Abstract
The extent to which the biodiversity and community composition of ecosystems affect their functions is an issue that grows ever more compelling as human impacts on ecosystems increase. We present evidence that supports a novel function of vertebrate biodiversity, the buffering of human risk of exposure to Lyme-disease-bearing ticks. We tested the Dilution Effect model, which predicts that high species diversity in the community of tick hosts reduces vector infection prevalence by diluting the effects of the most competent disease reservoir, the ubiquitous white-footed mouse (Peromyscus leucopus). As habitats are degraded by fragmentation or other anthropogenic forces, some members of the host community disappear. Thus, species-poor communities tend to have mice, but few other hosts, whereas species-rich communities have mice, plus many other potential hosts. We demonstrate that the most common nonmouse hosts are relatively poor reservoirs for the Lyme spirochete and should reduce the prevalence of the disease by feeding, but rarely infecting, ticks. By accounting for nearly every host species' contribution to the number of larval ticks fed and infected, we show that as new host species are added to a depauperate community, the nymphal infection prevalence, a key risk factor, declines. We identify important “dilution hosts” (e.g., squirrels), characterized by high tick burdens, low reservoir competence, and high population density, as well as “rescue hosts” (e.g., shrews), which are capable of maintaining high disease risk when mouse density is low. Our study suggests that the preservation of vertebrate biodiversity and community composition can reduce the incidence of Lyme disease.
The inexorable loss of species from ecological communities is a worldwide phenomenon that has prompted ecologists to critically examine the relationship between biodiversity and ecosystem function. Diversity has been causally linked to many ecosystem characteristics including productivity (1) variability (2, 3), resilience (4), and resistance to invasion and stressors (5–7). Here, we present evidence that diversity, in the form of species richness, can play an important role in determining disease risk to humans, using Lyme disease as a model system. Lyme disease (etiologic agent Borrelia burgdorferi), the most common vector-borne disease in North America, is transmitted by ticks of the family Ixodidae. We tested the Dilution Effect model (8, 9), which predicts that high species diversity in the community of hosts for ticks reduces the infection prevalence of ticks by diluting the effects of the ubiquitous white-footed mouse (Peromyscus leucopus), the principal natural reservoir for B. burgdorferi (10, 11).
In the forests of eastern North America, the immature stages of the Lyme disease vector, Ixodes scapularis, are extreme generalists, feeding on numerous mammalian, avian and reptilian host species, most of which are believed or documented to have low reservoir competence. The white-footed mouse, which infects from 40% to 90% of the larval ticks that feed on it (10, 11), has wide habitat tolerances, occurring in pristine forest as well as in degraded woodlots (12–14). Therefore, species-poor communities tend to have mice, but few other hosts, whereas species-rich communities have mice, plus many other potential hosts, which should dilute the impact of mice by feeding but rarely infecting ticks. The resulting expectation, the Dilution Effect hypothesis, is decreasing infection prevalence in the tick population with increasing host diversity. The Dilution Effect might not be a “pure” diversity effect (1), but could result from the observed correlations among vertebrate diversity and relative abundances of pathogen-carrying host species.
Of the three postegg life stages of the tick, the nymphal stage is most likely to infect humans (15). Because there is no transovarial transmission (16), the host from which the larval blood meal is taken is a key predictor of whether a nymph will be infected with B. burgdorferi. We use nymphal infection prevalence (NIP) as a measure of disease risk to humans and, by extending and parameterizing the model of Giardina et al. (17), investigate the change in NIP as the diversity of the host community is increased. NIP represents the probability of being exposed to Lyme bacteria if bitten by a nymphal tick and is a function of the distribution of larval meals among the community of vertebrates. The importance of NIP is well demonstrated in the varying prevalence of B. burgdorferi in North American ticks. Although I. scapularis occurs throughout the eastern half of the United States (18), B. burgdorferi prevalence is low in southern states (19, 20) and cases of Lyme disease are correspondingly low in these areas (21). Thus, despite high vector densities, disease risk to humans can be quite low if vector infection prevalence is also low.
This research was conducted in Dutchess County of southeastern New York at the Institute of Ecosystem Studies, a postagricultural landscape consisting largely of an oak forest matrix. Lyme disease incidence in this region is among the highest in the U.S., and NIP for questing ticks is ≈36%.
Materials and Methods
Model Parameters.
Our goal was to sample individuals from all potentially important avian and mammalian host species (reptilian hosts are rare or absent at our sites) to quantify the impact of each species of host on the infection prevalence of nymphal I. scapularis. For each species, we determined the mean number of engorged larvae collected during a 72-h captive period (“body burden”), the proportion of engorged larvae that molted into nymphs (“molting percentage”), and the infection prevalence of the resulting nymphs (“realized reservoir competence” see below). Data were collected during the seasonal peak in larval activity (August and September) of 2000 and 2001 unless otherwise noted. A random sample of mammalian and avian hosts was trapped in Sherman live traps (3 × 3.5 × 9 cm; ≈36,200 trapnights), dry pitfall traps (480 trapnights), Tomahawk traps (15 × 15 × 48 cm and 25 × 30 × 81 cm; 600 trapnights) and mist nets. All hosts were held in the laboratory for 72 h for tick collection and released at the site of capture. Engorged larvae dropping off animals were collected from pans of water held underneath caged animals and maintained at 22°C and high humidity until all specimens molted or died.
Molted nymphs were examined for the presence of B. burgdorferi by using direct immunofluorescence antibody microscopy (DFA) (ref. 22, see ref. 23 for detailed description of methods). We are aware of the limits of DFA to discern between B. burgdorferi and a recently discovered, sympatric genospecies of Borrelia (24), which was detected in 0–2.5% of nymphal I. scapularis sampled from parts of the northeastern and mid-Atlantic regions of the U.S. It is possible that our estimates of NIP and reservoir competence therefore may be slightly inflated if this novel Borrelia exists locally. However, an analysis of the ospA gene, specific to B. burgdorferi, from our site by Qui et al. (20) found a prevalence of 80% (±6.3%) in adult ticks (n = 40) versus 74.5% prevalence that we have observed by means of DFA (n = 1,493), suggesting that the novel Borrelia, if it exists at all, is at very low prevalence in our site. In any case, because identical DFA methods were used to assess infection in host-seeking and newly molted nymphs, our results should be robust to slight inflation of infection prevalence.
For all species except birds, molting percentage and realized reservoir competence were calculated as mean percentages per host rather than the gross percentages (i.e., number molted divided by number collected). The number of larvae tested per individual was the largest of the following: all nymphs that molted successfully, 25 nymphs or 10% of the body burden. Only animals from which at least five ticks were collected (for molting percentage) or tested (for reservoir competence) were included in calculations to avoid skewing the data. Body burdens of birds were frequently less than 5; therefore, gross percentages were substituted for mean percentages for these species.
Data were collected from mice in 1998 and from chipmunks in 1998, 1999, and 2000. Deer (Odocoileus virginianus) were shot under a depredation permit issued to a local farmer by the New York State Department of Environmental Conservation, and larvae were collected following the protocol of Telford (25). We collected few fully engorged larvae from deer, therefore we have incorporated published data (25) in our estimate of reservoir competence. Bird data are for songbirds [American Robin (Turdus migratorius), Ovenbird (Seiurus aurocapillus), Veery (Catharus fuscenscens), and Wood Thrush (Hylocichla mustelina)] that nest and/or forage on the ground and thus were expected to feed a significant number of ticks. As birds were sampled during the breeding season (June and July of 2000 and 2001) rather than the seasonal peak in larval activity, body burdens were adjusted by a correction factor equal to the mean difference in the body burden of mice collected in June and July vs. that of mice collected in August and September. Realized reservoir competence for birds incorporates original and published data (17) and is weighted by the relative abundance of each species at our site.
Density Estimation.
In the model, mouse density was hypothetically varied from 0 to 100 mice per hectare, to reflect the extreme fluctuations in numbers observed in this species (26). Eastern chipmunk (Tamias striatus) density was modeled as a function of mouse density, reflecting the tight correlation, with a slope of 0.5, between densities of the two species measured at the Institute of Ecosystem Studies over a period of 4 years (11). The densities of all other species were held constant as follows: we estimated songbird densities by using the point-count method (27) and corrected for season by multiplying breeding season densities by 2.4 (28). Deer densities were minimum number known alive estimated by spotlight counts in the autumn of 2000 and 2001. All other densities were taken from the literature and considered typical moderate densities for our habitat type (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org).
Reservoir Competence.
Reservoir competence is generally characterized by three components: susceptibility of the host to infection when bitten by an infected vector; the ability of the pathogen to magnify and persist in the host; and the efficiency of the host at transmitting the spirochete to feeding vectors (29); and is generally measured in the laboratory by using animals of known exposure. Our field-measured term “realized reservoir competence” (30) also incorporates the history of exposure of each host to infected vectors in the wild. Thus, realized reservoir competence is highly relevant to the role that each species plays in nature. The capacity of free-ranging animals to infect free-ranging populations of larval ticks is what will determine the natural infection prevalence in the nymphal cohort, and therefore the risk to humans entering that environment.
Model Details.
The model is an extension of Giardina et al. (17). It calculates the predicted NIP as the sum across all species of the nymphs infected by each species divided by the total number of nymphs fed by all species. Parameters of the model include density of host species (original data or literature value) Ni; species-specific body burdens (original data) Bi; species-specific molting percentage (original data) Si; and species-specific reservoir competence (original data) Ci. Therefore, mi = Ni Bi Si; where mi is the number of larval meals taken from species i; and Ii = mi Ci; where Ii is the number of nymphs infected from their larval meal on species i; and the total number of nymphs infected from their larval meal (IT) is IT = Σ mi Ci. The number of nymphs not infected in their larval meal (Ui); Ui = mi (1 − Ci) and UT = Σ mi(1 − Ci). Thus, the total NIP (NIPT): NIPT = IT/(IT+ UT). This allows us to describe the contribution of each species to NIP. This model makes the following assumptions: tick burdens are relatively constant among years (31); tick mortality, in addition to that captured in the molting percentage, is constant across all host species; mortality of ticks is independent of B. burgdorferi infection; infection prevalence in molted nymphs is the same as in engorged larvae. The inclusion of molting percentage as a parameter assumes that the species-specific molting success observed under controlled conditions in the lab is similar to that incurred in the field. The model outcome deviates only slightly (an increase of 0.9–2.7% points in the predicted NIP) when the molting percentage parameter is removed from the model (Table 3, which is published as supporting information on the PNAS web site). We used empirical data collected over a period of 7 years to validate the model. We estimated mouse densities and NIP on six 2.25-ha trapping grids as follows: mice were live trapped at least monthly for 2 or 3 consecutive days between late May and early November (for detailed trapping methods see Jones et al., ref. 32) and density was measured during the fall peak in larval abundance; NIP was estimated by transect drag-sampling (33) in each grid during the spring nymphal peak, followed by DFA to determine infection status with B. burgdorferi.
Results and Discussion
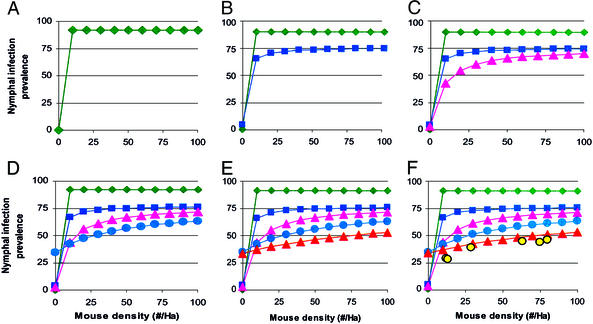
Our results demonstrate strong interspecific variation among host species in body burden and realized reservoir competence, with average body burdens ranging from 1.7 to 254 larvae per animal and reservoir competence ranging from 1.3% to 55% for nonmouse hosts (Table 1). This data set is, to our knowledge, the most complete accounting of the distribution of vector meals and infection probabilities across Ixodid tick hosts ever collected. The model results strongly support the predictions of the Dilution Effect hypothesis by demonstrating a dramatic decline in NIP as host diversity increases and community composition is concomitantly changed (Fig. 1). Hosts are added to the community in an order consistent with nested subsets of species observed in forest fragments in mid-western North America (12, 14). Different rules of community assembly may lead to somewhat different patterns of decline in NIP; however, our results are qualitatively robust because, for Lyme disease, all alternative hosts have lower competence than mice. If mice were the only species providing blood meals to larval ticks, the predicted NIP would be 92.5% (Fig. 1A). As the diversity of potential hosts is increased, NIP (and Lyme disease risk to humans) declines (Fig. 1 B–E). The highest diversity model performs well when compared with data collected at our site for the past 7 years (Fig. 1F).
Table 1.
Parameters used in model and the results of sensitivity analysis in which the density of each species was increased by 20%
| Species | Body burden
|
Molting percentage*
|
Reservoir competence*
|
Density, no. per hectare | Density source | Sensitivity of NIP to increase in host density†, percent change | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | N | Mean % (SE) | N | Mean % (SE) | N | ||||
| White-footed mouse | 27.8 (3.3) | 28 | 41.5 (3.5) | 47 | 92.1 (2.9) | 15 | 0–100 | Original data | NA |
| Eastern chipmunk | 36.0 (11) | 57 | 41.2 (6.0) | 43 | 55.0 (6.4) | 20 | 0–50 | Original data | 0.41% |
| White-tailed deer | 239 (99) | 12 | 56.3 (9.0) | 5 | 4.6 (2.3) | 19 | 0.25 | Original data | −0.22% |
| Raccoon | 127 (30) | 12 | 36.5 (7.3) | 13 | 1.3 (0.6) | 12 | 0.2 | Literature | −0.07% |
| Virginia opossum | 254 (115) | 21 | 44.1 (5.3) | 19 | 2.6 (1.1) | 18 | 1 | Literature | −0.78% |
| Striped skunk | 66.8 (12.7) | 4 | 63.9 (11) | 4 | 9.7 (8.4) | 4 | 0.05 | Literature | −0.01% |
| Short-tailed shrew | 62.9 (17.3) | 42 | 46.8 (4.5) | 41 | 41.8 (6.7) | 33 | 25 | Literature | 0.31% |
| Birds‡ | 1.7 (0.4) | 74 | 33.9 (NA) | 34 | 11.7 (NA) | 1,133 | 31.6 | Original and literature | −0.14% |
| Sorex shrews | 55.5 (32) | 8 | 49.6 (11) | 7 | 51.2 (15) | 6 | 25 | Literature | 1.4% |
| Red and grey squirrel | 142 (58) | 10 | 59.3 (11) | 9 | 14.7 (5.1) | 8 | 8.1 | Literature | −3.1% |
NA, not applicable.
Mean percentages per host, with the exception of birds. Body burdens of birds were frequently <5; therefore, gross percentages were substituted for means across individuals.
Sensitivity analysis performed at the long-term average mouse density of ≈20 mice per hectare.
Birds are American robin, ovenbird, veery, and wood thrush.
Figure 1.
Predicted NIP across a realistic range of mouse densities as host diversity is increased and host community composition is concomitantly changed. Host community consisting of only white-footed mice (green diamonds) (A); white-footed mice, chipmunks, and white-tailed deer (dark blue squares) (B); all hosts in B plus raccoons, opossums, and skunks (pink triangles) (C); all hosts in C plus short-tailed shrews and four species of ground nesting birds (light blue circles) (D); all hosts in D plus tree squirrels and Sorex shrews (red triangles) (E); and field data collected at our site over 7 years, showing the mouse density during the larval peak in year t and NIP for host-seeking nymphs in year t + 1 (yellow circles) (F).
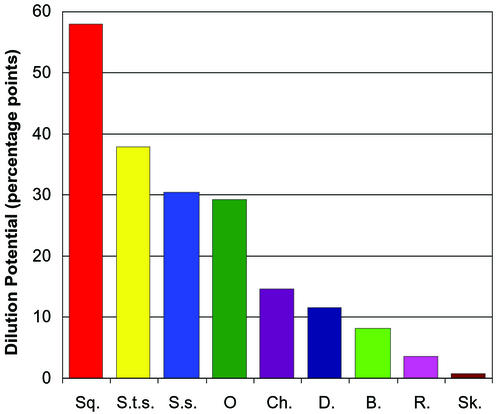
Although increasing host diversity in our system decreased NIP, not all species are equal in their dilution ability. The best dilution hosts provide meals for numerous ticks, occur at high densities and have low reservoir competence. We characterized the “dilution potential” (the difference in percentage points between the expected NIP in a two-host community consisting of mice plus the focal species and a community in which mice are the only possible host) of each host species in our system at the long-term (10-year) average mouse density at our site (≈20 mice per hectare; Fig. 2). Tree squirrels [gray squirrels (Sciurus carolinensis) and red squirrels (Tamiasciurus hudsonicus) combined] have the highest dilution potential, reducing infection prevalence by ≈58% points from what it would be if ticks were feeding on mice alone. Other hosts occur at too low densities [e.g., skunks (Mephitis mephitis)] and/or host too few ticks (e.g., birds) to function as effective dilution hosts. Because the densities of some hosts were not directly measured (see Tables 1 and 2), we performed a sensitivity analysis on this parameter. The results of the analysis, in which we increased the density of each species by 20% while holding mouse density constant at the long-term average, showed the model to be relatively insensitive to changes in host density, producing very minor changes in NIP ranging from a decrease of 3.1% to an increase of 1.4% (Table 1).
Figure 2.
The ability of each species to reduce the effect of white-footed mice (the most competent reservoir) on NIP. Dilution potential is the difference (in percentage points) between the expected NIP in a two-host community consisting of mice plus the focal species and a community in which mice are the only possible host. Sq., squirrel; S.t.s., short-tailed shrew; S.s., Sorex shrew; O, opossum; Ch., chipmunk; D, deer; B, birds; R, raccoon; Sk, skunk.
Some common tick hosts serve not only to dilute the effects of the most competent reservoirs, but also to maintain the spirochete in the community under conditions of low mouse density (the “rescue effect”; refs. 9 and 34). For example, in the most diverse community, NIP is predicted to be 34%, even at 0 mice per hectare (Fig. 1E). This is largely due to the effects of shrews, which have high reservoir competence, provide meals for many ticks, and can occur at high densities. In fact, if the densities used in our model are representative, shrews may function as rescue hosts in most years in our community. At the long-term average mouse density, 61% of the infected nymphs have fed on shrews, whereas mice infected only 20%. This situation changes as mouse density increases, with mice producing an increasing proportion of the infected nymphs.
Our model overestimates NIP slightly at low mouse densities. There are several possible explanations for this. First, we might have missed an important dilution host. Second, given that summer densities of mice and ground-nesting birds are inversely correlated because of strong predation by mice on nests (35, 36), birds may feed a larger proportion of the ticks at low mouse densities than is indicated in our model, resulting in lower NIP. Third, the deterministic nature of our model excludes the possibility that aggregated distributions of ticks on host individuals would reduce NIP faster than our model predicts. Finally, overestimation of NIP may occur because low mouse densities result in fewer infected nymphs to inoculate other hosts, reducing their realized reservoir competence (32, 37). This would be particularly likely if poor realized reservoir competence is a result of rapid loss of spirochetemia, something that has been demonstrated in several species in the laboratory (38, 39).
Another important metric of Lyme disease risk to humans is the density of infected nymphs (DIN), which is a complex function of abiotic factors that affect the survival of larvae and nymphs; biotic factors that dictate feeding success and availability of hosts for juveniles and adults; and the proportion of larvae feeding on host species of different reservoir competence. We believe that too little is known about the interactions among these factors to accurately predict DIN. However, we expect DIN to decline with increasing host diversity, similar to NIP. For example, the absolute, as well as the relative, density of white-footed mice is likely to decline with increasing diversity, as every species in our community is expected to either compete with or prey on mice (11). Indeed, mouse density has been negatively associated with mammalian diversity (14). In addition, larval body burden on mice declines with increasing density of chipmunks, thus decreasing the absolute number of tick meals provided by mice (40). The uncertainty about the indirect effects of diversity on DIN notwithstanding, assuming our order of species addition is realistic, then at any given vector density, a more diverse community always results in lower Lyme disease risk, regardless of the measure (NIP or DIN).
It should be noted that although at any given mouse density, a more diverse community results in lower NIP, the lowest NIP (and probably DIN) would be found in a mouse-free community. However, all available evidence indicates this to be an ecologically unrealistic, or at most transient, state (12–14, 41). The superior reservoir competence of mice may be related to the prominent position that they occupy in the community. Indeed, if tradeoffs prohibit efficient horizontal transmission between multiple hosts, we would expect pathogens to specialize on the numerically dominant host species (8). This result remains to be explored empirically and theoretically, but could yield insights into the functioning and characteristics of disease–host communities.
The Dilution Effect may provide incentive to maintain high diversity and stable community composition through wise land-use practices. For example, forest fragmentation decreases mammalian biodiversity and results in areas of very high mouse density (12–14, 41), which our model predicts would represent the highest Lyme disease risk. Both NIP and DIN have been shown to be inversely related to fragment size, with NIP of over 80% observed in extremely small forest fragments (<1 hectare) (42). The conditions necessary for the Dilution Effect (a generalist vector, horizontal transfer of the pathogen, variation in reservoir competence among hosts, and positive correlation between reservoir competence and the percentage tick meals supplied by hosts in the community) may occur in other disease systems (8). Thus, the reduction of biodiversity and anthropogenic changes in the composition of host communities may be increasing the risk of human exposure to other vector-borne diseases. The buffering of disease prevalence as a novel ecosystem function provided by high biodiversity deserves to be investigated on a larger scale.
Supplementary Material
Acknowledgments
We are grateful to the field assistants who helped to sample the host community, especially K. Oggenfuss, D. Whitaker, and V. Crossgrove. R. Winchcombe estimated deer density. E. Cougler and M. Cougler provided deer for analysis. Earlier drafts were improved by the comments of C. Canham and three anonymous reviewers. This research was funded by the Nathan Cummings Foundation, the National Science Foundation, the National Institutes of Health (National Institute of Allergy and Infectious Diseases), and the Institute of Ecosystem Studies. This is a contribution to the program of the Institute of Ecosystem Studies.
Abbreviations
- DFA
direct immunofluorescence antibody microscopy
- NIP
nymphal infection prevalence
- DIN
density of infected nymphs
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tilman D, Knops J, Wedin D, Reich P B, Ritchie M E, Siemann E. Science. 1997;277:1300–1302. [Google Scholar]
- 2.McGrady-Steed J, Harris P M, Morin P J. Nature. 1997;390:162–165. [Google Scholar]
- 3.Cottingham K L, Brown B L, Lennon J T. Ecol Lett. 2001;4:72–85. [Google Scholar]
- 4.Chapin F S I, Zavaleta E S, Eviner V T, Naylor R L, Vitousek P M, Reynolds H L, Hooper D U, Lavorel S, Sala O E, Hobbie S E, et al. Nature. 2000;405:242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 5.Levine J M. Science. 2000;288:852–854. doi: 10.1126/science.288.5467.852. [DOI] [PubMed] [Google Scholar]
- 6.Tilman D, Downing J. Nature. 1994;367:363–365. [Google Scholar]
- 7.Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime J P, Hector A, Hooper D U, Huston M A, Raffaelli D, Schmid B, et al. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 8.Ostfeld R S, Keesing F. Can J Zool. 2000;78:2061–2078. [Google Scholar]
- 9.Ostfeld R S, Keesing F. Conserv Biol. 2000;14:722–728. [Google Scholar]
- 10.Mather T N, Wilson M L, Moore S I, Ribeiro J M C, Spielman A. Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt K A, Ostfeld R S. Ecology. 2001;82:609–619. [Google Scholar]
- 12.Rosenblatt D L, Heske E J, Nelson S L, Barber D M, Miller M A, MacAllister B. Am Midl Nat. 1999;141:115–123. [Google Scholar]
- 13.Krohne D T, Hoch G A. Can J Zool. 1999;77:1247–1253. [Google Scholar]
- 14.Nupp T E, Swihart R K. J Mammal. 2000;81:512–526. [Google Scholar]
- 15.Barbour A G, Fish D. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 16.Patrican L A. J Med Entomol. 1997;34:52–55. doi: 10.1093/jmedent/34.1.52. [DOI] [PubMed] [Google Scholar]
- 17.Giardina A R, Schmidt K A, Schauber E M, Ostfeld R S. Can J Zool. 2000;78:2184–2197. [Google Scholar]
- 18.Dennis D T, Nekomoto T S, Victor J C, Paul W S, Piesman J. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 19.Piesman J, Clark K L, Dolan M C, Happ C M, Burkot T R. J Vector Ecol. 1999;24:91–98. [PubMed] [Google Scholar]
- 20.Qiu W G, Dykhuizen D E, Acosta M S, Luft B J. Genetics. 2002;160:833–849. doi: 10.1093/genetics/160.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Morbid Mortal Wkly Rep. 2002;51:29–31. [Google Scholar]
- 22.Lane R S, Burgdorfer W. Am J Trop Med Hyg. 1987;38:188–192. doi: 10.4269/ajtmh.1987.37.188. [DOI] [PubMed] [Google Scholar]
- 23.Ostfeld R S, Schauber E M, Canham C D, Keesing F, Jones C J, Wolff J O. Vector Borne Zoonotic Dis. 2001;1:55–63. doi: 10.1089/153036601750137688. [DOI] [PubMed] [Google Scholar]
- 24.Scoles G A, Papero M, Beati L, Fish D. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 25.Telford S R I, Mather T N, Moore S I, Wilson M L, Speilman A. Am J Trop Med Hyg. 1988;39:105–109. doi: 10.4269/ajtmh.1988.39.105. [DOI] [PubMed] [Google Scholar]
- 26.Ostfeld R S, Jones C G, Wolff J O. BioScience. 1996;46:323–330. [Google Scholar]
- 27.Bibby C J, Burgess N D, Hill D A. Bird Census Techniques. London: Academic; 1992. [Google Scholar]
- 28.Jȩdrzejewska B, Jȩdrzejewski W. Predation in Vertebrate Communities: The Białowieża Primeval Forest as a Case Study. New York: Springer; 1998. [Google Scholar]
- 29.Richter D, Spielman A, Komar N, Matuschka F R. Emerg Infect Dis. 2000;6:659–662. doi: 10.3201/eid0602.000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostfeld R S, Keesing F, Schauber E M, Schmidt K A. In: Conservation Medicine: Ecological Health in Practice. Aguirre A, Ostfeld R S, House C A, Tabor G, Pearl M, editors. New York: Oxford Univ. Press; 2002. [Google Scholar]
- 31.Goodwin B J, Ostfeld R S, Schauber E M. Vector Borne Zoonotic Dis. 2001;1:129–138. doi: 10.1089/153036601316977732. [DOI] [PubMed] [Google Scholar]
- 32.Jones C J, Ostfeld R S, Richard M P, Schauber E M, Wolff J O. Science. 1998;279:1023–1026. doi: 10.1126/science.279.5353.1023. [DOI] [PubMed] [Google Scholar]
- 33.Falco R C, Fish D. Exp Appl Acarol. 1992;14:165–177. doi: 10.1007/BF01219108. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert L, Norman R, Laurenson K, Reid H W, Hudson P J. J Anim Ecol. 2001;70:1053–1061. [Google Scholar]
- 35.Schmidt K A, Whelan C J. Conserv Biol. 1999;14:46–57. [Google Scholar]
- 36.Schmidt K A, Goheen J R, Naumann R, Ostfeld R S, Schauber E M, Berkowitz A. Ecology. 2001;82:2927–2936. [Google Scholar]
- 37.Schauber E M, Ostfeld R S. Ecol Appl. 2002;12:1142–1162. [Google Scholar]
- 38.Piesman J, Dolan M C, Schriefer M E, Burkot T R. Am J Trop Med Hyg. 1996;54:294–298. doi: 10.4269/ajtmh.1996.54.294. [DOI] [PubMed] [Google Scholar]
- 39.Levin M, Levine J F, Apperson C S, Norris D E, Howard P B. J Med Entomol. 1995;32:138–142. doi: 10.1093/jmedent/32.2.138. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt K A, Ostfeld R S, Schauber E M. J Med Entomol. 1999;36:749–757. doi: 10.1093/jmedent/36.6.749. [DOI] [PubMed] [Google Scholar]
- 41.Nupp T E, Swihart R K. Can J Zool. 1996;74:467–472. [Google Scholar]
- 42. Allan, B. F., Keesing, F. & Ostfeld, R. S. (2003) Conserv. Biol., in press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.