Abstract
In anautogenous mosquitoes, egg maturation requires a blood meal. As a consequence, mosquitoes are vectors of numerous devastating human diseases. Blood feeding triggers a 20-hydroxyecdysone (20E) hormonal cascade, which activates yolk protein precursor (YPP) genes in the female fat body, an insect metabolic tissue. An important adaptation for anautogeny is the previtellogenic arrest preventing activation of YPP genes. Equally essential is termination of their expression, so that another arrest is achieved after a batch of eggs is laid. Here, we report that mosquito Seven-up (AaSvp), a chicken ovalbumin upstream promoter-transcription factor homologue, is involved in regulating the cyclicity of vitellogenic ecdysteroid-mediated signaling through heterodimerization with a retinoid X receptor homologue Ultraspiracle (USP), the obligatory functional ecdysteroid receptor (EcR) partner. AaSvp inhibits 20E-dependent activation of the vitellogenin (Vg) gene in transfection assays. Two-hybrid and GST pull-down analyses demonstrate that in vitro AaSvp interacts with both AaUSP and AaEcR. However, the coimmunoprecipitation using fat body nuclear extracts reveals that at 33–36 h postblood meal, when the 20E titer sharply declines and YPP gene expression ceases, AaSvp replaces AaEcR in USP heterodimers. The chromatin immunoprecipitation assay indicates that protein–protein interaction rather than binding competition for the Vg ecdysteroid response element accounts for the inhibition of Vg expression by AaSvp.
Mosquitoes serve as vectors of many devastating human diseases because they require blood feeding for development of eggs. Vitellogenesis is the key process in egg maturation, which involves production of yolk protein precursors (YPPs) by the fat body, an insect metabolic tissue analogous to vertebrate liver and adipose tissue combined. In anautogenous mosquitoes, vitellogenesis is initiated only after a female mosquito ingests vertebrate blood. The requirement of a blood meal, called anautogeny, results in a highly regulated cyclicity of egg production as each cycle is tightly coupled with food intake. Anautogeny is one of the most fundamental phenomena underlying the vectorial capacity of mosquitoes. Therefore, understanding the molecular and genetic basis of anautogeny is of great importance for the future development of approaches to vector and pathogen control.
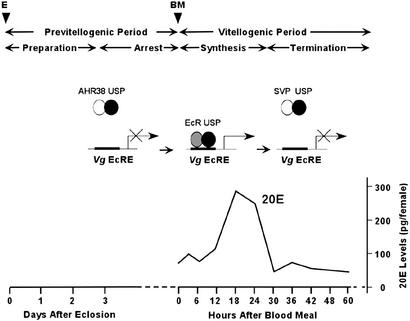
The events of vitellogenesis are best understood in the yellow-fever mosquito Aedes aegypti. In this mosquito, vitellogenesis in the fat body is separated into four phases: previtellogenic (PV) preparation, PV arrest, vitellogenic yolk protein synthesis, and termination of vitellogenesis (1). A newly emerged female needs about 3 days to become competent to endure the intense physiological demands of vitellogenesis. During this phase, the fat body acquires responsiveness to 20-hydroxyecdysone (20E) and becomes competent for massive yolk protein synthesis and secretion (2–4). After 3 days of PV development, the fat body and ovary enter a state of arrest that persists until a blood meal is taken, which triggers the release of the ovarian ecdysteroidogenic hormone from the medial neurosecretory cells of the brain for up to 12 h after feeding (5, 6). In response to ovarian ecdysteroidogenic hormone, the ovary produces ecdysone, which is converted into an active form of the hormone 20E (7). 20E titers are only slightly elevated at 4 h postblood meal (PBM), they begin to rise sharply at 6–8 h PBM, and they reach their maximum level at 18–24 h PBM. During the active synthesis stage, YPPs, vitellogenin (Vg), vitellogenic carboxypeptidase, and vitellogenic cathepsin B are produced by the fat body and accumulated by developing oocytes (1, 8). The massive yolk protein synthesis peaks at around 24 h PBM, then drops sharply and terminates by 36–42 h PBM. The fat body undergoes remodeling, and the first batch of eggs completes maturation.
The molecular basis of 20E action has been studied in detail during molting and metamorphosis in Drosophila melanogaster. 20E manifests the gene regulatory effect through its receptor that is a heterodimer of two members of the nuclear receptor gene family, ecdysone receptor (EcR) and a retinoid X receptor (RXR) homologue, Ultraspiracle (USP). The EcR–USP complex recognizes sequence-specific DNA motifs, called ecdysteroid response elements (EcREs). After binding to 20E, the EcR–USP complex directly induces several early response genes: E74, E75, and Broad. In turn, products of these early genes activate late target genes (9–11).
In the mosquito A. aegypti, this ecdysteroid regulatory hierarchy seems to be used reiteratively during vitellogenesis. Two EcR isoforms (AaEcR-A and AaEcR-B) and two USP isoforms (AaUSP-A and AaUSP-B) are expressed with unique patterns during pre- and vitellogenic periods (12–15). The mosquito EcR–USP heterodimer is capable of binding to various EcREs to modulate ecdysone regulation of target genes (16). However, regulatory regions of two YPP genes have similar EcREs, which are direct repeats with a single spacer (DR-1) (ref. 17; D. Martin and A.S.R., unpublished observations). It has been shown that EcRE is required for the activation of the Vg gene (17). The mosquito homologues of the Drosophila early genes E74 and E75 are induced in response to blood feeding in vitellogenic tissues, the fat body, and the ovary, and they are involved in mediating the ecdysteroid response during vitellogenesis (18, 19). Products of these early genes E74 and E75 are necessary for a high level of the Vg gene expression (20).
An essential adaptation for anautogeny is the establishment of PV developmental arrest preventing the activation of YPP genes in PV-competent females before blood feeding. Our studies have shown that the ecdysteroid receptor complex is a primary target of the 20E signaling modulation in mosquito target tissues at the state of arrest. At this stage, AaUSP exists as a heterodimer with the orphan nuclear receptor AHR38, the mosquito homologue of Drosophila DHR38, and vertebrate NGFI-B/Nurr1 orphan receptors. AHR38 is a repressor that disrupts the specific DNA binding of the ecdysteroid receptor and interacts strongly with AaUSP. In the presence of 20E, EcR can efficiently displace AHR38 and form an active heterodimer with USP. The latter event actually occurs after a blood meal (21).
The termination of YPP gene expression is important so that another arrest can be achieved after a female mosquito lays a batch of eggs. In search of other factors that might act as negative regulators of the AaEcR/AaUSP-mediated 20E response during vitellogenesis, we recently isolated a mosquito homologue (AaSvp) of vertebrate orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor (COUP-TF) and Drosophila Seven-up (Svp; ref. 22). Here, we report that AaSvp is involved in regulating the cyclicity of vitellogenic ecdysteroid-mediated signaling through heterodimerization with USP.
Materials and Methods
Plasmid Constructions.
To make the reporter construct 2xDR1-TATA-Luc, two copies of DR-1 (5′-AGCTTAGGTCAGAGGTCAAGAGAGGTCAGAGGTCACTCGA-3′) were placed in plasmid pLUC-MCS (Stratagene) between XhoI and HindIII. The AaSvp cDNA was cloned into the corresponding sites of the expression vector pcDNA 3.1/Zeo(+) (Invitrogen). The 0.6Vg-Luc reporter plasmid contains sequences from −600 to +115 bp of the A. aegypti Vg gene (17). pAc5-AaSvp was constructed by inserting the EcoRV fragments from pBS-AaSvp into the EcoRV site of pAc5/V5/HisA. The reporter pAc5-LacZ (Invitrogen) was used to normalize transfection efficiency. The construction of plasmids pAc5-AaEcR-B and pAc5-AaUSP-B has been described elsewhere (14). These expression plasmids used the same promoter, actin 5C. DNA fragment encoding the CDEF domains of AaSvp was subcloned in pGEX-4T-1 (Amersham Pharmacia) to create GST-AaSvp fusion. Other glutathione S-transferase fusions have been used previously (21). All constructs were verified with restriction digestions and partial sequencing.
Cell Culture and Transient Transfection Assay.
Transfection of Drosophila S2 cell line (Invitrogen) was conducted as described (17).
The green African monkey kidney CV-1 cell line (American Type Culture Collection) was used for the mammalian two-hybrid assay and transfected as described elsewhere (16). In brief, 3 × 105 cells were plated on 35-mm dishes for 18–24 h before transfection. Cells were transfected with 0.4 μg each of plasmids expressing GAL4 and VP-16 fusion proteins, cytomegalovirus-β-galactosidase expression plasmid, and 1.2 μg of the reporter plasmid pFR-Luc. Each well received 2.4 μg of total DNA. pM and pVP16 plasmid DNA were used as carriers for equalizing the amount of DNA allocated to each well.
GST Pull-Down Assay and Coimmunoprecipitation Assay.
Assays were performed as described by Zhu et al. (21). This coimmunoprecipitation was specific as none of these protein complexes could be detected in control immunoprecipitations with preimmune serum (data not shown).
Preparation of Nuclei and Chromatin Immunoprecipitation (ChIP).
Nuclei in fat body of 250 female adults were isolated for each time point as described (23). Nuclear proteins were then crosslinked to chromosomal DNA, as reported by Sachs and Shi (24).
The ChIP assay was performed by using a kit from Upstate Biotechnology (Lake Placid, NY). One milliliter of chromatin preparation was used for each ChIP assay with 5 μl of anti-histone H4 antiserum (Upstate Biotechnology), 10 μl of polyclonal antibodies against AaEcR, AaUSP, AHR38, and AaSvp, or 10 μl of cognate preimmune serum. Chromatin solution (100 μl) was used for the control of input DNA in the chromatin solution. After the ChIP protocol, semiquantitative PCR was performed for 20–25 cycles with the recovered DNA. The following primers were used (i) for the EcRE region (−380 to −81) of the Vg promoter (forward 5′-TCTGGAATCCATTGCAAGCTA-3′ and reverse 5′-ATTCACAGCATCCTTTCGTTCG-3′) and (ii) for a region ≈0.9 kb upstream from the EcRE (−1,670 to −1,282) on the Vg promoter (forward 5′-AAGGTTCCGTGCTCACTAATGC-3′ and reverse 5′-AAAGACCTTTCCGACGATTGTC-3′). PCR products were resolved on a 1.5% agarose gel and examined by means of Southern hybridization.
Antibodies.
Polyclonal antibodies were raised against the bacterially expressed DNA-binding domain (DBD) and the ligand-binding domain (LBD) of AaSvp in Leghorn chickens. Generation of rabbit polyclonal antibodies against AHR38, AaUSP, and AaEcR has been reported (21). Monoclonal mouse antibody against DmUSP (25) was a kind gift of F. Kafatos (European Molecular Biology Laboratory, Heidelberg).
Results
Mosquito Svp Homologue Represses the 20E-Mediated Transactivation.
To analyze the possible role of AaSvp in regulating 20E-mediated activation of yolk protein genes, we first carried out the cell transfection experiments with the 5′-regulatory region of the mosquito Vg gene. The 0.6-kb upstream Vg region, containing the Vg functional ecdysone response element, which has been shown to be sufficient for the in vitro 20E-mediated expression of the mosquito Vg gene in the Drosophila Schneider cell line (17), was used to drive the luciferase reporter. As reported (17), transfection of the 0.6Vg-Luc plasmid alone resulted in elevated expression of the reporter because of activity of endogenous ecdysteroid receptor (Fig. 1, lane 1). However, cotransfection of the 0.6Vg-Luc with the AaSvp expression plasmid led to 66% reduction of this basal activity of the reporter (Fig. 1, lane 2). Cotransfection of mosquito AaEcR-B and AaUSP-B expression plasmids with 0.6Vg-Luc in the presence of 20E increased the transcriptional activity of the reporter 2.3-fold (Fig. 1, lanes 3 and 4). Significantly, cotransfection of AaSvp reduced the 20E-dependent transcriptional activation of the 0.6Vg-Luc by a noteworthy 80% (Fig. 1, lane 5). This experiment indicated that AaSvp was capable of inhibiting Vg gene activation mediated by AaEcR-AaUSP in response to 20E.
Figure 1.
AaSvp inhibits basal and 20E-dependent activation of the A. aegypti Vg gene. 0.6Vg-Luc is a Vg-luciferase reporter gene containing 0.6 kb of the 5′ regulatory region of the Vg gene. Drosophila Schneider cells were transfected with 100 ng of 0.6Vg-Luc, along with pAc5-AaSvp (125 ng), pAc5-AaEcR-B (25 ng), and pAc5-AaUSP-B (25 ng) expression vectors, as specified. Cells were treated, where indicated, with 1 μM 20E for 36 h after transfection and incubated at 22°C.
Mosquito Svp Forms Heterodimers with EcR, USP, and HR38.
To investigate the mechanism governing the Svp inhibition of the 20E-dependent transcriptional activation further, we used the mammalian two-hybrid and the GST pull-down assays to test the ability of AaSvp to form heterodimers with AaEcR, AaUSP, AHR38, or a homodimer with itself.
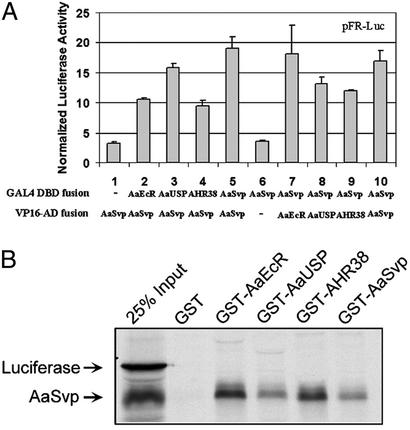
In the two-hybrid assay, the ligand (dimerization)-binding domains (LBDs) of mosquito nuclear receptors (AaSvp, AaEcR, AaUSP, and AHR38) were subcloned into pVP16 (CLONTECH) to construct fusions to the acidic activation domain of the herpes simplex virus transcriptional activator VP16. Similarly, these LBDs were cloned into pM vector (CLONTECH) to generate fusions to the DBD of GAL4. The GAL4 response element-controlled Firefly luciferase expression plasmid, pFR-Luc (5xUAS-TATA-Luc, Stratagene), was used as a reporter gene. Individual GAL4 fusion construct was transiently expressed in CV-1 cells together with VP16-AaSvp. Coexpression of VP16-AaSvp with GAL4-AaEcR resulted in the stimulation of the GAL4-responsive reporter (Fig. 2A, lane 2), indicating that AaSvp and AaEcR can dimerize in intact cells. Similarly, VP16-AaSvp was recruited to the reporter by the LBDs of AaUSP, AHR38, or AaSvp (Fig. 2A, lanes 3–5). However, when VP16-AaSvp was expressed alone, its transcriptional activity was negligible (Fig. 2A, lane 1).
Figure 2.
AaSvp directly interacts with AaEcR and AaSvp. (A) Mammalian two-hybrid assay. LBDs of AaEcR, AaUSP, AHR38, and AaSvp were individually fused with GAL4 DBD or VP16 AD. CV-1 cells were cotransfected with control reporter pCMV-LacZ and reporter construct pFR-Luc, together with indicated plasmids. Luciferase activity was normalized with LacZ activity. (B) In vitro GST pull-down assay. CDEF domains of AaEcR, AaUSP, AHR38, and AaSvp were fused to GST tag. Fusion proteins were expressed and incubated with 35S-labeled, TNT-expressed, full-length AaSvp. Luciferase was used as negative control.
Next, VP16 fusion constructs were tested separately with GAL4-AaSvp, which alone did not activate the reporter gene (Fig. 2A, lane 6). When GAL4-AaSvp was expressed together with VP16-AaEcR, VP16-AaUSP, VP16-AHR38, or VP16-AaSvp, the reporter gene was activated (Fig. 2A, lanes 7–10). Thus, in the mammalian cells, AaSvp was capable of forming not only homodimers but also heterodimers with AHR38, AaEcR, or AaUSP.
These AaSvp protein–protein interactions were further confirmed by using GST pull-down assays (Fig. 2B). In vitro synthesized and 35S-labeled, full-length AaSvp protein was retained by a GST protein, which was fused to AaEcR that lacked the A/B region (GST-AaEcR). Likewise, AaSvp was readily pulled down by GST-AHR38, GST-AaSvp, or GST-AaUSP, individually. As a negative control, luciferase protein was used, and it was not able to interact with any of these GST fusion proteins.
DNA Binding Is Not Required for the Repression Function of AaSvp.
To understand whether DNA binding was required for the repression function of AaSvp, we created a GAL4-AaSvp chimera by fusing the AaSvp LBD to the GAL4 DBD. After cotransfection of the reporter 2xDR1-TATA-Luc with AaEcR-B and AaUSP-B, a 6.3-fold induction was observed when 1 × 10−6 M Ponasterone A was added (Fig. 3, lanes 1 and 2). Consistently with the test performed in the Drosophila cell line (Fig. 1), this induction was abolished by cotransfection of the AaSvp expression vector (Fig. 3, lane 3). When the GAL4 DBD-AaSvp construct was cotransfected instead of AaSvp, this chimeric protein displayed a similar inhibitory effect. However, when GAL4 DBD was used, no repression was observed (Fig. 3, lanes 4 and 5). These results suggested that active repression function resided in the LBD of AaSvp, and AaSvp repressed EcR-USP-mediated activation of the reporter transcription in trans without binding to DNA.
Figure 3.
AaSvp represses AaEcR–AaUSP-mediated 20E response without DNA binding. Transfections were performed in CV-1 cells with a total of 500 ng of DNA, including 100 ng of 2xDR1-TATA-Luc, 25 ng of AaEcR-B and AaUSP-B each, and indicated amounts of AaSvp, GAL4 DBD-AaSvp LBD, and GAL4 DBD. The amount and type of DNA added to each transfection were balanced by adding empty expression vectors. Relative Luc activities were normalized to β-galactosidase activity. Ponasterone A (1 × 10−6 M) was added to the medium after transfection, as indicated.
AaSvp Associates with AaUSP, Not AaEcR, in the Fat Body During Vitellogenesis.
To investigate whether or not AaSvp formed heterodimers in vivo, coimmunoprecipitation experiments were carried out by using nuclei isolated from A. aegypti fat bodies at different stages of vitellogenesis. The anti-USP monoclonal antibody was used to precipitate AaUSP-containing protein complexes from nuclear extracts. Immunoprecipitated complexes were then subjected to SDS/PAGE followed by Western blot analysis using antibodies against AHR38, AaEcR, and AaSvp (Fig. 4A). Consistent with the previous report (21), AaUSP was found to be associated with AHR38 during the state of arrest (PV 3–5 days). After a blood meal, from 3–6 h PBM to 18–20 h PBM, when active synthesis of YPPs took place, AaUSP formed heterodimers with AaEcR. As a control, in vitro synthesized AaEcR-B is shown. A second larger band, visualized by means of anti-EcR antibodies, was likely EcR-A, which was also expressed in the fat body during vitellogenesis (15). Surprisingly, at 30–33 h PBM, when the active Vg synthesis was being terminated, AaUSP was found to be predominantly associated with AaSvp. Only traces of EcR were coimmunoprecipitated at this time. At 44–48 h PBM, when vitellogenesis was completely halted, only the AaUSP–Svp complex was found in coimmunoprecipitation reaction (Fig. 4A).
Figure 4.
AaUSP interacts with AHR38, AaEcR, and AaSvp in the fat body of A. aegypti during the vitellogenic cycle. Nuclear extracts were prepared from the fat bodies of 500 adult females for each time point. (A) Coimmunoprecipitation analysis. A protein aliquot equivalent to 100 mosquitoes was incubated with anti-DmUSP monoclonal antibody. The resulting immune complexes were then precipitated by the addition of protein A-agarose beads. After extensive washing, immune complexes were dissociated and separated by means of SDS/PAGE followed by immunoblotting using rabbit anti-AHR38, rabbit anti-AaEcR, or chicken anti-AaSvp antibodies, or the respective preimmune sera. In vitro translated proteins (AHR38, AaEcR-B, and AaSvp) were used as controls in Western blot analysis. (B) Western blot analysis of USP, EcR, HR38, and Svp proteins during the vitellogenic cycle. A protein aliquot equivalent to 25 fat bodies was loaded in each lane for SDS/PAGE.
These interactions were confirmed in reciprocal experiments in which nuclear extracts were first immunoprecipitated with AHR38, AaEcR, or AaSvp antibodies, and the immunoprecipitated complexes were probed for the presence of AaUSP (data not shown). By using the same approach, we found no evidence for the existence of the AaSvp–AaEcR or AaSvp–AHR38 heterodimer in fat body nuclei at any time of vitellogenesis (data not shown).
The presence of AaUSP, AaEcR, AHR38, and AaSvp proteins in fat body nuclear extracts was monitored by using Western blot analysis. Assaying the same time points revealed that both USP isoforms are present in approximately similar amounts throughout the first vitellogenic cycle (Fig. 4B).
Hormonal Effect on the Formation of USP Heterodimers.
To investigate possible involvement of 20E or juvenile hormone (JH III) in the switch from AaEcR–AaUSP to AaSvp–AaUSP heterodimerization, we conducted an in vitro heterodimerization assay. In vitro synthesized [35S]methionine-labeled AaUSP-B was incubated with equal amounts of in vitro synthesized unlabeled AaSvp and AaEcR-B (1 pmol each). 20E, JH III, or a mixture of these hormones was then added to the proteins. Formation of AaUSP–AaEcR and AaUSP–AaSvp heterodimers was examined by immunoprecipitation of these complexes with either anti-AaEcR or anti-AaSvp antibodies, respectively. Although AaEcR–AaSvp heterodimer might form under these conditions, the depletion of equal amount of AaEcR and AaSvp should display similar effects on the formation of AaUSP–AaEcR and AaUSP–AaSvp. In the presence of 5 × 10−6 M 20E, nine times more AaEcR–AaUSP heterodimer was detected than that of AaSvp–AaUSP (Fig. 5, lane 1). At the lower concentration of 20E (2 × 10−7 M), the amounts of the AaEcR–AaUSP heterodimer declined, whereas AaSvp–AaUSP increased slightly (Fig. 5, lane 2). In the absence of any hormones, AaEcR and AaSvp displayed similar affinities to AaUSP (Fig. 5, lane 3). The presence of 10−5 M JH III in the binding buffer had no effect on the formation of USP heterodimers with either EcR or Svp (data not shown). Likewise, addition of 10−5 M JH III in combination with 20E had no significant effect on the formation of AaEcR–AaUSP heterodimers (data not shown). These data suggested that the decline of 20E could be a key factor in both the disruption of AaEcR–AaUSP and the formation of AaSvp–AaUSP heterodimers in vivo.
Figure 5.
Effect of 20E on the formation of USP heterodimers. In vitro translated AaEcR-B and AaSvp (1 pmol each) were incubated with [35S]methionine-labeled AaUSP-B (1 × 106 cpm) in the binding buffer (20 mM Hepes, pH 7.9/100 mM NaCl/1 mM EDTA/4 mM MgCl2/1 mM DTT/0.02% Nonidet P-40/10% glycerol supplemented before use with 1 mg/ml BSA, 0.5 mM PMSF, and 5 mg/ml each of antipain, leupeptin, and pepstatin, 9 mg/ml aprotinin, and 2 mM benzamidine) for 2–4 h at 4°C. Antibodies against AaEcR (from rabbit) and AaSvp (from chicken) were then added to the reactions. The incubations were extended for an additional 2 h at 4°C, followed by sequential immunoprecipitations of AaEcR–AaUSP heterodimer with protein A agarose beads (Roche Diagnostics) and AaSvp–AaUSP complex with immobilized anti-chicken IgY (Promega). The amounts of each heterodimer were measured by scintillation counting of radiolabeled AaUSP-B in the precipitants. 20E (Sigma) was dissolved in ethanol and added to the binding buffer to a final concentration, as indicated. Ethanol was used as a negative control. This experiment was performed in two independent assays in triplicate. Each value represents the mean ± SD.
AaSvp Is Not Associated with the Vg Gene EcRE During the Vitellogenic Cycle.
To study the interaction of AaSvp with EcR and USP under biologically relevant conditions in vivo, we used ChIP analysis. We determined the timing of occupancy of the Vg gene EcRE by the EcR–USP heterodimer. We also addressed the question of whether or not AaSvp was forming a heterodimer directly on the EcRE of the Vg gene. The Vg EcRE occupancy was monitored for AaEcR, AaUSP, AHR38, AaSvp, and histone H4 with ChIP from fat body nuclear extracts at different times of the first vitellogenic cycle. Polyclonal antibodies specific for each of the five proteins and PCR primers flanking the 300-bp Vg gene fragment containing the Vg EcRE were used, as described in Materials and Methods.
At the state of arrest, only trace amounts of AaEcR could be detected in association with the Vg promoter. However, the amount of AaUSP associated with the Vg promoter at this stage was significant (Fig. 6A). After blood feeding, the Vg promoter occupancy by both AaEcR and AaUSP increased dramatically. At 10–12 h PBM, the time of exponential increase of Vg gene expression, the amount of AaEcR slightly decreased compared with those at 3–5 h PBM (Fig. 6A). Surprisingly, neither AaEcR nor AaUSP was associated with the Vg EcRE at 18–20 h PBM, when transcription of the Vg gene was still increasing, as well as later at 24–28 h PBM, when Vg reached its peak expression (not shown). At 36 h PBM, when expression of the Vg gene was terminated, the EcRE was not occupied by the AaEcR–AaUSP heterodimer. Neither AHR38 nor AaSvp was found on the assayed Vg promoter region at any time point of the first vitellogenic cycle (Fig. 6A). The specificity of factor association within the ecdysone-responsive region of the Vg promoter was confirmed by means of ChIP analysis using preimmune sera, which failed to immunoprecipitate Vg promoter sequences.
Figure 6.
ChIP analysis of the Vg gene EcRE. Crosslinked chromatin preparations from nuclei of the mosquito fat body dissected at different times of vitellogenic cycle were precipitated with antibodies against AaEcR, AaUSP, AHR38, AaSvp, or H4 antibodies. Immunoprecipitated and input material was analyzed by PCR with primers corresponding to the indicated region of the Vg promoter. The region from −380 to −81 contains EcRE of the Vg promoter (VgEcRE1). −, Preimmune sera used for negative control.
In a parallel experiment, primers flanking a 389-bp fragment of the Vg gene located 0.9 kb upstream from the Vg EcRE were used for the control PCR. This regulatory region of the Vg gene has been shown not to contain any EcREs (20). This control experiment demonstrated the lack of binding sites for the AaEcR–AaUSP heterodimer, confirming the specificity of the ChIP assay by using the Vg gene EcRE region. There was no positive reaction for either AHR38 or AaSvp in the ChIP using this upstream regulatory region of the Vg gene at any time point of the first vitellogenic cycle (Fig. 6B). These results revealed that AaSvp was not associated with AaUSP at the endogenous ecdysone-responsive Vg promoter under physiologically relevant conditions in vivo and that AaSvp likely modulated the 20E response by direct association with AaUSP without DNA binding.
Discussion
In this report, we have demonstrated that during mosquito vitellogenesis, Svp is involved in regulation of the cyclicity of the ecdysteroid-mediated signaling via its heterodimerization with USP, the homologue of RXR. The mammalian two-hybrid and GST pull-down assays have established that in vitro AaSvp can interact with AaEcR, AaUSP, and HR38. However, coimmunoprecipitation experiments using nuclear extracts have clearly shown that, in the mosquito female fat body, AaSvp associates only with AaUSP. Furthermore, the formation of AaSvp–AaUSP heterodimers occurs in a precise timely manner at 33–36 h PBM, after the 20E titer declines to the PV level and when the expression of YPP genes, including Vg, is terminated (1). Our in vitro experiments suggest that the declining titer of 20E could be a critical factor facilitating the formation of Svp–USP heterodimers. It is likely that in vivo additional unidentified cofactors determine specificity of AaSvp interaction with USP at the termination stage.
Western blot analyses presented in this paper and elsewhere have demonstrated that both AaSvp and AaUSP proteins are abundant throughout the entire vitellogenic cycle (14, 22). However, the active AaEcR–AaUSP heterodimers are not detectable in the nuclear proteins isolated from fat bodies of pre- and postvitellogenic stages (20, 23). This is also consistent with data from coimmunoprecipitation experiments showing that, at these stages, respectively, AaUSP forms heterodimers with AHR38 or AaSvp.
Transfection assays in Drosophila S2 and mammalian CV-1 cells show that AaSvp represses the 20E-dependent gene activation mediated by the EcR–USP receptor complex. Recently, we have shown that similarly to COUP-TF and DmSvp, AaSvp binds to direct repeats of the sequence AGGTCA and its variants. In addition, AaSvp is able to recognize IR1, although with much lower affinity (22). This broad binding specificity of AaSvp suggests that it could compete with the EcR–USP complex for binding sites. However, the inhibitory activity of the GAL4 DBD-AaSvp LBD chimera used in this work clearly indicates that AaSvp inhibits AaEcR–AaUSP-mediated activation of the reporter gene in trans as a result of a protein–protein interaction without DNA binding. This is consistent with the ChIP assay results showing that, at the time of formation of AaSvp–AaUSP heterodimers, they are not detectable on the Vg gene EcRE in vivo. COUP-TF-mediated transrepression has been reported for heterodimers with RXR, RAR, and TR (26).
Hence, we propose the following working model of modulation of the cyclicity of vitellogenic ecdysteroid-mediated signaling through alternative heterodimerization of the RXR homologue USP (Fig. 7). According to our hypothesis, AaUSP exerts its functions by associating with distinct partners at different stages of vitellogenesis. During the arrest stage, AHR38 prevents the formation of the functional EcR complex by sequestering AaUSP, and 20E-dependent transactivation is therefore blocked. After a blood meal, the AaEcR–AaUSP heterodimerization becomes dominant, and in the case of the Vg gene, AaEcR–AaUSP binding to the Vg EcRE permits the gene activation. When vitellogenesis proceeds to the termination stage, falling 20E titers shift AaUSP heterodimerization toward AaSvp, repressing USP-based hormone responses (Fig. 7).
Figure 7.
Working model of modulation of 20E response via different protein heterodimerizations. At the state of arrest, AaUSP was associated with AHR38, preventing activation of YPP production before blood feeding. After blood meal, AaUSP heterodimerizes with AaEcR and induces expression of YPP genes in the presence of elevated 20E titer. Around 33–36 h PBM, AaSvp attaches to AaUSP, decelerating the massive protein synthesis.
The regulatory network governing gene repression–activation cycles during mosquito vitellogenesis likely contains additional complexity. For example, the ChIP assay has shown that during the state of arrest the Vg EcRE is also occupied by USP. Previous studies suggest that mosquito isoform USP-B functions as a major heterodimerization partner of EcR during vitellogenic response (14). In contrast, the USP-A transcript is highly expressed during the PV period, and it is inhibited by 20E. Thus, it is conceivable that the USP-A isoform is involved in repression of the Vg gene, whereas USP-B is sequestered by HR38. However, without isoform-specific USP antibodies, it is impossible to determine which USP isoform is present at the Vg EcRE during the state of arrest and after the induction of vitellogenesis by a blood meal. In addition, Drosophila USP has been reported to form homodimers in the presence of JH III in vitro (27, 28). It is not clear whether AaUSP homodimers exist in the mosquito fat body at the time of elevated JH III titers.
Similar to some vertebrate nuclear receptors, such as RAR and TR, the ecdysteroid receptor in the fruit fly has dual action. In the presence of 20E, EcR–USP heterodimer activates transcription, whereas the unliganded receptor complex represses basal transcription in transient transfection assays (29). Recent functional studies of the usp gene have indicated that the unliganded EcR–USP heterodimer can mediate developmental silencing in vivo (30). During the state of arrest in fat body of the female mosquito, a very low amount of the EcR–USP complex was detected on the Vg EcRE. It may represent the second mechanism governing repression of the Vg expression prior blood feeding. Another finding, revealed by the ChIP assay, is the timing of the Vg EcRE occupancy by the EcR–USP heterodimer in vivo during vitellogenesis. Surprisingly, the maximal occupancy coincides with the onset and beginning of Vg expression. In contrast, at the time of the highest level of its expression, the EcR–USP heterodimer is not present on the EcRE. Our previous cell transfection analysis has shown that the EcRE is necessary for transactivation of the Vg gene by the EcR–USP heterodimer, although its effect is very low (17). Thus, it is likely that the interaction of the EcR–USP heterodimer and Vg EcRE is essential for switching on this gene but not for maintaining its expression. The transgenic study has shown that the products of early E74 and E75 genes are required for the high level of the ecdysteroid-mediated expression of the Vg gene (20). This implies that the EcR–USP heterodimer initiates the Vg expression in response to 20E, and the 20E-induced cascade of transcription activators maintains that expression. Future ChIP studies should determine the precise timing of occupancy of E74 and E75 binding sites at the Vg gene regulatory region by their respective transcription factors. The AaEcR-A isoform is highly expressed at the time of high titer of 20E (15), however, its role in regulation of YPP gene expression and other events driven by 20E regulatory hierarchy is unclear. The state of EcR isoforms during pre- and postvitellogenic gene repression also remains to be determined.
Likewise, factors other than 20E could also be involved in activating and maintaining the balance toward transition from AaEcR–AaUSP to AaSvp–AaUSP. Our in vitro experiments indicated that, in the absence of 20E, AaEcR and AaSvp are comparable in terms of binding to AaUSP. In fact, reverse-transcription PCR and Western blot assays demonstrated that the AaSvp gene expresses at a high level throughout the vitellogenic cycle (22). In contrast, the AaSvp–AaUSP complexes were detected only at the time of termination of the vitellogenesis, between 33 and 48 h PBM. The decline of 20E could be a key factor in the disruption of AaEcR–AaUSP and the formation of AaSvp–AaUSP heterodimers. However, other hormonal factors at the termination stage, such as oostatic peptide hormone (31), could account for a switch toward the formation of the AaSvp–AaUSP heterodimer complex.
Taken together, our findings indicate that the cyclicity of vitellogenic ecdysteroid-mediated signaling in the mosquito fat body is regulated through USP, which sequentially forms inactive or active heterodimers with either repressors (HR38 and Svp) or the activator (EcR).
Acknowledgments
We thank Professor F. Kafatos (European Molecular Biology Laboratory, Heidelberg) for the kind gift of monoclonal mouse antibodies against DmUSP. This work was supported by National Institutes of Health Grant AI-36959 (to A.S.R.).
Abbreviations
- COUP-TF
chicken ovalbumin upstream promoter-transcription factor
- DBD
DNA-binding domain
- 20E
20-hydroxyecdysone
- EcR
ecdysone receptor
- EcRE
ecdysteroid response element
- LBD
ligand-binding domain
- PV
previtellogenic
- PBM
postblood meal
- RXR
retinoid X receptor
- Svp
Seven-up
- USP
Ultraspiracle
- Vg
vitellogenin
- YPP
yolk protein precursor
- ChIP
chromatin immunoprecipitation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Raikhel A S. Adv Dis Vector Res. 1992;9:1–39. [Google Scholar]
- 2.Raikhel A S, Lea A O. Gen Comp Endocrinol. 1990;77:423–434. doi: 10.1016/0016-6480(90)90233-c. [DOI] [PubMed] [Google Scholar]
- 3.Dittmann F, Kogan P H, Hagedorn H H. Arch Insect Biochem. 1989;12:133–143. [Google Scholar]
- 4.Raikhel A S. In: Biology of Disease Vectors. Marquardt W C, editor. New York: Academic; 2002. [Google Scholar]
- 5.Lea A O. Gen Comp Endocrinol Suppl. 1972;3:602–608. [Google Scholar]
- 6.Lea A O, Brown M R. In: Molecular Insect Science. Hagedorn H H, Hildebrand J G, Kidwell M G, Law J H, editors. New York: Plenum; 1990. pp. 147–154. [Google Scholar]
- 7.Hagedorn H H, O'Connor J D, Fuchs M S, Sage B, Schlaeger D A, Bohm M K. Proc Natl Acad Sci USA. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raikhel A S, Kokoza V A, Zhu J, Martin D, Wang S-F, Li C, Sun G, Ahmed A, Dittmer N, Attardo G. Insect Biochem Mol Biol. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 9.Thummel C S. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 10.Riddiford L M, Cherbas P, Truman J W. Vitam Horm (San Francisco) 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- 11.Thummel C S. Insect Biochem Mol Biol. 2002;32:113–120. doi: 10.1016/s0965-1748(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho W-L, Kapitskaya M, Raikhel A S. Insect Biochem Mol Biol. 1995;25:19–27. doi: 10.1016/0965-1748(94)00045-j. [DOI] [PubMed] [Google Scholar]
- 13.Kapitskaya M, Wang S-F, Cress D E, Dhadialla T S, Raikhel A S. Mol Cell Endocrinol. 1996;121:119–132. doi: 10.1016/0303-7207(96)03847-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang S F, Li C, Zhu J, Miura K, Miksicek R J, Raikhel A S. Dev Biol. 2000;218:99–113. doi: 10.1006/dbio.1999.9575. [DOI] [PubMed] [Google Scholar]
- 15.Wang S-F, Li C, Sun G, Zhu J, Raikhel A S. Mol Cell Endocrinol. 2002;196:29–42. doi: 10.1016/s0303-7207(02)00225-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang S-F, Miura K, Miksicek R J, Segraves W A, Raikhel A S. J Biol Chem. 1998;273:27531–27540. doi: 10.1074/jbc.273.42.27531. [DOI] [PubMed] [Google Scholar]
- 17.Martin D, Wang S-F, Raikhel A S. Mol Cell Endocrinol. 2001;173:75–86. doi: 10.1016/s0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 18.Pierceall W E, Li C, Biran A, Miura K, Raikhel A S, Segraves W A. Mol Cell Endocrinol. 1999;150:73–89. doi: 10.1016/s0303-7207(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 19.Sun G, Zhu J, Chen L, Tu Z, Raikhel A S. Mol Cell Endocrinol. 2002;190:147–157. doi: 10.1016/s0303-7207(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 20.Kokoza V A, Martin D, Mienaltowski M, Ahmed A, Morton C, Raikhel A S. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Miura K, Chen L, Raikhel A S. EMBO J. 2000;19:253–262. doi: 10.1093/emboj/19.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura K, Zhu J, Dittmer N T, Chen L, Raikhel A S. J Mol Endocrinol. 2002;29:223–238. doi: 10.1677/jme.0.0290223. [DOI] [PubMed] [Google Scholar]
- 23.Miura K, Wang S-F, Raikhel A S. Mol Cell Endocrinol. 1999;156:111–120. doi: 10.1016/s0303-7207(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 24.Sachs L M, Shi Y-B. Proc Natl Acad Sci USA. 2000;97:13138–13143. doi: 10.1073/pnas.260141297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christianson A M K, King D L, Hatzivassiliou E, Casas J E, Hallenbeck P L, Nikodem V M, Mitsialis S A, Kafatos F C. Proc Natl Acad Sci USA. 1992;89:11503–11507. doi: 10.1073/pnas.89.23.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng X, Cooney A J, Tsai S Y, Tsai M-J. Mol Cell Biol. 1996;16:2332–2340. doi: 10.1128/mcb.16.5.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones G, Sharp P A. Proc Natl Acad Sci USA. 1997;94:13499–13503. doi: 10.1073/pnas.94.25.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones G, Wozniak M, Chu Y, Dhar S, Jones D. Insect Biochem Mol Biol. 2001;32:33–49. doi: 10.1016/s0965-1748(01)00077-7. [DOI] [PubMed] [Google Scholar]
- 29.Cherbas L, Lee K, Cherbas P. Genes Dev. 1991;5:120–131. doi: 10.1101/gad.5.1.120. [DOI] [PubMed] [Google Scholar]
- 30.Schubiger M, Truman J W. Development (Cambridge, UK) 2000;127:1151–1159. doi: 10.1242/dev.127.6.1151. [DOI] [PubMed] [Google Scholar]
- 31.Lea A O, Brown M R. In: Molecular Insect Science. Hagedorn H H, Hildebrand J G, Kidwell M G, Law J H, editors. New York: Plenum; 1990. pp. 147–154. [Google Scholar]