22.Leers M P, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers F C, Bjorklund B, Nap M, et al. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Caulin C, Salvesen G S, Oshima R G. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok V A, Bratton D L, Frasch S C, Warner M L, Henson P M. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 25.Janicke R U, Sprengart M L, Wati M R, Porter A G. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 26.Rojas M, Fuks A, Stanners C P. Cell Growth Differ. 1990;1:527–533. [PubMed] [Google Scholar]
- 27.Turbide C, Rojas M, Stanners C P, Beauchemin N. J Biol Chem. 1991;266:309–315. [PubMed] [Google Scholar]
- 28.Oikawa S, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Biochem Biophys Res Commun. 1992;186:881–887. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- 29.Izzi L, Turbide C, Houde C, Kunath T, Beauchemin N. Oncogene. 1999;18:5563–5572. doi: 10.1038/sj.onc.1202935. [DOI] [PubMed] [Google Scholar]
- 30.Kunath T, Ordonez-Garcia C, Turbide C, Beauchemin N. Oncogene. 1995;11:2375–2382. [PubMed] [Google Scholar]
- 31.Coucouvanis E, Martin G R. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 32.Schumann D, Chen C J, Kaplan B, Shively J E. J Biol Chem. 2001;276:47421–47433. doi: 10.1074/jbc.M109110200. [DOI] [PubMed] [Google Scholar]
- 33.Edlund M, Wikstrom K, Toomik R, Ek P, Obrink B. FEBS Lett. 1998;425:166–170. doi: 10.1016/s0014-5793(98)00222-1. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira A M, Fawcett J, Simmons D L, Watt S M. Blood. 1994;84:211–219. [PubMed] [Google Scholar]
- 35.Frangsmyr L, Baranov V, Prall F, Yeung M M, Wagener C, Hammarstrom S. Cancer Res. 1995;55:2963–2967. [PubMed] [Google Scholar]
- 36.Ocklind C, Forsum U, Obrink B. J Cell Biol. 1983;96:1168–1171. doi: 10.1083/jcb.96.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun H, Santoro S A, Zutter M M. Cancer Res. 1998;58:2224–2233. [PubMed] [Google Scholar]
- 38.Boudreau N, Sympson C J, Werb Z, Bissell M J. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brummer J, Ebrahimnejad A, Flayeh R, Schumacher U, Loning T, Bamberger A M, Wagener C. Am J Pathol. 2001;159:537–546. doi: 10.1016/s0002-9440(10)61725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthuswamy S K, Li D, Lelievre S, Bissell M J, Brugge J S. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.