Abstract
Transplanted cord blood (CB) hematopoietic stem cells (HSC) and progenitor cells (HPC) can treat malignant and nonmalignant disorders. Because long-term cryopreservation is critical for CB banking and transplantation, we assessed the efficiency of recovery of viable HSC/HPC from individual CBs stored frozen for 15 yr. Average recoveries (± 1 SD) of defrosted nucleated cells, colony-forming unit-granulocyte, -macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E), and colony-forming unit-granulocyte, -erythrocyte, -monocyte, and -megakaryocyte (CFU-GEMM) were, respectively, 83 ± 12, 95 ± 16, 84 ± 25, and 85 ± 25 using the same culture conditions as for prefreeze samples. Proliferative capacities of CFU-GM, BFU-E, and CFU-GEMM were intact as colonies generated respectively contained up to 22,500, 182,500, and 292,500 cells. Self-renewal of CFU-GEMM was also retained as replating efficiency of single CFU-GEMM colonies into 2° dishes was >96% and yielded 2° colonies of CFU-GM, BFU-E, and CFU-GEMM. Moreover, CD34+CD38− cells isolated by FACS after thawing yielded >250-fold ex vivo expansion of HPC. To assess HSC capability, defrosts from single collections were bead-separated into CD34+ cells and infused into sublethally irradiated nonobese diabetic (NOD)/severe combined immunodeficient (SCID) mice. CD45+ human cell engraftment with multilineage phenotypes was detected in mice after 11–13 wk; engrafting levels were comparable to that reported with fresh CB. Thus, immature human CB cells with high proliferative, replating, ex vivo expansion and mouse NOD/SCID engrafting ability can be stored frozen for >15 yr, can be efficiently retrieved, and most likely remain effective for clinical transplantation.
Cord blood (CB) is a viable alternative to bone marrow for related and unrelated allogeneic hematopoietic stem cell (HSC)/progenitor cell (HPC) transplantation (1–13). Since our initial preclinical (14–16) and clinical (1, 17–19) studies, there have been >2,000 CB transplants performed to treat a variety of malignant and nonmalignant disorders in children and adults (1–13, 17–19). CB HSC/HPC are frozen before use for transplantation (14, 20–22), but the longest that a CB collection has been stored frozen before use for clinical transplantation is in the 3- to 5-yr range. With >100,000 CBs stored frozen world-wide for prolonged periods in anticipation of their clinical use, information on longer-term storage of CB HSC and HPC is of critical importance.
The capacity to freeze and retrieve CB HPC cells was first reported when we suggested that CB could serve as a source of transplantable and engrafting HSC and HPC (14). We subsequently evaluated effects of 5-yr (16) and 10-yr (23) storage on retrieval of HPC in which postfreeze HPC numbers were compared directly to prefreeze numbers from the exact same CB samples. Thus, a true recovery rate could be calculated. At those times, we assessed only numbers and proliferation of HPC in vitro. In the present report, we extended analysis of HPC to 15-yr cryopreservation, and evaluated HSC content and engrafting capability of defrosted cells as well as additional important functional characteristics of HPC. This information included the replating capacity of frozen CB multipotential progenitor cells [(colony-forming unit-granulocyte, -erythrocyte, -monocyte, and -megakaryocyte (CFU-GEMM)], which estimates the “self-renewal” capacity of these cells (24, 25), the ex vivo expansion capability of frozen CB HPC/HSC, studies previously done on cells stored frozen for very short periods of time (26–28), and the engrafting capability in vivo of stored CB HSC in nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice. NOD/SCID mice are currently considered the best assay model for evaluating human HSC (29–32) and may provide a more direct assessment of the anticipated behavior of thawed CB cells in vivo. Our results demonstrate that CB HSC and HPC can be cryopreserved for at least 15 yr, and efficiently retrieved in functionally competent form.
Materials and Methods
CB Cells: Collection, Cryopreservation, and Separation.
CB cells used were scheduled for discard after delivery of the infant and prior needs, if any, for samples for clinical study had been satisfied. The Institutional Review Board of the Indiana University School of Medicine approved this study. Cells were frozen and subsequently retrieved after thawing as reported by us (14, 16, 23). In short, CB obtained within 36 h of collection were separated by Ficoll-Hypaque density cut procedure (density 1.070 g/cm3; Pharmacia) into a low-density (<1.070 g/cm3) mononuclear cell fraction. The cells were washed, and a small aliquot was used for in vitro colony assay. The remaining cells were cryopreserved by putting them into standard 3.6-ml cryotubes (Nalge Nunc) at 2 × 106 cells per ml in 2 ml with final concentrations of 10% dimethyl sulfoxide and 10% autologous plasma while being maintained at 4°C. These tubes were then placed into beakers with methanol, which were placed into −70°C freezers. After 24 h, the frozen samples were placed into liquid nitrogen, where they remained until being removed for assay. After thawing, cells were either used without further separation or were further subdivided by fluorescence-activated cell sorting (FACS; Becton Dickinson) into CD34+, CD34+CD38−, and CD34+CD38+ populations (each ≥98% CD34+) or by magnetic beads into CD34+ cells (≥95% CD34+) as described (31–33).
In Vitro Colony Assays for HPC.
Prefreeze and defrosted cells were plated at two to three different concentrations each to yield high enough colony numbers to get accurate colony counts with a minimal of colony overlap. Cells were plated in 1% methylcellulose culture medium with 30% FBS (HyClone) and with the following mixture of growth factors [1 unit/ml recombinant human (rhu) erythropoietin (Epo; Amgen Biologicals), 10 ng/ml rhu IL-3 (Immunex), 10 ng/ml rhu granulocyte–macrophage colony-stimulating factor (GM-CSF, Immunex), and 50 ng/ml rhu steel factor (SLF, also called stem cell factor; R & D Systems), or 1 unit/ml rhu Epo plus 10 ng/ml rhu IL-3] for analysis of granulocyte-macrophage [colony-forming unit-granulocyte, -macrophage (CFU-GM)], erythroid [burst-forming unit-erythroid(BFU-E)], and CFU-GEMM progenitors, or in 0.3% agar culture medium with 10% FBS, 10 ng/ml rhu GM-CSF alone, or with 50 ng/ml rhu SLF with or without 100 ng/ml rhu Flt3-ligand (FL, Immunex) for analysis of CFU-GM. Colonies were scored after 14 days incubation in a humidified chamber at 5% CO2 and lowered (5%) O2. Details regarding the assays, what they measure, and the exact ingredients used have been published (34).
Secondary Replating of CFU-GEMM.
Replating of CFU-GEMM-colonies from a 1° to a 2° dish are considered a means to estimate the “self-renewal” capacity of CFU-GEMM and were performed as described (24) but without the use of CB plasma, which is known to enhance detection of replatable CFU-GEMM (25). Individual CFU-GEMM colonies that grew in methyl cellulose plates after 14 days incubation with Epo, IL-3, GM-CSF, and SLF were removed, each colony made into a single cell suspension and plated in a single 2° plate in methylcellulose culture mixture containing the same ingredients and growth factors as in the primary culture. Assayable 2° colonies were scored as CFU-GM, BFU-E, or CFU-GEMM 14 days later.
Ex Vivo Expansion.
Frozen CB samples were thawed and washed with complete medium (CM, consisting of Iscove's modified Dulbecco's medium supplemented with 10% FCS, 1% l-glutamine and penicillin and streptomycin). Cells were stained with CD34 FITC and CD38 phycoerythrin [PE; Becton Dickinson Immunocytometry Systems (BDIS)], and total CD34+ cells or subfractions of CD34+ cells were isolated by flow cytometric cell sorting by using a FACSVantage SE (BDIS). Sorted cells were cultured ex vivo in individual wells of 24-well plates of CM supplemented with FL and megakaryocyte growth and differentiation factor (MGDF, also called thrombopoietin) at 50 ng/ml each; SLF, IL-3, and IL-6 at 100 ng/ml each; and GM-CSF at 20 ng/ml. Cytokines used for ex vivo expansion were a kind gift from Amgen Biologicals. Cytokines were supplemented at the initiation of culture and every 48 h thereafter. Cultures were demidepopulated every 7–10 days, and care was taken not to allow cell numbers to exceed 106 cells per ml. Cells recovered from culture at every time point were counted and plated in clonogenic assays as described above. Data are reported as cumulative cell or progenitor cell production in culture after correcting for the number of cells removed after each demidepopulation as described (35). Results were compared with the ex vivo expansion capability of nonfrozen freshly isolated CB collected from different donors than those used for the frozen samples, because prefreeze data on the frozen cells was not available.
NOD/SCID Mouse Assay.
CD34+ cells were isolated by magnetic beads from defrosts of 15-yr frozen CB samples and assayed as detailed (31). Between 2 × 104 and 3 × 104 CD34+ cells were isolated from each donor sample separately, and the purified cells (≥95% CD34+) from each donor sample were injected into two sublethally irradiated NOD/SCID mice whereby each recipient received between 1 × 104 and 1.5 × 104 CD34+ cells. These NOD/SCID mice were given 300 rad from a 137Cs source, and cells were injected into mice 2 h later. Controls were aged-matched NOD/SCID mice that received no irradiation or cells. NOD/SCID mice were purchased from The Jackson Laboratory.
Results
Recovery of Viable Nucleated Cells and HPC.
We had previously demonstrated high-efficiency recovery of immature and more mature subsets of HPC from human CB that had been cryopreserved for up to 10 yr (23). In that study, recovery of nucleated cells averaged 88%, whereas 74–92% recovery of HPC was found; some samples in each category were recovered at 100% efficiency. This was a direct comparison of the same samples assayed prefreeze and postthaw after 10 yr by using the same culture conditions and growth factors. In the present study, we also compared recovery of nucleated cells and HPC after thawing of the exact same frozen donor samples evaluated after 10 yr by using the same prefreeze cytokines and growth conditions (Table 1). There was no statistical difference in the recovery after 10 and 15 yr compared with prefreeze values of nucleated cells, or of CFU-GM, BFU-E, or CFU-GEMM that were detected by colony formation stimulated by the combination of Epo and IL-3 in methylcellulose cultures or of CFU-GM that were detected by colony formation stimulated by GM-CSF in agar cultures. As with the 10-yr defrost, we found up to 100% recovery of the prefreeze nucleated cell and colony numbers.
Table 1.
Percent recovery of cryopreserved CB cells after 15 and 10 yr
| Parameter, yr | Mean ± 1 SD | Range | No. |
|---|---|---|---|
| Nucleated cells | |||
| 15 | 83 ± 12 | 64–100 | 9 |
| 10 | 88 ± 20 | 47–100 | 10 |
| CFU-GM | |||
| 15 | 95 ± 15 | 56–100 | 9 |
| 10 | 92 ± 11 | 81–100 | 10 |
| BFU-E | |||
| 15 | 84 ± 25 | 29–100 | 9 |
| 10 | 91 ± 17 | 55–100 | 8 |
| CFU-GEMM | |||
| 15 | 85 ± 25 | 29–100 | 9 |
| 10 | 74 ± 25 | 40–100 | 8 |
Data from the 10-yr defrost were reported (23) and are shown here for comparison.
When the original cells were frozen, potent costimulating factors such as SLF (36) and FL (37) had not yet been identified. The addition of SLF or FL with a CSF allowed detection of more immature subsets of HPC with a higher proliferative capacity than was noted with cells stimulated with CSFs in the absence of SLF or FL (16, 36, 37). By the time we analyzed the 5- and 10-yr defrosts, we were also able to assess the numbers and size of colonies formed from defrosted cells cultured with growth factors in the presence of SLF; the recovered HPC manifested a very high proliferative capacity after stimulation with SLF plus a CSF such as Epo or GM-CSF (16, 23). A direct comparison of 10- vs. 15-yr defrosts is shown in Table 2 for CFU-GM responsive to stimulation by either GM-CSF, GM-CSF plus SLF (in agar), or Epo,IL-3 plus SLF; for BFU-E responsive to stimulation by Epo plus IL-3; and CFU-GEMM responsive to stimulation by Epo, IL-3 and SLF (in methylcellulose). As noted previously (16), CFU-GEMM, but not BFU-E, colonies are seen when SLF is added with Epo plus IL-3 (data not shown). There were no significant differences in the retrieval of HPC (responsive to stimulation with a CSF or CSFs plus SLF) after 10 and 15 yr. Additionally, we were able to quantitate the retrieval of CFU-GM stimulated with FL in combination with GM-CSF, in the absence and presence of SLF, although we could not directly compare this to prefreeze or 5- or 10-yr defrosts of these same samples, as we had not used FL in the previous studies.
Table 2.
Recovery of immature HPC from cryopreserved CB after 15 and 10 Yr, expressed per 106 cells frozen
| Parameter, yr | Mean ± SD | Range | No. |
|---|---|---|---|
| CFU-GM colonies stimulated with GM-CSF | |||
| 15 | 318 ± 217 | 140–840 | 9 |
| 10 | 101 ± 83 | 16–234 | 8 |
| CFU-GM colonies stimulated with GM-CSF + SLF | |||
| 15 | 1621 ± 833 | 380–3441 | 9 |
| 10 | 1501 ± 746 | 545–2565 | 8 |
| CFU-GM colonies stimulated with GM-CSF + FL | |||
| 15 | 1513 ± 733 | 380–3159 | 9 |
| 10 | ND | ||
| CFU-GM colonies stimulated with GM-CSF + FL + SLF | |||
| 15 | 2214 ± 925 | 860–4400 | 9 |
| 10 | ND | ||
| CFU-GM colonies stimulated with Epo + IL-3 + SLF | |||
| 15 | 1329 ± 479 | 440–2160 | 9 |
| 10 | 1710 ± 902 | 669–3116 | 8 |
| BFU-E colonies stimulated with Epo + IL-3 | |||
| 15 | 1235 ± 655 | 160–2119 | 9 |
| 10 | 627 ± 351 | 212–1178 | 8 |
| CFU-GEMM colonies stimulated with Epo + IL-3 + SLF | |||
| 15 | 2018 ± 1025 | 280–3480 | 9 |
| 10 | 1390 ± 1004 | 378–3254 | 8 |
ND, not done.
Defrosted CB HPC stored frozen for 15 yr had extensive proliferative capacity as assessed by the number of cells contained in individual colonies. The results given are the average results of 40 BFU-E, 40 CFU-GEMM, and 34 CFU-GM colonies each assessed separately from a total of defrosts from seven different donors. BFU-E stimulated with Epo and IL-3 gave rise to 39,000 ± 29,000 (± SD; range 10,000–182,500) erythroid cells per colony. CFU-GEMM stimulated with Epo, IL-3 and SLF gave rise to colonies containing 198,100 ± 52,900 (range 70,000–292,500) erythrocytes, granulocytes, macrophages, and megakaryocytes, although not all colonies contained megakaryocytes. CFU-GM stimulated with the combination of Epo, IL-3 and SLF gave rise to colonies containing 12,100 ± 5,400 (range 2,500–22,500) granulocytes and macrophages. However, the proliferative capacity of these CFU-GM is likely a large underestimate of the true proliferative capability of these cells; CFU-GM colonies in methyl cellulose were not nearly as large as those growing in agar culture stimulated with GM-CSF plus SLF, with or without FL. We did not count the number of cells contained in individual colonies formed in agar as this is more technically difficult to do accurately. However, actual sizes of the largest colonies, as visualized in the semisolid medium plates, can be seen in Fig. 1 (CFU-GM colonies stimulated with GM-CSF, GM-CSF plus SLF, GM-CSF plus FL, or GM-CSF plus SLF and FL). As previously reported for noncryopreserved cells (36, 37), CFU-GM colonies stimulated with GM-CSF plus either SLF or FL are larger than those stimulated with only GM-CSF, and the addition of SLF plus FL to cultures with GM-CSF yields the largest colonies (37). The ratios of CFU-GM colonies that formed from the 15-yr defrosts (n = 9) in the presence of GM-CSF plus SLF, GM-CSF plus FL, or GM-CSF, SLF and FL compared with those stimulated only by GM-CSF were, respectively, 6.1 ± 3.3 (mean ± 1 SD; range 2.6–12.4), 5.8 ± 3.0 (2.6–11.9), and 8.5 ± 3.6 (4.7–15.0). Thus, CFU-GM subsets from defrosted CB, similar to that of fresh noncryopreserved CB, that had greater proliferative capacity were in greater quantity than those with lesser proliferative capacity. Visualization of the actual largest representative BFU-E colonies formed in methylcellulose with Epo and IL-3, and representative CFU-GEMM colonies in methylcellulose with Epo, IL-3 and SLF are shown in Fig. 1. The sizes of these colonies are similar to those shown for the 10-yr defrosts stimulated with the same cytokine combinations (23) and to those of freshly obtained CB. The above results demonstrate high-efficiency recovery of HPC with extensive proliferative capacity after 15-yr storage in a frozen state.
Figure 1.
Colonies formed from CB CFU-GM, BFU-E, and CFU-GEMM stored frozen for 15 yr, defrosted, and plated in semisolid agar culture medium for 14 days in the presence of GM-CSF alone or in combination with SLF, FL, or SLF plus FL for CFU-GM, and in semisolid methyl cellulose culture medium for 14 days, respectively, in the presence of Epo plus IL-3 and of Epo and IL-3 plus SLF for BFU-E and CFU-GEMM. These are representatives of the largest colonies formed. Numbers of these colonies per 106 defrosted cells can be found in Table 2.
Recovery of CFU-GEMM with Replating Capability.
In previous studies, we had shown that CFU-GEMM colonies from nonfrozen CB could be replated as single colonies per plate with the formation of 2° CFU-GEMM, BFU-E, and CFU-GM colonies (24, 25). Many of the 2° CFU-GEMM colonies were as large as the 1° CFU-GEMM colonies from which they were derived, suggesting a degree of self-renewal capacity for CFU-GEMM. In the present study, for the first time, we evaluated the capacity of cryopreserved CFU-GEMM to generate 2° colonies of CFU-GEMM, BFU-E, and CFU-GEMM (Table 3). The CFU-GEMM present in defrosts of 15-yr frozen CB had extensive replating characteristics. In fact, 96.6% of the 2° plates contained at least one colony that was either a CFU-GEMM, BFU-E, or CFU-GM. More impressively, 37.9% of the 2° plates contained at least one CFU-GEMM, and every plate that contained a 2° CFU-GEMM colony also contained BFU-E and CFU-GM colonies. On average, every 1° CFU-GEMM colony gave rise, respectively, to 37.3 (range 0–98), 14.1 (range 0–166), and 2.0 (range 0–18) CFU-GM, BFU-E, and CFU-GEMM colonies per 2° plate. Because we did not add CB plasma to either the 1° or 2° plates, and CB plasma enhances the replating efficiency of 1° CFU-GEMM (25), it is likely that we have underestimated the replating (self-renewal) capacity of individual CFU-GEMM from frozen CB. Although we did not do replating evaluations on the original CB samples used in the present study, the replating capacity seen is similar to that of fresh CB (24). Thus, these results demonstrate the retention of self-renewal capacity of CFU-GEMM after 15-yr storage in a frozen state.
Table 3.
Percent secondary replating capability of single CFU-GEMM retrieved from 15-yr frozen CB
| Any progenitor | 96.6 |
| CFU-GM, BFU-E, and CFU-GEMM | 37.9 |
| CFU-GM and BFU-E | 79.3 |
| CFU-GM and CFU-GEMM | 37.9 |
| BFU-E and CFU-GEMM | 37.9 |
| CFU-GM | 93.1 |
| BFU-E | 87.8 |
| CFU-GEMM | 37.9 |
Results are based on 29 replated colonies from a total of six different donors. Both 1° and 2° colonies were plated in methylcellulose culture medium with Epo, IL-3, and SLF. At least one secondary colony was detected in 28 of the 29 primary colonies plated.
Recovery of HPC/HSC That Can Be Expanded ex Vivo.
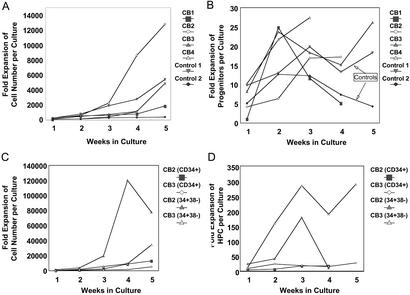
Numerous investigators have shown the extensive capacity of unfrozen CB HPC/HSC for ex vivo expansion (reviewed in refs. 11, 16, and 35–39). This extensive expansion capability has also been seen with CB stored frozen for short periods of time (26–28). To assess the effects of longer storage of frozen CB, we evaluated the capacity of CB frozen for 15 yr to give rise, in suspension cultures supplemented with growth factors, to nucleated cells and HPC in long-term cultures (Fig. 2). Over the course of 35 days in culture, freshly isolated (control) and cryopreserved CB CD34+ cells supported the production of >1,000- to 13,000-fold higher numbers of cells than used to initiate the long-term cultures (Fig. 2A). Kinetics of expansion of assayable progenitors in cultures initiated with CD34+ cells from cryopreserved samples were also similar to those observed in cultures initiated with control cells, whereby, at peak production in all cultures, in excess of 10- to 25-fold more progenitor cells were detected relative to the initial number of clonogenic cells contained in each culture (Fig. 2B).
Figure 2.
Fold expansion in total cell number and clonogenic progenitors in long-term cultures of CD34+ or CD34+CD38− cells from fresh (control) or cryopreserved CB samples. CD34+ cells from fresh CB samples or CD34+ and CD34+CD38− cells from cryopreserved CB samples were expanded in long-term cultures as described in Materials and Methods and were demidepopulated every 7 to 10 days. The total cell number contained in each culture at different time points was calculated by using the formula X = (number of cells per culture) × (1/2)n where X is the number of total cells in culture and n is equal to the number of previous demidepopulations. The total number of cells at different time points was divided by the number of cells used to initiate each culture to calculate fold expansion of cells (A and C). The same formula was used to calculate the total number of clonogenic cells produced in culture, and, similarly, fold expansion of total progenitors was determined (B and D). CD34+ cells from a total of four cryopreserved CB samples and two fresh samples were used in these studies, and long-term cultures were initiated with CD34+CD38− cells from two of the four cryopreserved samples.
CD34+ cells can be subphenotyped with the CD38 marker into populations of more immature (CD34+CD38−) and more mature (CD34+CD38+) HPC, with HSC found in the CD34+CD38− population. CD34+CD38− and CD34+CD38+ cells were isolated from cryopreserved CB samples, and long-term cultures were initiated with both phenotypes of cells. Both total cell production and expansion of progenitor cells were considerably higher among CD34+CD38− cells than their CD38+ counterparts (data not shown). Fold expansion of both total cells (Fig. 2C) and clonogenic cells (Fig. 2D) were substantially higher in cultures initiated with CD34+CD38− cells compared with similar cultures originated with CD34+ cells from the same cryopreserved CB samples, confirming the primitive nature and extensive proliferative potential of CD34+CD38− cells. Fold increase in the number of HPC in cultures initiated with CD34+CD38− CB cells exceeded 150 in both long term cultures.
Recovery of NOD/SCID Repopulating Human HSC.
To assess the cryopreservation of CB HSC more directly then what is described above, which mainly examines the activity of HPCs, we took advantage of the NOD/SCID mouse as an assay for engrafting and repopulating human stem cells (29–32, 39). Magnetic bead separated CD34+ cells isolated from defrosted cells of four donors were each injected into two NOD/SCID mice. Mice were evaluated for human cell engraftment 11–13 wk after injection of CB cells. As shown in Table 4 and Fig. 3, human NOD/SCID repopulating cells were present in the four samples, and were noted in both recipients of each donor cell inoculum in three of the four samples tested as determined by the percentage of CD45+, CD45+/CD19+, and CD45−/CD11b+ human cells in mouse bone marrow. Engraftment of NOD/SCID mice 11–13 wk after injection of human cells is considered a measure of relatively long-term engrafting human stem cells (32). The low percentage of human cells engrafting these mice is due to the limiting numbers of CD34+ cells available for transplantation in the NOD/SCID mice, because we wished to assess the repopulating capacity of single collections of CB. However, the level of human chimerism in these mice is consistent with that seen previously (31) by using similar low numbers of transplanted fresh CD34+ cells. These results demonstrate that CD34+ cells isolated from CB stored frozen for 15 yr have the capacity to engraft the marrow of NOD/SCID mice at a frequency equivalent to that previously shown for freshly isolated CB CD34+ cells.
Table 4.
In vivo repopulation of mouse NOD/SCID bone marrow with CD34+ cells isolated from CB cells stored frozen for 15 yr
| Sample | % CD45+ | % CD45+/CD19+ | % CD45+/CD11b+ |
|---|---|---|---|
| Untransplanted control | 0.04 | 0.1 | 0.01 |
| 0.02 | 0.05 | 0.02 | |
| 0.01 | 0.12 | 0.01 | |
| 1 | 0.3 | 1.2 | 7.4 |
| 1a | 0.03 | 0.0 | 0.0 |
| 2 | 2.4 | 68.1 | 19.3 |
| 2a | 0.5 | 49.7 | 10.2 |
| 3 | 1.5 | 5.7 | 4.1 |
| 3a | 0.8 | 20.6 | 5.6 |
| 4 | 1.44 | 42.3 | 16.3 |
| 4a | 0.5 | 58.0 | 25.2 |
Percentages shown are for human cells in the windows shown in Fig. 3 set on the CD19 or CD11b isotype at a background level of 1%. Sample numbers reflect two recipients (e.g., 1, 1a or 2, 2a, etc.) of each CB donor. Mice 1, 2, 3, and 4 were evaluated 11 wk after injection of cells into NOD/SCID mice. Mice 1a and 2a were evaluated 13 wk after injection, and mice 3a and 3b were evaluated 12 wk after injection.
Figure 3.
Flow cytometric analysis of human CD45+ cells with CD19 and CD11b surface phenotypes found in the bone marrows of NOD/SCID mice 4 mo after infusion of bead-separated CB obtained from defrosts of cells cryopreserved and stored frozen for 15 yr. The histograms are of cells that express human CD45 above background levels (set at 1% of isotype). The actual percentages of human cells that are CD45+, CD45+CD19+, and CD45+CD11b+ are reported in Table 4.
Discussion
CB transplantation is a relatively recent clinical procedure, with the first CB transplant performed in October 1988 (1, 11, 19). Since then, hundreds of thousands of CB collections have been stored frozen throughout the world, in anticipation of their potential use to treat a multitude of malignant and nonmalignant disorders in children and adults. However, the longest that a CB collection has been in frozen storage before actual clinical use is likely between 3 and 5 yr. Our own studies have previously demonstrated high-efficiency recovery of immature and mature subsets of CFU-GM, BFU-E, and CFU-GEMM after 5 (16) and 10 (23) yr of frozen storage. These recovery calculations were possible because of direct comparisons of post- and prefreeze HPC numbers obtained from the same CB samples using the exact same culture conditions. Since our last report in 1997 (23), papers published in 1998 and 1999, respectively, described results of analysis of 15- and 12-yr defrosts of CB. The 15-yr study (40) reported on recovery of relatively mature subsets of CFU-GM (as detected by colony formation after stimulation with 5637 conditioned medium), but, because no prefreeze data were available for these same samples, the efficiency of recovery of the CFU-GM could not be calculated. The 12-yr study (41) reported recovery of CFU-GM, BFU-E, and CFU-GEMM based on comparing numbers of cells to 1-mo defrost values from the same samples. These authors noted >90% recovery of HPC after 10 yr. Because the recovery calculations were not based on prefreeze control numbers, the actual efficiency of recovery could not be absolutely calculated. Although it is clear from the above studies that one could retrieve viable HPC, there was the reasonable and more important concern of whether or not the more immature HSC population survived the freezing procedure and long-term storage.
The practical aims of the present study were to evaluate recovery of HPC after 15-yr storage in a frozen state, and most importantly to determine not only the in vitro proliferative, self-renewal and expansive properties of recovered HPC, but also to evaluate the capacity of recovered cells to engraft and repopulate the hematopoietic system of sublethally irradiated NOD/SCID mice. Our results clearly demonstrate that HPC with extensive proliferative, self-renewal and ex vivo expansion capabilities, and HSC with NOD/SCID repopulating ability can be effectively recovered after 15-yr storage in a frozen state. As part of our initial preclinical studies that suggested the feasibility of CB transplantation (14), we have had CB samples stored frozen since the mid 1980s. HPCs responsive to the stimulatory effects of combinations of cytokines, including CSFs (Epo, GM-CSF, and IL-3) and the potent costimulating cytokines (SLF and FL), are considered more immature than HPC responsive to single cytokines (e.g., GM-CSF, IL-3, or Epo), conclusions supported by the more extensive proliferative capacity of HPC responsive to stimulation by multiple vs. single cytokines. In fact, some of the HPC responsive to stimulation by multiple cytokines including SLF and/or FL may actually reside within the HSC compartment (16, 24, 25). That single colonies from CB CFU-GEMM in such culture conditions can be replated in 2° dishes with generation of CFU-GEMM, BFU-E, and CFU-GM colonies is highly suggestive of the self-renewal capacity of these CB CFU-GEMM. Self-renewal capacity is a hallmark of stem cells. Whereas assessment of CFU-GEMM activity in vitro may not represent long-term repopulating stem cell function, the data from these studies are consistent with these cells being a subset, although a more mature subset, of HSC. Because the replating capacity of 15-yr defrosts of CB CFU-GEMM is as extensive as that of freshly isolated CB CFU-GEMM, and the recovered CD34+CD38− population had the capacity, at least as potent as such fresh cells, to be ex vivo expanded, it was clear that very early cells within the HSC/HPC compartments had withstood the cryopreservation and storage conditions used. However, ultimately, whether or not any stem cell collection, whether from a freshly obtained or frozen sample, will be useful for clinical transplantation depends on the ability of the cells to appropriately home to a microenvironment niche conducive for their self-renewal, growth, and differentiation. Whether this homing has occurred is usually measured by the long-term engrafting capability of the infused cells. Whereas assays for HSC homing/engraftment have been well established for decades with rodent cells, it is only recently that assays that apparently detect such human HSC have become available. The most common and widely used assay for human HSC engrafting capabilities is the human mouse SCID repopulating cell assay (29–32). In fact, this in vivo assay has shown that human CB HSC are more effective than their bone marrow counterparts in engrafting SCID mice, results consistent with the lower number of CB, compared with adult bone marrow cells, needed for clinical engraftment (1–13, 17–18, 20, 22). Our data herein show that 15-yr defrosts of frozen CB engraft and repopulate the hematopoietic system of NOD/SCID mice in a quantitative and qualitative manner consistent with that of freshly isolated CB cells.
Whereas final proof for the long-term engrafting capability of CB cryropreserved and stored frozen for long periods of time requires clinical results demonstrating long-term engraftment in humans, our inclusive results are highly suggestive that CB can be stored frozen for at least 15 yr with highly efficient recovery of viable and highly functional HSC and HPC needed for successful CB transplantation.
Acknowledgments
These studies were supported by U.S. Public Health Service National Institutes of Health RO1 Grants RO1 HL56416, RO1 HL67384, and RO1 DK53674 from the National Institutes of Health (to H.E.B.) and RO1 HL55716 from the National Heart, Lung, and Blood Institute (to E.F.S.).
Abbreviations
- BFU-E
burst-forming unit-erythroid
- CFU-GM
colony-forming unit-granulocyte, -macrophage
- CFU-GEMM
colony-forming unit-granulocyte, -erythrocyte, -monocyte, and -megakaryocyte
- CB
cord blood
- HSC
hematopoietic stem cell
- HPC
hematopoietic progenitor cell
- NOD
nonobese diabetic
- SCID
severe combined immunodeficient
- rhu
recombinant human
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- SLF
steel factor
- Epo
erythropoietin
- FL
Flt3-ligand
References
- 1.Gluckman E, Broxmeyer H E, Auerbach A D, Friedman H, Douglas G W, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Wagner J E, Kernan N A, Steinbuch M, Broxmeyer H E, Gluckman E. Lancet. 1995;346:214–219. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- 3.Wagner J E, Rosenthal J, Sweetman R, Shu X-O, Davies S M, Ramsay N K C, McGlave P B, Sender L, Cairo M S. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 4.Kurtzberg J, Laughlin M, Graham M L, Smith C, Olson J F, Halperin E C, Ciocci G, Carrier C, Stevens C E, Rubinstein P. N Engl J Med. 1996;3:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte J P, et al. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio A R, Berkowitz R L, Cabbad M, Dobrila N L, Taylor P E, et al. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 7.Rocha V, Wagner J E, Sobocinski K A, Klein J P, Zhang M-J, Horowitz M M, Gluckman E. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 8.Barker J, Davies S M, DeFor T, Ramsay N K C, Weisdorf D J, Wagner J E. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin M J, Barker J, Bambach B, Koc O N, Rizzieri D A, Wagner J E, Gerson S L, Lazarus H M, Cairo M, Stevens C E, et al. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 10.Sanz G F, Saavedra S, Planelles D, Senent L, Cervera J, Barragan E, Jimenez C, Larrea L, Martin G, Martinez J, et al. Blood. 2001;98:2332–2338. doi: 10.1182/blood.v98.8.2332. [DOI] [PubMed] [Google Scholar]
- 11.Broxmeyer H E, Smith F O. In: Stem Cell Transplantation. Forman S I, Blume K G, Thomas E D, editors. Cambridge, MA: Blackwell Scientific; 1999. pp. 431–443. [Google Scholar]
- 12.Hawes J M. J Clin Pathol. 2001;54:428–434. [Google Scholar]
- 13.Barker J N, Wagner J E. Curr Opin Oncol. 2002;14:160–164. doi: 10.1097/00001622-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Broxmeyer H E, Douglas G W, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broxmeyer H E, Kurtzberg J, Gluckman E, Auerbach A D, Douglas G, Cooper S, Falkenburg J H F, Bard J, Boyse E A. Blood Cells. 1991;17:313–329. [PubMed] [Google Scholar]
- 16.Broxmeyer H E, Hangoc G, Cooper S, Ribeiro R C, Graves V, Yoder M, Wagner J, Vadhan-Raj S, Rubinstein P, Broun E R. Proc Natl Acad Sci USA. 1992;89:4109–4113. doi: 10.1073/pnas.89.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner J E, Broxmeyer H E, Byrd R L, Zehnbauer B, Schmeckpeper B, Shah N, Griffin C, Emanuel P, Zuckerman K, Cooper S, et al. Blood. 1992;79:1874–1881. [PubMed] [Google Scholar]
- 18.Kohli-Kumar M, Shahidi N T, Broxmeyer H E, Masterson M, Delaat C, Sambrano J, Morris C, Auerbach A D, Harris R E. Br J Haematol. 1993;85:419–422. doi: 10.1111/j.1365-2141.1993.tb03192.x. [DOI] [PubMed] [Google Scholar]
- 19.Broxmeyer H E. In: Cellular Characteristics of Cord Blood and Cord Blood Transplantation. Broxmeyer H E, editor. Bethesda: AABB Press; 1998. pp. 1–9. [Google Scholar]
- 20.Gluckman E. In: Cellular Characteristics of Cord Blood and Cord Blood Transplantation. Broxmeyer H E, editor. Bethesda: AABB Press; 1998. pp. 147–164. [Google Scholar]
- 21.Peterson R K, Clay M, McCullough J. In: Cellular Characteristics of Cord Blood and Cord Blood Transplantation. Broxmeyer H E, editor. Bethesda: AABB Press; 1998. pp. 165–197. [Google Scholar]
- 22.Ballen K, Broxmeyer H E, McCullough J, Piacibello W, Rebella P, Verfaille C M, Wagner J E. Biol Blood Marrow Transplant. 2001;7:635–645. doi: 10.1053/bbmt.2001.v7.pm11787526. [DOI] [PubMed] [Google Scholar]
- 23.Broxmeyer H E, Cooper S. Clin Exp Immunol. 1997;107:45–53. [PubMed] [Google Scholar]
- 24.Carow C, Hangoc G, Cooper S, Williams D E, Broxmeyer H E. Blood. 1991;78:2216–2221. [PubMed] [Google Scholar]
- 25.Carow C E, Hangoc G, Broxmeyer H E. Blood. 1993;81:942–949. [PubMed] [Google Scholar]
- 26.Lu L, Ge Y, Li Z-H, Freie B, Clapp D W, Broxmeyer H E. Cell Transplant. 1995;4:493–503. doi: 10.1177/096368979500400510. [DOI] [PubMed] [Google Scholar]
- 27.Li Z H, Broxmeyer H E, Lu L. Leukemia. 1995;9:S12–S16. [PubMed] [Google Scholar]
- 28.DiGiusto D L, Lee R, Moon J, Moss K, O'Toole T, Voytovich A, Webster D, Mule J J. Blood. 1996;87:1261–1271. [PubMed] [Google Scholar]
- 29.Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer H E, Dick J. Blood. 1994;83:2489–2497. [PubMed] [Google Scholar]
- 30.Orazi A, Braun S E, Broxmeyer H E. Blood Cells. 1994;20:323–330. [PubMed] [Google Scholar]
- 31.Bock T A, Orlic D, Dunbar C E, Broxmeyer H E, Bodine D M. J Exp Med. 1995;182:2037–2043. doi: 10.1084/jem.182.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodine D M. In: Cellular Characteristics of Cord Blood and Cord Blood Transplantation. Broxmeyer H E, editor. Bethesda: AABB Press; 1998. pp. 45–65. [Google Scholar]
- 33.Lu L, Xiao M, Shen R-N, Grigsby S, Broxmeyer H E. Blood. 1993;81:41–48. [PubMed] [Google Scholar]
- 34.Cooper S, Broxmeyer H E. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, Coico R, editors. New York: Wiley; 1996. , Suppl. 18, pp. 6.4.1–6.4.12. [Google Scholar]
- 35.Traycoff C M, Abboud M R, Laver J, Brandt J E, Hoffman R, Law P, Ishizawa L, Srour E F. Exp Hematol. 1994;22:215–222. [PubMed] [Google Scholar]
- 36.Broxmeyer H E, Cooper S, Lu L, Hangoc G, Anderson D, Cosman D, Lyman S D, Williams D E. Blood. 1991;77:2142–2149. [PubMed] [Google Scholar]
- 37.Broxmeyer H E, Lu L, Cooper S, Ruggieri L, Li Z H, Lyman S D. Exp Hematol. 1995;23:1121–1129. [PubMed] [Google Scholar]
- 38.Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, Fagioli F, Berger M, Aglietta M. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 39.Piacibello W, Sanavio F, Severino A, Dane A, Gammaitoni L, Fagioli F, Perissinotto E, Cavalloni G, Kollet O, Lapidot T, Aglietta M. Blood. 1999;93:3736–3749. [PubMed] [Google Scholar]
- 40.Kobylka P, Ivanyi P, Breur-Vriesendorf B S. Transplantation. 1998;65:1275–1278. doi: 10.1097/00007890-199805150-00024. [DOI] [PubMed] [Google Scholar]
- 41.Mugishima H, Harada K, Chin M, Suzuki T, Hayakawa S, Sato K, Klein J P, Gale R P. Bone Marrow Transplant. 1999;23:395–396. doi: 10.1038/sj.bmt.1701580. [DOI] [PubMed] [Google Scholar]