Abstract
Hex is a homeobox gene that is expressed in all stages of B cell development except plasma cells. We studied lymphocyte development in the absence of Hex by using the RAG1-deficient blastocyst complementation system because homozygous disruption of Hex is embryonic lethal. Hex−/−;RAG1−/− chimeric mice had severely reduced numbers of mature B cells, pre-B cells, and CD5+ B cells with a striking 15-fold increase in the percentage of B220−CD19+ cells in the bone marrow. Hex−/−;RAG1−/− chimeric mice failed to generate IgG antibodies to T cell-independent antigens, although their serum IgM levels and antibody responses to T cell-dependent antigens were intact. Therefore, Hex is necessary for B cell development and function and its absence results in a dramatic increase in B220−CD19+ cells.
The gene Hex encodes a homeobox-containing protein that is highly conserved among vertebrates and is necessary for normal embryonic development. Deletion of Hex by homologous recombination in mice is embryonic lethal and results in abnormalities in forebrain, thyroid, and liver development (refs. 1 and 2; C.W.B., unpublished results). Hex has also been implicated in endothelial and blood differentiation based on its expression in early hematopoietic and vascular cells in multiple species (3–6), the results of gain and loss of function experiments in zebrafish (7), and on the finding of decreased granulocytic-macrophage colony counts in methylcellulose colony-forming assays using Hex−/− yolk sacs (2). Interestingly, the expression of Hex is significantly up-regulated in B cell leukemias in AKXD mice, a recombinant inbred strain that develops predominantly B cell leukemia and lymphoma due to retrovirally mediated insertional activation of cellular proto-oncogenes (8). These data suggest that Hex is necessary for early stages of B cell development, that expression levels of Hex must drop for terminal differentiation to occur, and that dysregulated expression of Hex may play a role in the pathogenesis of B cell malignancy.
Due to the fact that a null mutation in Hex is embryonic lethal, we generated Hex−/−;RAG1−/− chimeric mice to determine the necessity of Hex for lymphocyte development and function. RAG1-deficient mice fail to rearrange antigen receptor genes, resulting in a complete absence of mature T or B cells (9). Injection of pluripotent embryonic stem (ES) cells into RAG1−/− blastocysts gives rise to chimeric animals in which any lymphocytes that develop past the pro-B cell or pro-T cell stage must be derived from the injected ES cells (10). On generating Hex−/−;RAG1−/− chimeric mice we found that, in the absence of Hex, B cell development was almost completely arrested at the pro-B cell stage in the bone marrow, with a severe reduction of mature B cells in the periphery. Additionally, the small number of B cells that did mature failed to generate IgG antibodies to T cell-independent antigens and there was a marked increase in the proportion of B220−CD19+cells, a rare population of cells that has previously been shown to contain bipotential B-macrophage progenitor cells (11). Our results demonstrate that the homeobox transcription factor Hex is necessary for B cell development and function. In addition, mutation of Hex results in a dramatic increase in the rare B220−CD19+cell population.
Methods
Generation of Hex+/− Mice, Hex−/− ES Cells, and RAG1-Deficient Blastocyst Complementation.
The Hex genomic clone was isolated as a P1 clone by screening a commercially available 129/SVJ ES cell library by using Hex-specific PCR primers as described (12). To construct the targeting vector, a 2.2-kb Xba fragment containing the first exon of Hex, including the transcriptional and translational start sites, was replaced with a pgk-neo cassette. In addition, a 250-bp BamHI/PstI fragment containing the second exon, which includes most of the homeodomain, was deleted. Finally, a 5′ 7-kb Xba/Xba and a 3′ 1.5-kb PstI/Xba homologous region was inserted on either side of the pgk-neo cassette. This vector was linearized and electroporated (25 μg) into JET ES cells (2 × 107). JET cells were derived from 129/SVJ blastocysts (J.M., unpublished results). Two hundred twenty heterozygous clones were selected by using G418 (0.3 mg/ml), and five of these were identified as having undergone homologous recombination by Southern blot analysis using a probe external to the 3′ recombination site. Two heterozygous clones were injected into C57BL/6J blastocysts to generate chimeric mice. Chimeric mice from one clone transmitted the mutant allele in the germ line, and these mice were interbred to produce Hex+/− as well as Hex−/− mice. Homozygous mutant ES cell lines were isolated by selecting heterozygous cells in increased concentrations of G418 (4–6 mg/ml). High resolution karyotyping was performed on a total of five Hex−/− clones. Two independently derived clones with 40 chromosomes and no observable cytogenetic abnormalities were used for injection into RAG1−/− blastocysts as described (10). Similar results were obtained in chimeras generated with either Hex−/− ES cell clone. Therefore, the data from both Hex−/− ES cell clones is combined. Genotyping of mice and tissues was performed by PCR analysis on DNA isolated from chimeric mouse tissues or mouse tail DNA by using a multiplex PCR reaction. Primer no. 2 (5′-ccctgtagcggtgagaagag-3′) corresponds to sequence that is present in the first intron of Hex and is present in both the WT and targeted alleles. Primer no. 1 (5′-agacgcaccaccatcaattt-3′) corresponds to sequence in intron 1 that is present only in the WT allele, and primer no. 3 (5′-ccacacgcgtcaccttaata-3′) corresponds to sequence in the neo gene, which is present only in the targeted allele. Therefore, one PCR reaction amplified both the WT and targeted allele, depending on which alleles are present.
Analysis of Lymphocyte Development.
Single-cell suspensions were isolated from bone marrow, spleen, and peritoneum, and red blood cells were lysed in hypotonic buffer. Cells were stained with FITC- and phycoerythrin-labeled antibodies (BD PharMingen) and analyzed on a FACStar with cellquest software (Becton Dickinson). Data are presented as the mean percent ± SEM.
Analysis of B Cell Function.
Serum IgM levels were determined by isotype-specific ELISA following the manufacturer's recommendations using horseradish peroxidase-conjugated detection antibodies (Southern Biotechnology Associates). Plates were analyzed on a Bio-Rad model 550 microplate reader at 415 nm. Serum IgM concentrations were determined based on the titration curve of standard IgM.
T cell-independent antibody responses were determined after immunization with 25 μg of 2,4,6,-trinitrophenyl (TNP; ref. 24)-Ficoll (Biosearch) i.p. IgM and IgG3 anti-TNP antibody levels were measured on day 7 after immunization as described for isotype-specific ELISA except that plates were coated with 1 mg/ml TNP in PBS for 16 h at 4°C. Relative levels of anti-TNP antibodies were determined based on the optical density measurements.
T cell-dependent antibody responses were determined after immunization with keyhole limpet hemocyanin (KLH; Calbiochem) in complete Freund's adjuvant (50 μg per mouse) i.p. IgM and IgG1 anti-KLH antibody levels were measured on days 7 and 19 after immunization, respectively, as described for isotype-specific ELISA except that plates were coated with 1 mg/ml KLH in PBS for 16 h at 4°C. Relative levels of anti-KLH antibodies were determined based on the optical density measurements.
Results
B Cell Development Is Severely Impaired in the Absence of Hex.
We generated chimeric mice in which Hex−/− ES cells were injected into RAG1−/− blastocysts. We first isolated two independent Hex−/− ES cell clones with a homozygous disruption of the Hex gene in which exons 1 and 2 were deleted and replaced by the neomycin resistance gene (Fig. 1) and then injected each of these Hex−/− clones into RAG1−/− blastocysts. As controls, we generated chimeric mice with Hex+/− ES cell clones as well as WT (Hex+/+) JET ES cells that had not undergone gene targeting or G418 selection. We also used WT C57BL/6J as an additional control. In all of the analyses that follow, similar results were obtained from Hex+/+;RAG1−/− chimeras and WT C57BL/6J mice. Hex−/−;RAG1−/−, Hex+/−;RAG1−/−, and Hex+/+;RAG1−/− chimeric offspring were found to have ES cell-derived lymphocytes as determined by PCR genotyping of lymphocytes as well as positive staining with antibodies to the Ly9.1 allelic marker (data not shown), which is not expressed in C57BL/6J mice and therefore distinguishes ES cell-derived lymphocytes (129/SVJ strain) from RAG1−/− cells (C57BL/6J strain).
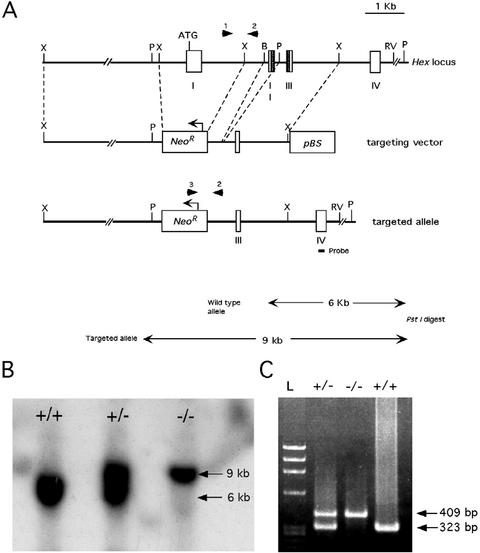
Figure 1.
Targeted disruption of the mouse Hex gene. (A) Schematic representation of the mouse WT Hex locus, targeting vector, and targeted allele. The homeobox is present in exons 2 and 3 (shaded regions). Arrows show the direction of transcription. The genomic probe, which identifies different PstI fragment sizes for the WT and targeted allele, is shown. (B) Southern blot analysis of genomic DNA cut with PstI from WT, Hex+/−, and Hex−/− ES cell clones. (C) PCR genotyping of ES cell clones. Primers 1, 2, and 3 (arrowheads in A) were used to amplify sequences present in the WT or targeted allele.
The development of T and B cells in Hex−/−;RAG1−/−, Hex+/−;RAG1−/− and Hex+/+;RAG1−/− chimeras was assessed by flow cytometry of lymphocytes stained with antibodies to various surface antigens. T cell development seemed to proceed normally in Hex−/−;RAG1−/−, Hex+/−;RAG1−/−, and Hex+/+; RAG1−/− chimeras. As expected, spleen cells from RAG1−/− mice contained no CD4- or CD8-positive cells. Spleen cells from Hex−/−;RAG1−/−, Hex+/−;RAG1−/−, and Hex+/+;RAG1−/− chimeras contained normal percentages of CD4 and CD8 T cell populations (Fig. 2), indicating that T cell development was not affected in the absence of Hex.
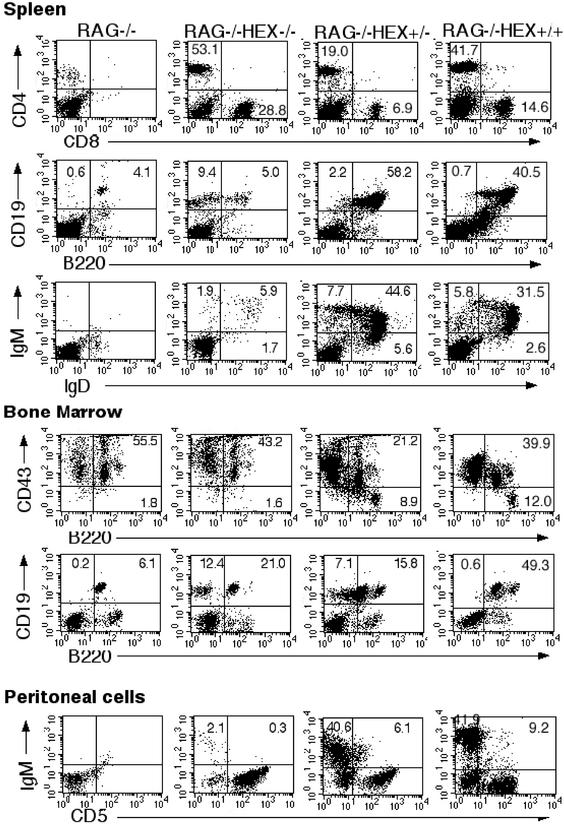
Figure 2.
B cell development is impaired in the absence of Hex. Representative results from flow cytometric analysis of lymphocytes from RAG1−/−, Hex−/−;RAG1−/− chimeras, Hex+/−;RAG1−/− chimeras, and Hex+/+;RAG1−/− chimeras. The staining antibodies are indicated along the axes. The numbers indicate the percentage of cells within the quadrant. Similar results were seen in at least six RAG1−/−, six Hex−/−;RAG1−/− chimeras from two different Hex−/− ES cell clones, three Hex+/−;RAG1−/− chimeras, and three Hex+/+;RAG1−/− chimeras.
However, B cell development was markedly impaired in Hex−/−;RAG1−/− chimeras. The absence of Hex resulted in a profound decrease in the percentage of B cells characterized by the surface expression of B220 and CD19. Analysis of spleen cells showed that only 6.2 ± 0.7% of the cells in Hex−/−;RAG1−/− were B220+CD19+, which was at least 7-fold less than in Hex+/−;RAG1−/− chimeras (58.8 ± 4.8%) and Hex+/+;RAG1−/− chimeras (44 ± 16.4%, Fig. 2) as well as WT mice (46 ± 10.9%). Consistent with the finding of markedly reduced percentages of B220+CD19+ cells, only 5.6 ± 0.6% of the spleen cells of Hex−/−;RAG1−/− chimeras were IgM+ and IgD+ (mature B cells) as compared with 42.6 ± 9.8% in Hex+/−;RAG1−/−, 39.4 ± 17.1% in Hex+/+;RAG1−/− chimeras (Fig. 2) and 46.2 ± 7.8% in WT mice. A similar severe reduction in mature B cells was found in the blood and lymph nodes from Hex−/−;RAG1−/− chimeras (data not shown).
As B cells mature in the bone marrow from pro-B to pre-B cells, they down-regulate expression of the surface antigen CD43. In the bone marrow of Hex−/−;RAG1−/− chimeras, pre-B cells (CD43−B220+) were undetectable (Fig. 2) in marked contrast to Hex+/−;RAG1−/− and Hex+/+;RAG1−/− chimeras (Fig. 2) or to WT mice (data not shown), indicating that the absence of Hex impaired B cell development at or before the pro-B cell stage. Because RAG1−/− mice have pro-B cells in their bone marrow, it is difficult to determine whether pro-B cell development is affected in the Hex−/− chimeras. However, at least 20% of the B220+ cells in Hex−/−;RAG1−/− chimeras were Ly9.1 positive (data not shown), indicating that some of the pro B cells were derived from Hex−/− ES cells.
B1 cells (IgM+CD5+) represent fetal/neonatal B cells in mice and are primarily found in the peritoneum (13). To determine the effect of the absence of Hex on B1 cell development, cells were isolated from the peritoneum and stained with IgM and CD5 mAbs. Hex−/−;RAG1−/− chimeras contained markedly reduced numbers of IgM positive cells with barely any detectable CD5+ IgM+ cells, in contrast to Hex+/−;RAG1−/− and Hex+/+; RAG1−/− chimeras (Fig. 2) as well as WT mice (data not shown), indicating that Hex is required for both B1 and B2 cell development.
Absence of Hex Results in Striking Accumulation of B220−CD19+ Cells.
One surprising finding was the presence of an over 15-fold increase in the percentage of B220−CD19+ cells in Hex−/−; RAG1−/− chimeras as compared with RAG1−/− and Hex+/+; RAG1−/− chimeras (Fig. 2 and Table 1). In both the spleen and bone marrow, B220−CD19+ cells made up <1% of the cells in Hex+/+;RAG1−/− chimeras, RAG1−/− mice (Fig. 2 and Table 1), and WT mice (Table 1 and data not shown) but 14.9 ± 7.5% of the cells in Hex−/−;RAG1−/− chimeric mice (Table 1). These cells were also found in the bone marrow (4.8 ± 1.7%) and to a lesser extent, in the spleens of Hex+/−;RAG1−/− chimeras (1.6 ± 0.6%, Fig. 2 and Table 1), suggesting that heterozygous disruption of Hex may result in an intermediate phenotype. Because of the increased percentage of B220−CD19+ cells in Hex+/−;RAG1−/− chimeras, we performed FACS analysis on bone marrow of non-chimeric Hex+/− mice to determine whether the increase in this unusual population of cells was an artifact of the blastocyst complementation assay or truly represented haploinsufficiency at the Hex locus. We found that Hex+/− mice had an increased percentage of B220−CD19+ cells (2.4 ± 1.4%) compared with control mice [both WT C57BL/6 mice (0.6 ± 0.43%) and Hex+/+ littermates of the Hex+/− mice (0.2 ± 0.02%)]. This increase is not as great as that seen in Hex−/−;RAG1−/− chimeric mice. Therefore, the abnormal accumulation of B220−CD19+ cells in both Hex+/− and Hex+/−;RAG1−/− chimeras represents an intermediate phenotype and is due to haploinsufficiency at the Hex locus and is not an artifact of the RAG1-deficient blastocyst complementation assay. The B220−CD19+ cells are unusual because B220 normally appears on the surface of B cell progenitors before CD19. However, this phenotype is similar to that of a recently described population of cells in adult bone marrow that contain bipotential B-macrophage progenitor cells (11).
Table 1.
Frequency of B220−CD19+ cells in bone marrow
| Genotype | % B220−CD19+ cells in bone marrow |
|---|---|
| Hex−/−; RAG1−/− (n = 5) | 14.9 ± 7.5 |
| Hex+/−; RAG1−/− (n = 3) | 4.8 ± 1.7 |
| Hex+/+; RAG1−/− (n = 3) | 0.6 ± 0.17 |
| WT − C57BL/6 (n = 4) | 0.6 ± 0.43 |
| WT − Hex+/+ littermates of Hex+/− mice (n = 4) | 0.2 ± 0.02 |
| Hex+/− (n = 4) | 2.4 ± 1.4 |
B Cell Function Is Perturbed in the Absence of Hex.
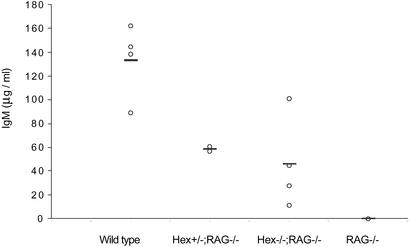
We assessed the ability of the residual mature B cells in Hex−/−;RAG1−/− chimeras to synthesize Ig and to undergo Ig isotype switching in response to T cell-independent and to T cell-dependent antigens. Contrary to our expectation, serum IgM was detected in most of the Hex−/−;RAG1−/− chimeras at levels that were comparable to Hex+/−;RAG1−/−, although lower than WT mice (Fig. 3). This result indicates that the small number of B cells generated in the absence of Hex were capable of synthesizing IgM. The capacity of these B cells to synthesize IgM is consistent with other genetic mutations that result in decreased numbers of mature B cells such as BTK and PI3K, which also result in the presence of serum IgM although at reduced levels (14, 15).
Figure 3.
Serum IgM level in the absence of Hex. Mean serum IgM concentrations in RAG1−/−, Hex−/−;RAG1−/− chimeras, Hex+/−;RAG1−/− chimeras, and C57BL/6 mice were determined by ELISA from at least three mice in each group.
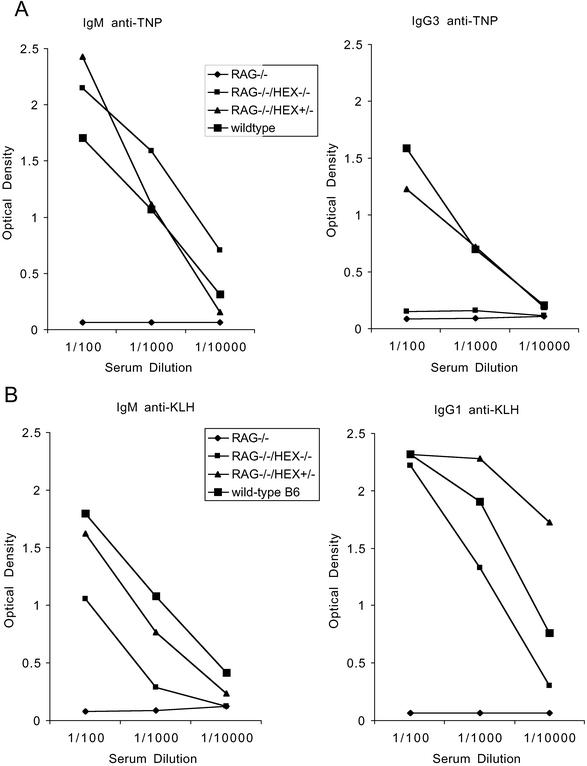
To evaluate antibody responses to T cell-independent antigens, mice were immunized with TNP-Ficoll, and serum levels of IgM anti-TNP and IgG3 anti-TNP were assayed 7 days later. All of the mice tested, including the Hex−/−;RAG1−/− chimeras, generated IgM anti-TNP antibodies. However, in contrast to Hex+/−;RAG1−/− and WT mice, Hex−/−; RAG1−/− chimeras did not synthesize any detectable IgG3 anti-TNP antibodies (Fig. 4A), indicating that T cell-independent Ig isotype switching was defective in the absence of Hex.
Figure 4.
Role of Hex in antibody responses. T cell-independent antibody responses to TNP-Ficoll (A) and T cell-dependent antibody responses to KLH (B) in RAG1−/−, Hex−/−;RAG1−/− chimeras, Hex+/−;RAG1−/− chimeras, and C57BL/6 mice. The results are the mean antibody titers from at least three mice for each group. Similar results were found in Hex+/− chimeras and in Hex+/− mice, and a total of three mice were grouped together.
The ability of Hex−/−;RAG1−/− B cells to synthesize antibodies to T cell-dependent antigens was assessed by immunization with KLH and determination of serum levels of IgM and IgG1 anti-KLH antibodies 7 and 19 days later, respectively. Hex−/−; RAG1−/− chimeras synthesized both IgM and IgG1 antibodies to KLH (Fig. 4B) but at lower titers than Hex+/−;RAG1−/− and WT mice. Nevertheless, these results indicate that Hex is not required for T cell-dependent Ig isotype switching.
Discussion
We have demonstrated a critical and unique role for the homeobox gene Hex in B cell development. In the absence of Hex, B cell development seems to be arrested at the pro-B cell (B220+CD43+) stage in the bone marrow and there is a severe reduction in mature B1 and B2 cells in the periphery with a small number of B cells that express IgM and IgD in the spleen. These cells seem to be capable of synthesizing IgM and undergoing T cell-dependent Ig isotype switching to IgG1, indicating that the small numbers of Hex−/− B cells that do develop can differentiate into mature B cells and produce IgG1. Furthermore, these results suggest that Hex is not necessary for CD40-mediated isotype switching in mouse B cells, and that T cell helper function is normal in the absence of Hex. However, Hex-deficient B cells are not capable of undergoing T cell-independent Ig isotype switching, indicating that T cell-independent B cell function is defective in the absence of Hex. The capacity of Hex−/− B cells to synthesize IgM and antigen-specific IgM antibodies also suggests that recombination of the heavy and light chain variable regions is not dependent on Hex, although inefficient recombination of the variable regions cannot be ruled out.
The presence of decreased numbers of mature B cells, and absent T cell-independent Ig isotype switching with normal T cell-dependent immune responses suggests that the block in B cell development in the absence of Hex seems to occur at a later stage than in mice with homozygous disruption of Ikaros, E2A, or Pax 5 genes (16–19). However, the unique accumulation of B220−CD19+ cells in the absence of Hex indicates that Hex has a different role during B cell development than these transcription factors. Further study of Hex and its interaction with other transcription factors or signaling molecules that have been shown to be necessary for B cell development is likely to provide important new insight into B cell development and function.
The accumulation of a population of cells that are CD19+ but B220− in Hex−/−;RAG1−/− chimeras, especially in the bone marrow, is novel and may reflect an increased accumulation of a recently described cell population in adult bone marrow that contains bipotential B cell-macrophage progenitor cells (11). It is also consistent with previous data suggesting a potential role for Hex in monocyte development (2). The B220−CD19+ cells in Hex−/−;RAG1−/− chimeras are likely to be immature progenitor cells because there were no CD43− cells in the bone marrow (Fig. 2). The fact that as many as 44% of the cells in the spleen and marrow of Hex−/−;RAG−/− chimeras were B220−CD19+ is particularly striking because <1% of the marrow and spleen cells of WT mice are B220−CD19+, a percentage that is consistent with that reported by Montecino-Rodriguez (11). However, without further evidence, we cannot speculate as to whether the B220−CD19+ cells that are accumulating in the absence of Hex have the same biphenotypic potential as the cells reported by Montecino-Rodriguez or represent a novel cell population. In vitro differentiation studies will be needed to determine the developmental potential of these cells.
Another interesting feature related to the increased accumulation of B220−CD19+ cells is the presence of elevated percentages of these cells in both Hex+/−;RAG1−/− and in non-chimeric Hex+/− mice. The fact that the percentage of B220−CD19+ is <1% in Hex+/+;RAG1−/− chimeras demonstrates that the abnormal accumulation of these cells in Hex+/−;RAG1−/− and in non-chimeric Hex+/− mice is not due to strain difference, clonal variation due to ES cell selection in culture, or to some intrinsic inability of JET ES cells to give rise to normal lymphopoiesis in RAG1−/− mice. Rather, these data indicate that the defect is due solely to mutation of the Hex gene and that haploinsufficiency at the Hex locus results in an abnormal phenotype. Abnormalities of lymphopoiesis in heterozygous mice have previously been reported with germ line mutations in the transcription factors E2A (18) and Pax5 (20, 21). However, this is the only example of an abnormal phenotype associated with heterozygosity at the Hex locus. Neither of the previous reports of mutations of Hex demonstrated any discernible phenotype in Hex heterozygotes (1, 2). It is certainly possible that there are other phenotypic abnormalities in Hex+/− mice that have yet to be described.
In summary, mutation of the homeobox gene Hex results in a number of abnormalities of lymphopoiesis, including a severe reduction in the percentages of mature B cells, pre-B cells, and CD5+ (peritoneal) B cells. Additionally, the absence of Hex results in a failure to generate IgG antibodies to T cell-independent antigens. Finally, there is a dramatic accumulation of B220−CD19+ cells with both homozygous and heterozygous Hex mutant cells. The Hex−/−;RAG1−/− chimera is the only example of genetic mutation resulting in a markedly expanded population of B220−CD19+ cells and provides a novel opportunity to examine the origin and development of this unique population of cells.
Acknowledgments
We thank C. A. Janeway, Jr., D. Schatz, M. K. Hostetter, and W. Khan for suggestions and critical reading of the manuscript. This research was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant K08 HL03471 (to C.W.B.) and Charles Hood Foundation Child Health Research Grants (to C.W.B., H.C.J., and R.L.F.).
Abbreviations
- ES
embryonic stem
- KLH
keyhole limpet hemocyanin
- TNP
2,4,6-trinitrophenyl
References
- 1.Martinez Barbera J P, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington R S. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- 2.Keng V W, Yagi H, Ikawa M, Nagano T, Myint Z, Yamada K, Tanaka T, Sato A, Muramatsu I, Okabe M, Sato M, Noguchi T. Biochem Biophys Res Commun. 2000;276:1155–1161. doi: 10.1006/bbrc.2000.3548. [DOI] [PubMed] [Google Scholar]
- 3.Bedford F K, Ashworth A, Enver T, Wiedemann L M. Nucleic Acids Res. 1993;21:1245–1249. doi: 10.1093/nar/21.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crompton M R, Bartlett T J, MacGregor A D, Manfioletti G, Buratti E, Giancotti V, Goodwin G H. Nucleic Acids Res. 1992;20:5661–5667. doi: 10.1093/nar/20.21.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hromas R, Radich J, Collins S. Biochem Biophys Res Commun. 1993;195:976–983. doi: 10.1006/bbrc.1993.2140. [DOI] [PubMed] [Google Scholar]
- 6.Manfioletti G, Gattei V, Buratti E, Rustighi A, De Iuliis A, Aldinucci D, Goodwin G H, Pinto A. Blood. 1995;85:1237–1245. [PubMed] [Google Scholar]
- 7.Liao W, Ho C, Yan Y L, Postlethwait J, Stainier D Y. Development. 2000;127:4303–4313. doi: 10.1242/dev.127.20.4303. [DOI] [PubMed] [Google Scholar]
- 8.Hansen G M, Justice M J. Oncogene. 1999;18:6531–6539. doi: 10.1038/sj.onc.1203023. [DOI] [PubMed] [Google Scholar]
- 9.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Lansford R, Stewart V, Young F, Alt F W. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montecino-Rodriguez E, Leathers H, Dorshkind K. Nat Immunol. 2001;2:83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh B, Jacobs H C, Wiedemann L M, Brown A, Bedford F K, Nimmakayalu M A, Ward D C, Bogue C W. Mamm Genome. 1999;10:1023–1025. doi: 10.1007/s003359901152. [DOI] [PubMed] [Google Scholar]
- 13.Hardy R R, Hayakawa K. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 14.Khan W N, Alt F W, Gerstein R M, Malynn B A, Larsson I, Rathbun G, Davidson L, Muller S, Kantor A B, Herzenberg L A, et al. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 15.Fruman D A, Snapper S B, Yballe C M, Davidson L, Yu J Y, Alt F W, Cantley L C. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 16.Georgopoulos K, Bigby M, Wang J H, Molnar A, Wu P, Winandy S, Sharpe A. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 17.Bain G, Maandag E C, Izon D J, Amsen D, Kruisbeek A M, Weintraub B C, Krop I, Schlissel M S, Feeney A J, van Roon M, et al. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Y, Soriano P, Weintraub H. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 19.Urbanek P, Wang Z Q, Fetka I, Wagner E F, Busslinger M. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 20.Nutt S L, Busslinger M. Biol Chem. 1999;380:601–611. doi: 10.1515/BC.1999.077. [DOI] [PubMed] [Google Scholar]
- 21.Nutt S L, Vambrie S, Steinlein P, Kozmik Z, Rolink A, Weith A, Busslinger M. Nat Genet. 1999;21:390–395. doi: 10.1038/7720. [DOI] [PubMed] [Google Scholar]