Abstract
Recent global-scale analyses indicate that climate variability affects net carbon storage but regard temperature and precipitation to be the main contributors. Seasonal and interannual variation in light availability may also limit CO2 uptake. As an experimental test of light limitation by cloud cover during tropical rainy seasons and by the unusually heavy cloud cover associated with La Niña, we installed high-intensity lamps above the forest canopy to augment light for Luehea seemannii, a tropical canopy tree species, during cloudy periods of 1999–2000. Light augmentation only partially compensated for the reduction in photosynthetic photon flux density caused by clouds. Nonetheless, leaves acclimated to the augmented irradiance, and photosynthesis, vegetative growth, and reproduction increased significantly. Light, rather than water, temperature, or leaf nitrogen, was the primary factor limiting CO2 uptake during the rainy season.
Keywords: El Niño‖canopy‖photosynthesis‖light limitation
We ask whether year-to-year variation in cloud cover and light intensity limits photosynthesis and carbon uptake by a widespread tropical tree. This question is important for several reasons. Heavy tropical cloud cover can reduce irradiance and limit photosynthesis by fully exposed sun leaves (1–3). Recent eddy covariance studies indicate that day-to-day variation in cloud cover and irradiance also affects net carbon uptake by forests (4–6). Tropical cloud cover varies seasonally (7, 8) and interannually with fluctuations in the El Niño Southern Oscillation (9). Tropical cloud cover also underwent strong longitudinal and latitudinal redistributions during the 1990s (10), and global solar irradiance reaching the Earth's surface has declined by 2.7% per decade since the 1950s, probably as a consequence of increases in anthropogenic particulates and cloud cover (11). Finally, tropical forests account for 32–36% of global terrestrial net primary production (12, 13). The implications for global carbon uptake are significant if carbon uptake by tropical trees is indeed limited by year-to-year variation in cloud cover and irradiance.
To determine whether natural year-to-year variation in cloud cover limited carbon gain, we supplemented light levels artificially whenever cloud cover reduced photosynthetic photon flux density (PPFD; 400–700 nm) by 700 μmol⋅m−2⋅s−1 for two replicate adult canopy trees in a tropical forest located in the Republic of Panama. Supplemental illumination compensated only partially for natural reductions in PPFD. This experimental manipulation was maintained for 2 years, including 1999, the particularly cloudy La Niña year. We studied the response to augmented light of leaf-level photosynthesis, branch-level sap flow, leaf- and branch-level carbohydrate storage, branch extension growth, and fruit production.
Materials and Methods
Site, Species, and Treatments.
The study was conducted at the Parque Natural Metropolitano (PNM; lat. 9°04′N, long. 79°23′W), a 256-hectare natural reserve located near Panama City, Republic of Panama. A 51-m-tall construction crane provided access to the forest canopy. Air temperature and PPFD have been recorded continuously since 1996 from a micrometeorological station located above the canopy on the crane tower. Annual rainfall averages 1,740 mm, >90% of which occurs during the wet season between May and December. PPFD averages 33% greater during the dry season between January and April because cloud cover is reduced. El Niño events are associated with below-normal precipitation (14), and La Niña events are associated with an extra 1.5–2 mm⋅d−1 of precipitation from November to March (data from the Climate Diagnostics Center of the National Oceanic and Atmospheric Administration and the Cooperative Institute for Research in Environmental Sciences). Air temperatures averaged 25.6 ± 0.03°C (mean ± SE) with an instantaneous maximum of 33.2°C and minimum of 20.9°C during this study.
Luehea seemannii Tr. & Planch. (Tiliaceae), a late-successional canopy tree species (15), is abundant at the site. Two control and two experimental trees were randomly selected from within the 0.85 hectares of forest canopy accessible from the crane. A 30-m-tall lamp tower was installed above each experimental tree in December 1998. Each tower held two horizontal banks of four 1,500-W metal halide lamps. Lamps were separated by ≈1 m and were positioned ≈2 m above the canopy to augment PPFD by 700 μmol⋅m−2⋅s−1 for the uppermost canopy leaves closest to the lamps. The lamps were ignited whenever 10-min average solar PPFD, measured with a quantum sensor (LI-190SA, Li-Cor, Lincoln, NE) located above the canopy on one of the lamp towers, fell >700 μmol⋅m−2⋅s−1 below ideal, cloud-free PPFD. The lamps were extinguished when the 10-min average solar PPFD was above this threshold. Ideal, cloud-free daily courses of PPFD were calculated from solar angles by using date, time of day, and the maximum observed PPFD per solar angle in 5 years of historical data collected on the crane tower. Ambient light thus was augmented during cloudy periods from June 1999 through March 2000 and then again from July through December 2000. Trees were leafless from mid-April through June 2000. PPFD data from both the micrometeorological station and from the lamp tower were collected continuously during the 2-year study.
The spectral quality of lamp light was measured 1 m from the lamps and 1 h after ignition with a spectroradiometer (LI-1800, Li-Cor). The spectrum was similar to those of other metal halogen lamps used indoors or in glasshouses for plant growth studies. Thus, no unusual photomorphogenic effects were expected or observed for branches under the lamps.
The penetration of supplemental light into the canopy was measured with a 1-m line-integrating quantum sensor (LI-191SA, Li-Cor) inserted horizontally into the canopy on a cloudy day. The instantaneous decrease in PPFD when the lamps were turned off under continuously cloudy conditions was recorded for three horizontal locations beneath the lamps per canopy depth.
Horizontal coverage of supplemental light was measured by linear arrays of gallium arsenide phosphide photodiodes (Hamamatsu, Bridgewater, NJ) (16) arranged at 0.5-m intervals throughout the canopies of illuminated trees. The photodiodes were referenced to the quantum sensor located above the canopy on one of the lamp towers. All sensors were connected to a data logger (21X Micrologger, Campbell Scientific Instruments, Logan, UT), and 10-s data were averaged every 10 min.
Leaf temperatures were measured by using 0.127-mm-diameter copper-constantan thermocouples placed under the leaf near the midvein and secured with tape or plastic putty. Leaf temperatures were recorded as 5-min averages during multiple 24-h cycles during both 1999 and 2000 and were collected by a data logger protected from direct sunlight (LI-1400, Li-Cor). Leaf temperatures were rarely >3°C above ambient air temperatures, primarily because leaf angles changed to reduce leaf surface area exposed to solar and lamp radiation. Illuminated leaf temperatures normally did not exceed exposed canopy leaf temperatures occurring on sunny days (≈35°C). Photosynthetic temperature response curves (methods below) indicated that photosynthesis under saturating light was constant for leaf temperatures from 26 to 34°C, decreased by 28 ± 12.2% (n = 4) for leaf temperatures from 34 to 38°C, and decreased by 41.7 ± 4.7% (n = 4) for leaf temperatures from 38 to 40°C. In a few cases, after branch growth raised foliage height, proximity to the lamps increased leaf temperatures; however, these branches were excluded from all measurements.
A long-term record of solar irradiance was collected on Barro Colorado Island, ≈29 km northwest of PNM, and it was used to examine interannual variation in light availability. Solar radiation was recorded hourly from late 1989 through July 2002 by using a pyranometer (LI-200X, Li-Cor) mounted above the Barro Colorado Island canopy on a 45-m tower. Historical hourly PPFD data (for two quantum sensors, LI-190SA, Li-Cor) and air temperatures were collected from a meteorological station positioned above the canopy on the crane tower beginning in 1996 at PNM. The hourly diffuse fraction of ambient light was estimated by using the observed PPFD and the historical values of ideal, cloud-free PPFD excluding early morning and late evening values (17).
Gas Exchange, Laboratory Analyses, and Growth Measurements.
Gas exchange and respiration measurements were taken approximately monthly from mature, fully expanded leaves in the canopy by using an LI-6400 Portable Photosynthesis System (Li-Cor) to compare the effect of ambient and supplemental light. All leaves were carefully selected to be from the same age-cohort to avoid differences in photosynthetic capacity associated with leaf age (18). Temperature was maintained within ≈3°C of ambient (except when measuring photosynthetic temperature responses), relative humidity was maintained at ≈10% less than ambient but always <80%, and CO2 entering the chamber was maintained at 380 μmol⋅mol−1. A red and blue diode light source was used during light–response measurements. Leaf fluorescence was measured on Sept. 4, 1999, on dark-adapted leaves in the morning and then on the same leaves at the end of the day by using an OS5-FL Modulated Fluorometer (Opti-Sciences, Tyngsboro, MA). Sap flow was measured from Oct. 19 to Dec. 18, 1999, and then again from March 30 to Apr. 7, 2000, in branches ≈5 cm in diameter with 2-cm-long home-built probes by using the constant heating method (19).
Total nonstructural carbohydrates (TNCs; total of sugars and starch) were sampled from mature leaves and from 5-cm branch samples collected 10 cm from the tips of branches at dawn on 1 day each month in 1999 and in February 2000. Leaves were sampled from the same branch at dusk on the previous day to estimate the daily fluctuation of carbohydrate in leaves. Three roots, each ≈1 cm in diameter, 10 cm in length, and from a depth of ≈20 cm, also were collected from each tree for TNC analysis. Dried and ground samples were analyzed by using a modified phenolic–sulfuric method after extracting sugars with ethanol and digesting starch with amyloglucosidase (20). Nitrogen content of leaves was measured by using an NCS 2500 Elemental Analyzer (CE Instruments, Milan).
Branch extension growth (measured during the growing season from May to January) and counts of reproductive buds were recorded for 103 illuminated branches (118 for mature fruits), 66 nonilluminated branches on illuminated trees (90 for fruits), and 91 branches on control trees (90 for fruits). Fruit masses were determined for 1,274 individual fruits from illuminated branches, 509 from nonilluminated branches on illuminated trees, and 684 from control trees. TNC data were analyzed by using ANOVA, sap flow data by paired t tests, and other data by unpaired t tests.
Results
Variation in Light Intensity.
Barro Colorado Island tends to have more cloud cover than the PNM site, but a longer record of irradiance is available for comparison of interannual events. Relatively cloud-free, sunny conditions and positive irradiance anomalies were sustained throughout the 1991–1992 and 1997–1998 El Niño events, and relatively cloudy, overcast conditions and negative irradiance anomalies were sustained throughout the 1998–1999 La Niña event. This association between cloud cover, irradiance anomalies, and the El Niño Southern Oscillation is likely to be widespread throughout the tropics (9).
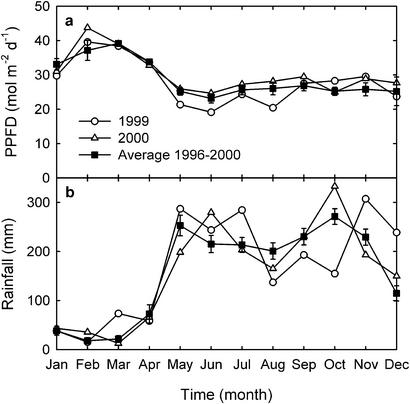
At the PNM site, cumulative PPFD measured from May through September was 17% lower for 1999 than for 2000 and 11% lower for 1999 than for the site-specific, 5-year average (Fig. 1a). Reduced PPFD was associated with cloud cover during the rainy season between May and December (Fig. 1b). The estimated diffuse fraction of hourly PPFD during the 1999 rainy season was 0.71 ± 0.01 (mean ± 1 SE) compared with 0.61 ± 0.01 for 2000 (t = 12.5, P < 0.001). The estimated diffuse fraction of PPFD during the 1999 dry season was 0.54 ± 0.02, compared with 0.51 ± 0.01 for 2000 (t = 2.91, P < 0.005).
Figure 1.
Monthly averages for PNM of total daily PPFD (400–700 nm) for 1999 and 2000 (open symbols) and the 5-year average (1996–2000, filled symbols) (a), and total monthly rainfall for 1999 and 2000 (open symbols) and the 20-year average (filled symbols) (b). Filled symbols represent means ± 1 SE.
Lamps were ignited for an average of 7.04 ± 0.16 h⋅d−1 vs. 3.36 ± 1.93 h⋅d−1 during the 1999 and 2000 rainy seasons, respectively. Approximately one-third of the branches on illuminated trees received >100 μmol⋅m−2⋅s−1 PPFD from the lamps. The total PPFD received by such illuminated branches from the lamps from July through December averaged 4.3 ± 0.1 mol⋅m−2⋅d−1 vs. 2.0 ± 0.1 mol⋅m−2⋅d−1 during 1999 and 2000, respectively.
Light from the lamps decreased rapidly with depth into the canopy. Supplemental PPFD decreased by 77.9 ± 1.8% at 0.5 m of depth, 94.1 ± 1.2% at 1.0 m of depth, 97.1 ± 1.2% at 1.5 m of depth, and 99.0 ± 0.4% at 2 m of depth.
Responses to Experimental Illumination.
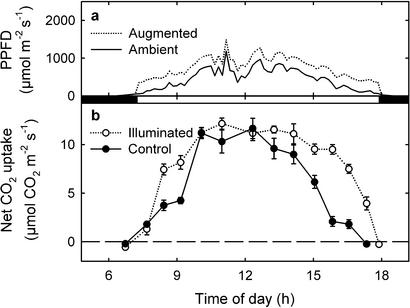
Instantaneous PPFD was often <50% of the historically observed maximum for a particular solar angle throughout the day during the rainy season (Fig. 2a). On such days, experimental lamps remained ignited continuously from 7:30 a.m. until 5:30 p.m. When lamps were ignited, canopy leaf photosynthesis for illuminated branches was higher than for nonilluminated branches on experimental trees in the late mornings and late afternoons, but it was similar during midday, when ambient PPFD was higher (Fig. 2b) and leaves were closer to light saturation (Fig. 4). During a representative cloudy day, the increase in photosynthesis due to the lamps averaged 4.1 ± 1.0 μmol⋅m−2⋅s−1 for randomly chosen leaves exposed to average solar PPFD of 219.3 ± 18.2 μmol⋅m−2⋅s−1 and an additional PPFD of 173.9 ± 57.0 μmol⋅m−2⋅s−1 provided by the lamps (n = 16 leaves). Fluorometry measurements taken for leaves directly under the lamps indicated no additional photoinhibition due to the lamps.
Figure 2.
Instantaneous ambient PPFD (solid line) and average instantaneous augmented PPFD for illuminated leaves (dotted line) for a representative rainy season day (Sept. 2, 1999) (a), and instantaneous net CO2 uptake for illuminated leaves (open symbols) and nonilluminated leaves on experimental plus control trees (filled symbols) for the same day (b). Data are means ± 1 SE (n = 10 leaves per treatment). Dark bars between a and b indicate when lamps were extinguished.
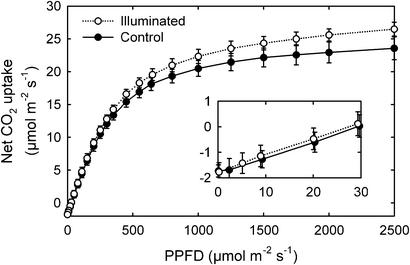
Figure 4.
The relationship between the rate of net CO2 uptake and PPFD for illuminated leaves and nonilluminated leaves on experimental plus control trees. Symbols represent means ± 1 SE (n = 10) for 1999; data for nonilluminated leaves on experimental trees and for leaves on control trees were not statistically different and so are shown combined.
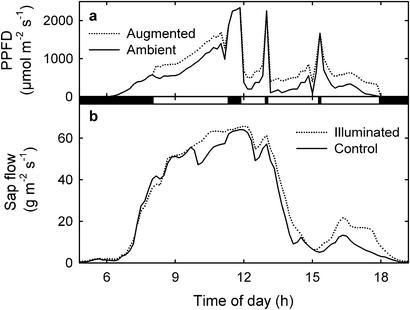
Before lamps were ignited, instantaneous sap flow was similar for illuminated and control branches of equal diameter on the same experimental tree (Fig. 3). After lamps were ignited, sap flow was greater in illuminated branches and was less sensitive to fluctuations in solar PPFD. Total daily sap flow averaged 28.3% higher for illuminated branches than for nonilluminated branches when the lamps were ignited (t = 2.26, P < 0.05, n = six branches).
Figure 3.
Instantaneous ambient PPFD (solid line) and average instantaneous augmented PPFD for illuminated leaves (dotted line) for a second representative rainy season day (Oct. 23, 1999) (a), and instantaneous sap flow for an illuminated branch (dotted line) and a nonilluminated branch (solid line) in the same tree for the same day (b). Dark bars between a and b indicate when lamps were extinguished.
Photosynthesis increased with increasing PPFD up to 2,500 μmol⋅m−2⋅s−1 for fully exposed canopy leaves (Fig. 4). The net CO2 uptake rate for PPFD >450 μmol⋅m−2⋅s−1 was greater for leaves that developed under supplemental illumination than for leaves on nonilluminated branches on either experimental or control trees (t = 2.77, P = 0.013, n = 10 leaves). Data for 2000 showed a similar pattern of increased photosynthetic ability of leaves that developed under supplemental illumination, with the maximum rate of photosynthesis averaging 9.7% higher for illuminated than for nonilluminated leaves (t = 5.46, P < 0.001, n = 54 leaves). The maximum photosynthetic rates of shade leaves located >0.5 m deep in the canopy were not affected by the lamps. Neither leaf respiration rates (Fig. 4, Inset), nitrogen per dry mass (combined average of 21.7 ± 0.8 mg of nitrogen⋅g−1), nor leaf specific mass (combined average of 115.8 ± 1.8 g⋅m−2, n = 20 leaves per treatment) differed between treatments.
TNC concentrations were greater for illuminated than for control leaves at dawn and were indistinguishable for leaves at dusk and for terminal branch wood (Table 1). TNC concentrations were lower for roots of illuminated trees than control trees (Table 1).
Table 1.
Growth, reproduction, and TNCs for year 2000
| Treatment | Branch growth
|
Reproduction
|
TNCs, mg/g dry mass
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Branch length, cm | Nodes per branch | Buds per branch | Fruits per branch | Fruit mass, g | Root | Branch wood | Leaf, dawn | Leaf, late P.M. | |
| Illuminated | 185.7 ± 13.6 | 85.5 ± 5.7 | 219.2 ± 16.2 | 128.9 ± 14.7 | 0.285 ± 0.004 | 34.2 ± 6.2 | 58.1 ± 16.2 | 60.6 ± 8.4 | 77.7 ± 10.4 |
| Nonilluminated, experimental | 141.6 ± 8.0 | 63.0 ± 3.2 | 154.2 ± 16.7 | 153.5 ± 17.7 | 0.251 ± 0.004 | — | — | — | — |
| Nonilluminated, control | 111.2 ± 5.5 | 45.9 ± 2.1 | 108.8 ± 10.2 | 60.8 ± 7.5 | 0.255 ± 0.006 | 70.0 ± 21.6 | 54.1 ± 11.6 | 50.0 ± 9.2 | 82.2 ± 7.6 |
Branch growth is the length of current year's shoot extension growth measured between June 2000 and mid-January 2001. The number of fruits per branch was quantified after fruits were mature in mid-March 2001. TNCs were determined for samples collected in late August. Values are means ± 1 SD.
Branch extension growth averaged 31% greater for illuminated branches than for nonilluminated branches on experimental trees and 67% greater for illuminated branches than for branches on control trees in 2000 (Table 1). The number of nodes and associated leaves produced per branch was greatest under artificial illumination (Table 1). Internode lengths were longest for branches on control trees (2.42 ± 0.05 cm), shorter for branches on illuminated trees, and indistinguishable for illuminated vs. nonilluminated branches on illuminated trees (2.20 ± 0.04 cm, combined). The number of reproductive buds averaged 42%, 59%, and 102% greater for illuminated branches than for nonilluminated branches on experimental trees in both years, branches on control trees in 1999, and branches on control trees in 2000, respectively (Table 1, data for 1999 not shown). The number of mature fruits averaged 125% greater for illuminated branches than for nonilluminated branches on the same trees in 1999, but these values were similar in 2000, possibly because of damage received by the branches during the removal of the lamps from the site. The number of mature fruits averaged 190% and 112% greater for illuminated branches than for branches on control trees for 1999 and 2000, respectively (Table 1, 1999 data not shown).
Discussion
Light availability limited carbon gain of canopy leaves and sap flow of canopy branches of a widespread tropical tree species during the rainy season and especially during a particularly cloudy La Niña event. Supplemental illumination was applied only when cloud cover reduced incoming solar PPFD by at least 700 μmol⋅m−2⋅s−1 and only increased PPFD by an average of 173.9 μmol⋅m−2⋅s−1 (and never by >700 μmol⋅m−2⋅s−1). Nonetheless, supplemental illumination increased daily net carbon gain by an average of 18.4% (from 270.6 to 320.4 mmol⋅m−2⋅d−1) for exposed canopy leaves between May and December during the La Niña event. This increase illustrates the large effect that interannual variation in cloud cover can have on net CO2 uptake.
In addition, fully sun-exposed canopy leaves acclimated to the augmented illumination through an increase in photosynthetic potential (Fig. 4). Leaves of Luehea seemannii produced in the relatively sunny dry season also have higher photosynthetic potentials than do leaves produced on the same tree in the relatively cloudy rainy season (21). The observed acclimation of photosynthetic potentials further suggests that this widespread tropical tree species responds to seasonal and interannual variation of solar irradiance with concomitant changes in carbon uptake. Photosynthetic rates increased with increasing PPFD up to 2,500 μmol⋅m−2⋅s−1 for both illuminated and control leaves; thus, canopy leaves are rarely light-saturated, similar to leaves of other tropical tree species (21), and potentially could benefit from a general increase in PPFD.
Neither starch inhibition nor water supplies was likely to have limited productivity during the rainy season. TNCs measured in terminal branch wood, which is likely to be fed by the most physiologically active leaves, did not differ between illuminated and control tree branches (Table 1). Starch inhibition is also unlikely because illuminated leaves did not accumulate more starch at the end of the day than control leaves (Table 1). Sap flow in illuminated and nonilluminated branches on illuminated trees indicated that water was not limiting during the rainy season (Fig. 3). Light augmentation increased transpiration without adversely affecting the water relations of the tree. Midmorning maximum stomatal conductance was not lower during or after days of illumination by the lamps, and midday stomatal depression occurred whether lamps were ignited or not (Fig. 3b) (22). Thus, the increased net CO2 uptake in illuminated branches should have become available to support vegetative growth and reproduction.
Branch extension growth and reproductive responses are consistent with this prediction, and similar branch extension into tree-fall gaps has been observed (23), although replication in just two trees demands a cautious interpretation. Branch extension growth, the number of new nodes, and the number of reproductive buds were all greatest on illuminated branches, intermediate for nonilluminated branches on illuminated trees, and least for control trees. The observed increases on nonilluminated branches on illuminated trees relative to control trees suggest that the extra carbon gained through supplemental illumination increased growth and reproduction throughout the entire crown of illuminated trees. However, nonilluminated branches were arbitrarily defined as branches that received <100 μmol⋅m−2⋅s−1 PPFD from the lamps. Growth and reproduction may have responded to even smaller increases in light availability. In any event, there is no evidence that growth and reproductive responses reflect reallocation of resources within crowns because entire crowns responded. Tissue carbohydrate concentration, however, was lower for roots of illuminated trees than control trees. This may reflect an overall increase in the growth of the tree; root carbohydrate reserves may have been used to support an increase in root growth to balance the documented increase in shoot growth (24).
Clouds reduce direct beam and total irradiance but increase diffuse irradiance. Diffuse irradiance penetrates canopies more efficiently than direct beam irradiance, raising the possibility that clouds may increase whole canopy carbon uptake even when total irradiance is reduced (25, 26). We added direct beam radiation, and its rapid attenuation with depth into the canopy (just 1% of supplemental illumination penetrated 2 m) was expected relative to natural light, which includes a substantial diffuse component. In any event, the tradeoff between reduced total irradiance, increased diffuse irradiance, and a higher radiation-use efficiency determines an optimal cloud cover and diffuse fraction for canopy-level carbon uptake. This optimum diffuse fraction can be ≈50% for forest trees (6). The diffuse fraction of PPFD always averaged >50% at the PNM site and reached a sustained maximum mean of 71% during the rainy season of the 1999 La Niña event. Against this high background diffuse fraction, any increase in cloud cover and reduction in total irradiance would be expected to reduce canopy-level carbon uptake. Similarly high diffuse fractions must be characteristic of cloudy wet seasons throughout the tropics.
Interannual climate variability thus may influence net CO2 uptake and growth through variation in solar irradiance caused by clouds and atmospheric particulates (27, 28) coupled with the photosynthetic flexibility of tropical canopy species. Recent global-scale analyses have demonstrated that year-to-year climate variation influences global carbon storage, but they have emphasized variation in temperature and precipitation and ignored cloud cover (29–31). In our experiment, supplemental illumination compensated for an average of 25% of the reduction in PPFD induced by heavy cloud cover. This falls well within the normal variation in solar irradiance that occurs between years with El Niño Southern Oscillation variation in central Panama (Fig. 1). Clearly, more long-term data on forest carbon fluxes and continuous radiation data at tropical sites are needed to assess the impacts of seasonal and interannual events on forest productivity. Nevertheless, terrestrial ecosystem models that use historical data to estimate net primary production may miscalculate the global carbon budget, especially during El Niño and La Niña events, because of the acclimation of photosynthesis by canopy tree species to interannual changes in solar irradiance demonstrated here.
Acknowledgments
We thank Stephen Paton for meteorological data; Juan Posada, Mirna Samaniego, and Katia Silvera for field assistance; and Chad Reddick and Lou Santiago for laboratory assistance. This work was supported by National Science Foundation Grant IBN-9805908, the Andrew W. Mellon Foundation, and an anonymous foundation.
Abbreviations
- PPFD
photosynthetic photon flux density
- PNM
Parque Natural Metropolitano
- TNC
total nonstructural carbohydrate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mulkey S S, Kitajima K, Wright S J. Trends Ecol Evol. 1996;11:408–412. doi: 10.1016/0169-5347(96)10043-4. [DOI] [PubMed] [Google Scholar]
- 2.Pearcy R W. Funct Ecol. 1987;1:169–178. [Google Scholar]
- 3.Zotz G, Winter K. Ecol Stud. 1996;114:312–323. [Google Scholar]
- 4.Kellomäki S, Wang K-Y. Ecol Modell. 2000;128:63–88. [Google Scholar]
- 5.Chen J M, Liu J, Cihlar J, Goulden M L. Ecol Modell. 1999;124:99–119. [Google Scholar]
- 6.Hollinger D Y, Kelliher F M, Byers J N, Hunt J E, McSeveny T M, Weir P L. Ecology. 1994;75:134–150. [Google Scholar]
- 7.Wright S J, Van Schaik C P. Am Nat. 1994;143:192–199. [Google Scholar]
- 8.Wright S J. In: Tropical Forest Plant Ecophysiology. Mulkey S S, Chazdon R L, Smith A P, editors. New York: Chapman & Hall; 1996. pp. 440–460. [Google Scholar]
- 9.Klein S A, Soden B J, Lau N. J Clim. 1999;12:917–932. [Google Scholar]
- 10.Chen J, Carlson B I, Del Genio A D. Science. 2002;295:838–841. doi: 10.1126/science.1065835. [DOI] [PubMed] [Google Scholar]
- 11.Stanhill G, Cohen S. Agric For Meteorol. 2001;107:255–278. [Google Scholar]
- 12.Melillo J M, McGuire A D, Kicklighter D W, Moore B, Vörösmarty C J, Schloss A L. Nature. 1993;363:234–240. [Google Scholar]
- 13.Field C B, Behrenfeld M J, Randerson J T, Falkowski P. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 14.Estoque M A, Luque J, Chandeck-Monteza M, Garcia J. Effects of El Niño on Panama Rainfall. Panama City, Republic of Panama: Instituto de Recursos Hidraulicos y Electrificacion; 1985. [Google Scholar]
- 15.Croat T B. Flora of Barro Colorado Island. Palo Alto, CA: Stanford Univ. Press; 1978. [Google Scholar]
- 16.Pontailler J Y. Funct Ecol. 1990;4:591–596. [Google Scholar]
- 17.Roderick M L. Agric For Meteorol. 1999;95:169–185. [Google Scholar]
- 18.Kitajima K, Mulkey S S, Wright S J. Am J Bot. 1997;84:702–708. [PubMed] [Google Scholar]
- 19.Granier A. Ann Sci For. 1985;42:193–200. [Google Scholar]
- 20.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Anal Chem. 1956;28:350–356. [Google Scholar]
- 21.Kitajima K, Mulkey S S, Wright S J. Oecologia. 1997;109:490–498. doi: 10.1007/s004420050109. [DOI] [PubMed] [Google Scholar]
- 22.Meinzer F C, Andrade J L, Jackson P. Plant Cell Environ. 1997;20:1242–1252. [Google Scholar]
- 23.Young T P, Hubbell S P. Ecology. 1991;72:1464–1471. [Google Scholar]
- 24.Sprugel D G, Hinckley T M, Schaap W. Annu Rev Ecol Syst. 1991;22:309–334. [Google Scholar]
- 25.Roderick M L, Farquhar G D, Berry S L, Noble I R. Oecologia. 2001;129:21–30. doi: 10.1007/s004420100760. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury B J. Remote Sens Environ. 2001;75:1–21. [Google Scholar]
- 27.Thompson A M, Witte J C, Hudson R D, Guo H, Herman J R, Fujiwara M. Science. 2001;291:2128–2132. doi: 10.1126/science.291.5511.2128. [DOI] [PubMed] [Google Scholar]
- 28.Siegert F, Ruecker G, Hinrichs A, Hoffmann A A. Nature. 2001;414:437–440. doi: 10.1038/35106547. [DOI] [PubMed] [Google Scholar]
- 29.Tian H, Melillo J M, Kicklighter D W, McGuire A D, Helfrich J V K, III, Moore B, III, Vörösmarty C J. Nature. 1998;396:664–667. [Google Scholar]
- 30.Kindermann J, Wurth G, Kohlmaier G H, Badeck F W. Global Biogeochem Cycles. 1996;10:737–755. [Google Scholar]
- 31.Braswell B H, Schimel D S, Linder E, Moore B., III Science. 1997;278:870–872. [Google Scholar]