Abstract
Heparin and low-molecular weight heparins (LMWHs), complex, sulfated polysaccharides isolated from endogenous sources, are potent modulators of hemostasis. Heparin and LMWHs interact with multiple components of the coagulation cascade to inhibit the clotting process. Pharmaceutical preparations of these complex polysaccharides, typically isolated from porcine intestinal mucosa, are heterogeneous in length and composition and, hence, highly polydisperse. Because of the structural heterogeneity of heparin and LMWHs, correlating their activity with a particular structure or structural motif has been a challenging task. Herein, we demonstrate a practical analytical method that enables the measurement of a structural correlate to in vivo anticoagulant function. With this understanding we have developed LMWHs with increased anticoagulant activity and decreased polydispersity. In addition to the pronounced anti-Xa and anti-IIa activity of these LMWHs, we also demonstrate that they possess desirable in vivo pharmacokinetic properties, the ability to cause the release of tissue factor pathway inhibitor (TFPI) from the endothelium, complete bioavailability through s.c. delivery, and the ability to inhibit both venous and arterial thromboses. Importantly, from a clinical safety point of view, unlike LMWHs presently used in the clinic, we show that these LMWHs are rapidly and completely neutralized by protamine. Together, the findings presented herein demonstrate a facile approach for the creation of designer LMWHs with optimal activity profiles.
Thrombosis formation is a major health concern of the Western world, leading to significant patient morbidity and mortality (1). The creation of efficient, specific inhibitors of blood coagulation remains critical for the appropriate clinical management of thromboses. As such, heparin, a highly sulfated polysaccharide isolated from mast cells, has been an anticoagulant and antithrombotic agent of choice for >60 years. While heparin's effect on the coagulation cascade is multifaceted and complex, one of the primary mechanisms by which heparin elicits its anticoagulant function is through binding the serpin antithrombin III (AT-III). Binding of AT-III to a specific heparin pentasaccharide sequence, HNAc/S,6SGHNS,3S,6SI2SHNS,6S (see abbreviations footnote), induces a protein conformational change that activates AT-III (2, 3). Activated AT-III binds to and inhibits the activity of a number of proteases in the coagulation cascade. First, activated AT-III can directly bind to and inhibit the activity of factor Xa. Second, thrombin (factor IIa) also binds to heparin at a site proximate to the AT-III pentasaccharide-binding site (4). Formation of a ternary complex between AT-III, thrombin, and heparin results in the inactivation of thrombin. Unlike its anti-Xa activity, which requires only the AT-III pentasaccharide-binding site, heparin's anti-IIa activity is size-dependent, requiring at least 18 saccharide units for the efficient formation of an AT-III, thrombin, and heparin ternary complex (4). Importantly, in current pharmaceutical preparations of heparin, only one of three polysaccharide chains contains the AT-III pentasaccharide sequence and is an active anticoagulant (5).
Although heparin is a highly efficacious anticoagulant and antithrombotic agent for a variety of clinical indications, there are many limitations associated with it. For example, heparin's chemical heterogeneity and polydispersity lead to nonspecific protein binding, yielding poor pharmacokinetics on s.c. injection (6), and there are side effects associated with heparin therapy, including heparin-induced thrombocytopenia (7, 8).
To circumvent the shortcomings associated with heparin therapy while maintaining its potent anticoagulant activity, numerous strategies have been designed to create low-molecular weight heparins (LMWHs), molecules with reduced chain length. Because of their lower molecular weight and less polydispersity compared with heparin, such LMWH molecules, including Lovenox (enoxaparin) and Fragmin (dalteparin), have higher, more reproducible bioavailability after s.c. administration and more predictable pharmacokinetics, leading to greater safety (9, 10). However, Lovenox and Fragmin suffer from shortcomings of their own. Importantly, the LMWHs have less anticoagulant activity than parent heparin (11). Critically, from a safety point of view, none of the LMWHs can be efficiently neutralized by protamine, leading to their limited use or lack of use in important unmet medical conditions (12, 13). Thus, an ideal LMWH preparation should couple the strengths of heparin, namely, high activity and the ability to be neutralized, with the strengths of LMWH, namely, consistent bioavailability and predictable pharmacokinetics. However, at the present time, creation of such a LMWH has not been proven possible because there is no facile method of monitoring the AT-III-binding sites on heparin as a function of depolymerization and purification. Such a structure–function correlate involving the AT-III binding site would enable the creation of tailored LMWHs, including ones with tunable anti-Xa:anti-IIa ratios, defined tissue factor pathway inhibitor (TFPI) release profiles, and the ability to be neutralized by agents such as protamine.
Herein, we detail a structural correlate that is an accurate predictor of heparin's anti-Xa and anti-IIa activity both in vitro and in vivo. Furthermore, we use this understanding to rationally design LMWHs with low polydispersity that readily reduce the formation of arterial and venous thrombi in vivo compared with existing LMWHs. Importantly, we also show that these LMWHs are rapidly and completely neutralized by protamine. Together, these findings provide proof-of-concept that the structure–function correlation can be used to create rationally designed LMWHs with desired in vitro and in vivo attributes.
Methods and Materials
Materials.
Heparin was purchased from Celsus Laboratories (Cincinnati) and molar concentrations of stocks were calculated based on an average molecular weight of 13,000. Disaccharide standards were purchased from Sigma. Heparinases I, II, and III were produced as described (14–16).
Structural Analysis of Heparin and LMWHs.
Heparin and LMWHs were subjected to compositional analysis under conditions described in refs. 3, 17, and 18.
Generation of LMWH.
The LMWHs rdLMWH-1 and -2 were generated through the controlled cleavage of porcine intestinal mucosa heparin with a mixture of heparinases (19). Briefly, to 1 g of porcine intestinal mucosa in 50 ml of 50 mM calcium acetate buffer, pH 6.7, 0.1 molar equivalent of a heparinase mixture was added, and the solution was maintained at 37°C for 4–8 h. After precipitation of the enzyme, the supernatant was loaded onto a 1-m long, 10-cm diameter P10 size exclusion column. Saccharide fragments were eluted by using a running buffer of 100 mM ammonium bicarbonate, pH 9.0. The eluent was tracked by UV absorption at 232 nm, and 3-ml fractions were collected after the initial void volume. The fractions yielding positive UV absorption at 232 nm were collected and pooled. The sample was lyophilized to remove ammonium bicarbonate and redissolved in ultrapure water.
Molecular Weight Measurement.
The absolute average molecular weight and polydispersity of the LMWHs used in this study were determined by size-exclusion chromatography (SEC) coupled with multiangle laser light scattering (MALLS) detection (20). Briefly, a Shodex OH pak SB-803 HQ column (8.0 × 300 mm; Showa Denko, Tokyo) was used for the chromatographic analysis of heparin and LMWHs. The SEC eluant (0.1 M ammonium acetate, pH 7, containing 0.05% sodium azide) was prepared in vacuum-degassed, double-distilled water and vacuum filtered through a 0.2-μm filter. Column effluent was monitored sequentially with a miniDAWN light scattering detector (Wyatt Technology, Santa Barbara, CA) and a Waters R401 differential refractometer.
Anti-Xa and -IIa Activity Assay.
All reagents for these assays were obtained by modification of the amidolytic method of Teien and colleagues (21, 22) by using S-2222 as the chromogenic substrate for the anti-Xa assay. The detailed procedure is described elsewhere (22). The concentration of LMWH in unknown samples was calculated by comparing to the calibration curve derived from the first international standard for LMWH which was linear in the range between 0 and 0.7 international units (IU)/ml (r2 > 0.99). The results were expressed in anti-Xa IU/ml. The anti-IIa assay was done similarly, but the thrombin-specific substrate S-2238 was substituted. Both Xa and IIa assays were performed on an automated coagulation machine (Coag-A-Mate MTX II, Organon Teknika–Cappel).
Measurements of Plasma TFPI Activity.
TFPI activity in rabbit plasma after a single s.c. administration of heparin or LMWH was determined by a two-step colorimetric assay as described (23, 24). Briefly, in the first step, a dilution of the test sample is incubated with a saturating concentration of factor VIIa/IIa complex. In the second step, a saturating concentration of factor X is added to the reaction mixture as a substrate for the residual factor VIIa/tissue factor catalytic activity; the factorXa generated is measured with the specific chromogenic substrate, S-2222 (American Diagnostica, Greenwich, CT). The resulting absorbance is read at 405 nm. Linear calibration curves were obtained with standard plasmas provided by the manufacturer (American Diagnostica). All test samples were assayed at a 5% dilution. Results are expressed as percent of TFPI activity in pooled rabbit plasma.
Venous Thrombosis Model.
Male New Zealand rabbits, 2.5–3 kg, were anesthetized with ketamine (45 mg/kg) and xylazine (5 mg/kg) by means of i.m. injection. Both sides of the jugular veins (2 cm) were exposed and separated from surrounding tissue (25, 26). The saline or heparins or LMWHs were injected through the right ear marginal vein. Three minutes later, 1 ml/kg human pooled plasma heated to 37°C was injected through the right ear marginal vein. Fifteen seconds later, the jugular veins were ligated with 2-0 silk thread in situ to produce stasis. After 30 min, the veins were segregated and opened in a Petri dish filled with 3.8% sodium citrate. Thrombus formation was evaluated using the Wessler 0–4 scale. In addition, the wet weight of the thrombus was measured.
Arterial Thrombosis Model in Rats.
The arterial thrombosis model was prepared essentially as described with minor modifications (27, 28). Briefly, male Sprague–Dawley rats weighing 350–400 g were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg). The right side carotid artery was carefully isolated free of surrounding tissues (≈2 cm). A perivascular probe connected to an ultrasonic flow meter (Transonic, Ithaca, NY) was placed under and surrounding the carotid artery to monitor the blood flow rate. The experiments began with the injection of 0.2 ml of saline or heparin solution through the penile vein. Exactly 1 min after injection, a piece of filter paper (6 mm in diameter, Whatman no. 5) soaked with 50% FeCl3 was placed on top of the freed carotid artery. The filter paper was removed 15 min later. The experiment was terminated 1 h after FeCl3 treatment and the carotid artery (2 cm) was removed. The thrombus (if formed) was removed and weighed wet. The time to total occlusion (TTO), the time it takes for the blood flow to completely stop, and the thrombus weight were recorded.
Protamine Neutralization of Heparin and LMWHs.
The ability of protamine to neutralize heparin or LMWH was assessed in vitro (29, 30). For this study, 200 μl of human pooled plasma was mixed with 4 μl of either saline or 100 μg/ml heparin, enoxaparin, rdLMWH-1, or rdLMWH-2. For each sample, different ratios of protamine to anti-Xa units of heparin, namely, 0, 0.25, 0.5, 1, 1.5, 2, and 3, were prepared. After incubation for 20 min, the anti-Xa, anti-IIa activity, and activated partial thromboplatin time of the samples was measured.
Results and Discussion
3-O-Sulfated Tetrasaccharide as an Indicator of Anticoagulant Function.
We reasoned that quantification of the AT-III-binding pentasaccharide sequence, namely, HNAc,6SGHNS,3S,6SI2SHNS,6S, pre-sent within the heparin chain could potentially be an indicator of anti-Xa and/or anti-IIa activity of heparin and LMWHs. In previous studies, we investigated the action of the heparinases toward both heparin fragments containing the AT-III-binding sequence and model AT-III-binding pentasaccharide compounds (19). We found that virtually all glucosamine-uronic acid glycosidic linkages in heparin are cleaved by a mixture of heparinases I, II, and III under exhaustive digestion conditions. However, we found that the presence of a 3-O sulfate on the reducing end glucosamine renders the glycosidic bond between N-acetylated, 6-O-sulfated glucosamine and the unsulfated glucuronic impervious to the action of the heparinases (ΔUHNAc,6S↓GHNS,3S,6S, where the arrow represents the uncleavable glycosidic linkage). Thus, under exhaustive digestion conditions with heparinases I–III, a tetrasaccharide product containing the critical 3-O-sulfated residue is reproducibly formed.
In an effort to detect and quantify this tetrasaccharide structure in complex samples of heparin or LMWH, we digested a number of heparins and LMWHs with heparinases I–III and separated the resulting saccharides by capillary electrophoresis. Under exhaustive digestion conditions, eight major entities were typically observed in the electrophoretogram (see Fig. 7, Table 3, and Supporting Text, which are published as supporting information on the PNAS web site, www.pnas.org). Seven of these were identified as disaccharides; the eighth was identified as a tetrasaccharide. Isolation of this tetrasaccharide and sequencing of it indicated that it has the expected structure [ΔUHNAc,6SGHNS,3S,6S, or ±4–7 in the property-encoded nomenclature(17)]. That this is the major tetrasaccharide derived from exhaustive depolymerization of heparin is expected given our understanding of the substrate specificities of heparinases I–III as discussed above.
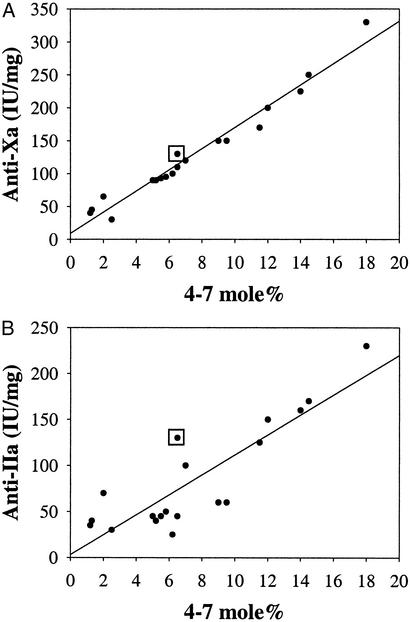
To test whether quantification of ±4–7 could be used to predict anticoagulant function, we determined the ±4–7 content for a variety of heparins from various sources as well as LMWHs that are currently clinically available, including enoxaparin and dalteparin. For instance, we find that enoxaparin, with a measured anti-Xa activity of 100 IU/mg, contains 6.2 mol % of the ±4–7 tetrasaccharide; whereas dalteparin, with a higher anti-Xa activity of 150 IU/mg, contains 9.0 mol % of this tetrasaccharide. We then plotted the anti-Xa or anti-IIa activity of the heparin or LMWH vs. its ±4–7 content. In the case of anti-Xa activity, we find that the ±4–7 content is highly correlated and thus ±4–7 is a good predictor of anti-Xa activity (r2 = 0.98; Fig. 1A). The correlation of ±4–7 with anti-IIa activity is also reasonably good (r2 = 0.8; Fig. 1B). That the ±4–7 correlation is not as good as for anti-IIa activity as compared with anti-Xa activity is consistent with the fact that additional structural motifs, beyond the pentasaccharide sequence, are required for efficient anti-IIa activity of heparins (4). Importantly, these correlations hold, regardless of the biological source of heparin or LMWH or the means by which the LMWH is generated, i.e., chemical or enzymatic cleavage of the parent heparin (see Table 4, which is published as supporting information on the PNAS web site).
Figure 1.
Correlation of the ±4–7 content with anticoagulant activity. The ±4–7 content was plotted vs. anti-Xa (A) or anti-IIa (B) activity. Heparins and LMWHs from various sources were subjected to compositional analysis and the molar percentage of ±4–7 was quantified and compared with its anti-Xa and anti-IIa activities. For reference, the values for heparin (anti-Xa of 130 IU/mg, anti-IIa of 130 IU/mg, and ±4–7 content of 6.5) are boxed. mole% is defined as the ratio of moles ±4–7 to total moles of saccharide products expressed as a percentage.
Generation of Novel LMWHs.
Based on the above findings, we examined whether it would be possible to create a LMWH with increased anti-Xa and anti-IIa activities in vitro by optimizing the ±4–7 content and chain length. With these points in mind, we digested porcine intestinal mucosa heparin under controlled conditions with a mixture of the heparinases and monitored the ±4–7 content as a result of enzymatic digestion. LMWHs thus generated were purified by size fractionation. After purification, physical characterization of the molecules included assessment of their molecular weight, composition, and anti-Xa and anti-IIa activities. The in vitro profile of these new LMWHs were compared with that of enoxaparin and dalteparin (Table 1).
Table 1.
Physical characteristics of rdLMWH-1 and -2 compared to dalteparin and enoxaparin
| Characteristic | Enoxaparin | Dalteparin | rdLMWH-1 | rdLMWH-2 |
|---|---|---|---|---|
| 4–7 mol % | 6.2 | 9.0 | 18.0 | 12.0 |
| Anti-Xa, IU/mg | 100 | 150 | 330 | 200 |
| Anti-IIa, IU/mg | 25 | 60 | 230 | 155 |
| Polydispersity | 1.35 ± 0.05 | 1.40 ± 0.04 | 1.01 ± 0.02 | 1.00 ± 0.03 |
| Average molecular weight | 4,200 ± 100 | 6,000 ± 200 | 5,000 ± 100 | 4,500 ± 100 |
Notably, under two separate digestion and separation conditions, slightly different LMWHs were created. The first is hereafter referred to as rationally designed LMWH-1 (rdLMWH-1) and the second as rationally designed LMWH-2 (rdLMWH-2). Molecular weight measurement of the two indicated that rdLMWH-1 possessed a molecular weight of 5,000, whereas that of rdLMWH-2 was 4,500 (Table 1). Importantly, both rdLMWHs have a polydispersity of very near 1.0, i.e., less than other LMWHs that are currently available (which typically have polydispersities of 1.35 ± 0.05 to 1.40 ± 0.04). Lower polydispersity has been implicated in more predictable activity and lower side effects in vivo, effects that we sought to explore directly (see below).
In vitro assessment of the activities of rdLMWH-1 and -2 indicated that, as predicted, rdLMWH-1 and -2 had higher anti-Xa activity than other LMWHs. rdLMWH-1 had a measured anti-Xa activity of 330 IU/mg, over twice as high as heparin, and 2–3 times as high as existing LMWHs (Table 1). rdLMWH-2 was also a potent inhibitor of Xa, with an activity almost 1.5 times as high as heparin and twice as great as existing LMWHs (Table 1). These pronounced anti-Xa activities agreed well with the measured mol % of the ±4–7 tetrasaccharide in each preparation; rdLMWH-1 possessed 18 mol % of the tetrasaccharide, whereas rdLMWH-2 possessed 12%. Taken together with the homogenous nature of rdLMWH-1 and -2, these results indicate that rdLMWH-1 and -2 are significantly more enriched in antithrombotic activity as compared with existing LMWHs and represent LMWH preparations that truly are enriched for anticoagulant action well beyond the parental heparin compound.
rdLMWH-1 and -2 possessed significant anti-IIa activities of 230 IU/mg and 155 IU/mg, respectively. This finding is in contrast to existing LMWHs which exhibit 4–10 times less anti-IIa activity as compared with anti-Xa activity (9). The pronounced anti-Xa and anti-IIa activities of these molecules indicates that not only do rdLMWH-1 and -2 contain a higher percentage of the ±4–7 sequence but they also have optimal positioning of this site within the context of the heparin chain, thus allowing for the efficient formation of an AT-III/thrombin ternary complex. Taken together, these results indicate that rational design of rdLMWH-1 and -2 enables an enrichment of the AT-III-binding sequence leading to (i) higher anti-Xa activity, (ii) significant anti-IIa activity, and (iii) lower polydispersity. To further assess the effects of rdLMWH-1 and -2, we directly tested the activity of these molecules in vivo.
In Vivo Pharmacokinetic Measurements.
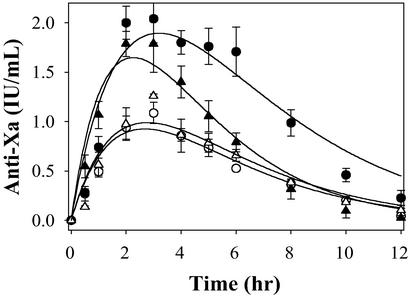
As a next step in assessing whether our in vitro findings can be successfully translated into improved in vivo activity, we conducted a series of pharmacokinetic experiments. For these experiments, we compared rdLMWH-1 and -2 to the leading LMWHs, namely, enoxaparin and dalteparin. As a first step, each of these was administered to rabbits by s.c. injection at 3 mg/kg. After administration, blood was collected at various time points and the pharmacokinetic parameters were determined by following anti-Xa activity.
At an equivalent weight dose of 3 mg/kg, the bioavailability of rdLMWH-1 is over twice as high as enoxaparin or dalteparin, whereas that of rdLMWH-2 is ≈1.5 times as high (Fig. 2 and Table 2). Thus, the potent in vivo activity of rdLMWH-1 and -2 observed here is consistent with their activity in vitro. In addition, rdLMWH-1 and -2, after s.c. administration, exhibit comparable absorption (ka) and elimination (ke) rate constants compared with enoxaparin or dalteparin, demonstrating that the increased bioavailability is because of the higher inherent anti-Xa activity (IU/mg) and lower polydispersity of rdLMWH-1 and -2 (1.35–1.4 for enoxaparin and dalteparin compared with 1.0 for rdLMWH-1 and -2). Based on the increased potency of rdLMWH-1 and -2 in vitro and in vivo, we anticipated that rdLMWH-1 and -2 should be more effective in preventing and/or treating thrombosis formation. We directly tested this hypothesis in two settings, namely, in animal models of venous and arterial thrombosis. In the former situation, we anticipated that the potent anti-Xa activities of rdLMWH-1 and -2 would make these molecules effective inhibitors of venous thrombosis formation. In the latter situation, we expected the potent anti-IIa activities to play an important role in inhibiting arterial thromboses.
Figure 2.
Pharmacokinetics of enoxaparin, dalteparin, rdLMWH-1, and rdLMWH-2. The anti-Xa activity of enoxaparin (○), dalteparin (▵), rdLMWH-1 (●), or rdLMWH-2 (▴) was measured after s.c. administration at 3 mg/kg.
Table 2.
Pharmacokinetic parameters of LMWH after s.c. injection of 3 mg/kg
| Parameter | Enoxaparin | Dalteparin | rdLMWH-1 | rdLMWH-2 |
|---|---|---|---|---|
| ka, h−1 | 0.48 ± 0.08 | 0.40 ± 0.02 | 0.34 ± 0.01 | 0.49 ± 0.04 |
| ke, h−1 | 0.31 ± 0.02 | 0.35 ± 0.03 | 0.29 ± 0.01 | 0.41 ± 0.04 |
| t1/2,a, h | 1.5 ± 0.2 | 1.7 ± 0.1 | 2.04 ± 0.04 | 1.4 ± 0.1 |
| t1/2,e, h | 2.3 ± 0.2 | 2.0 ± 0.2 | 2.39 ± 0.03 | 1.7 ± 0.2 |
| AUC, IU⋅h/ml | 6.4 ± 0.4 | 7.5 ± 0.6 | 15 ± 2 | 9.2 ± 0.8 |
| Cmax, IU/ml | 0.92 ± 0.08 | 1.1 ± 0.2 | 1.9 ± 0.2 | 1.6 ± 0.1 |
| tmax, h | 2.6 ± 0.1 | 2.6 ± 0.2 | 3.2 ± 0.1 | 2.2 ± 0.2 |
| MRT, AUMC/AUC | 4.9 ± 0.2 | 4.6 ± 0.4 | 5.3 ± 0.2 | 3.9 ± 0.4 |
ka, absorption rate constant; ke, elimination rate constant; AUC, area under the curve; AUMC, area under the moment curve; MRT, mean residence time; Cmax, maximum plasma concentration; tmax, time to Cmax.
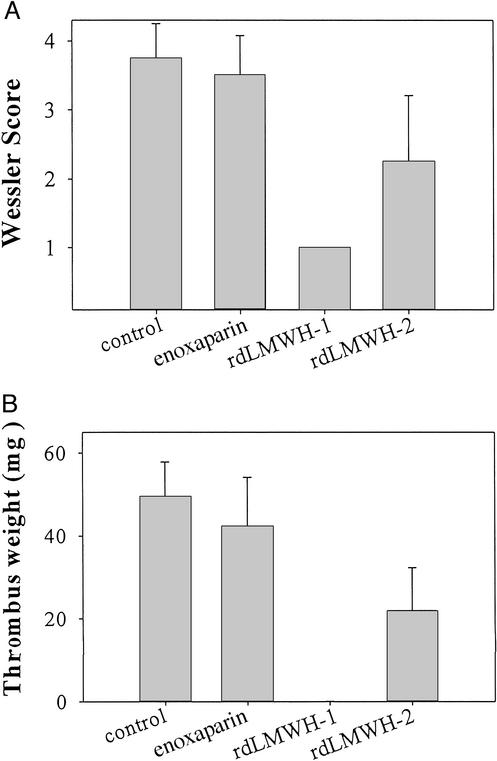
rdLMWH-1 and -2 Inhibit Venous Thrombosis.
Clinically, LMWHs and the pentasaccharide, agents with high anti-Xa activity, are used in the prevention and treatment of venous thrombosis. Therefore, as a direct comparison to existing agents, we tested the ability of rdLMWH-1 and -2 to inhibit venous thrombosis in a rabbit model (Fig. 3) and compared their inhibitory activity to enoxaparin as well as a saline control. At equivalent weight doses of 0.05 mg/kg, rdLMWH-1 and -2 were more potent at inhibiting venous thrombosis formation than enoxaparin, as measured by the Wessler score (Fig. 3A) and thrombus weight (Fig. 3B). Notably, the rdLMWHs are at least as potent as, if not more potent than, enoxaparin when dose matching studies were completed (5 IU/kg, data not shown). Together, these findings illustrate that rdLMWH-1 and -2 are potent anti-Xa agents able to efficiently inhibit the formation of thrombi. Given their potent in vitro activity, we sought to explore whether rdLMWH-1 and -2 would be capable of inhibiting thrombosis formation in other circumstances, specifically arterial thrombosis, for which existing LMWHs are currently not effective inhibitory agents.
Figure 3.
Inhibition of venous thrombosis by LMWH. Enoxaparin, rdLMWH-1, or rdLMWH-2 (0.05 mg/kg each) were measured for their ability to inhibit venous thrombosis and compared with a saline control (far left bar). Clot formation was measured on the basis of the 0–4 Wessler score (A) and the weight of the resulting thrombus (B).
rdLMWH-1 and -2 Are Potent Inhibitors of Arterial Thrombosis.
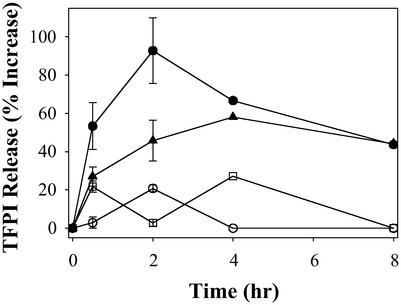
Accumulating evidence indicates that factor VIIa, activated by tissue factor, is a key initiator of arterial thrombosis in vivo (31). TFPI is a potent modulator of this arm of the coagulation pathway. It exerts its function by neutralizing the catalytic activity of factor Xa as well as through feedback inhibition of the factor VIIa/tissue factor complex (32). In addition to their well-studied ability to promote the inhibitory activity of AT-III, heparin and LMWHs may also mediate the release of TFPI from endothelial cells. Given the importance of TFPI release in the overall function of pharmacological doses of heparin and the LMWHs, we sought to measure the effect of rdLMWH-1 and -2 on TFPI release in vivo. We measured the activity of TFPI in the plasma after s.c. administration of rdLMWH-1, rdLMWH-2, heparin, or enoxaparin. To establish a release profile, plasma samples were collected at different time points and tested.
Compared with heparin and enoxaparin, s.c. administration of rdLMWH-1 or -2 is associated with a more pronounced release of TFPI into the circulation (Fig. 4). The peak TFPI activity is reached ≈4 h after s.c. administration. TFPI activity is also elevated in the plasma from heparin-treated animals, albeit to a lesser extent, whereas enoxaparin (or dalteparin) resulted in a minimal increase of plasma TFPI activity (33). These results strongly suggest that the administration of rdLMWH-1 or -2 is associated with superior mobilization of TFPI from the endothelium as compared with other heparin-derived molecules.
Figure 4.
TFPI release from the endothelium after the administration of enoxaparin (○), heparin (□), rdLMWH-1 (●), or rdLMWH-2 (▴).
Thus, given the pronounced anti-IIa activity of rdLMWH-1 and -2, coupled with their ability to release efficiently TFPI from the endothelium, we reasoned that these molecules might be potent inhibitors of arterial thrombosis in vivo. Therefore, we tested the ability of heparin, enoxaparin, rdLMWH-1, and rdLMWH-2 to inhibit arterial thrombosis in an animal model (Fig. 5).
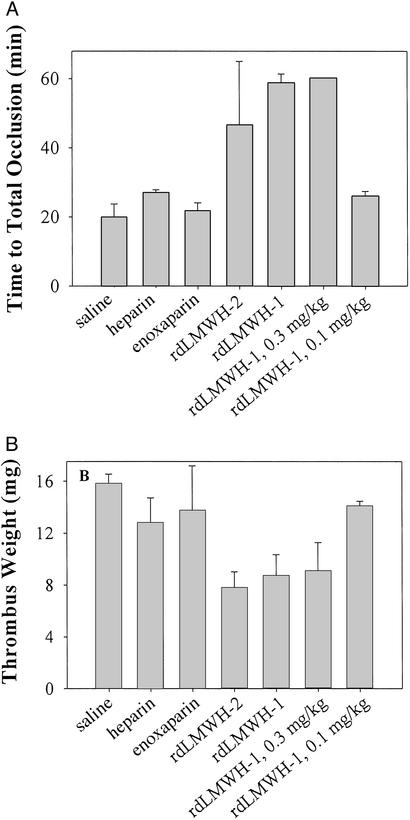
Figure 5.
Antithrombotic activity of heparin, enoxaparin, rdLMWH-1, and rdLMWH-2 in a rat arterial thrombosis model. The ability of 0.5 mg/kg enoxaparin, rdLMWH-1, or rdLMWH-2 to inhibit arterial thrombosis was assessed by measurement of TTO (A) and thrombus weight 60 min after the administration of thrombus stimulus (FeCl3) (B). Lower doses of rdLMWH-1, namely 0.3 and 0.1 mg/kg, were also used (two rightmost bars).
At 0.5 mg/kg, heparin prolonged the TTO to ≈27 min compared with 17 min for the control group (saline injection) (Fig. 5A). A weaker inhibition was also observed for 0.5 mg/kg enoxaparin (TTO = 23 min). Inhibition of thrombus formation was also observed in the final thrombus weight (Fig. 5B). In contrast, at the same dose of 0.5 mg/kg, rdLMWH-1 completely prevented thrombi occlusion of the artery. In this case, the blood flow rate never reached 0 within the 60-min observation window. This result is also reflected by the significantly reduced thrombus weight at the end of 60 min. At 0.3 mg/kg, essentially the same response was observed, namely complete occlusion never occurred within the observation window. At 0.1 mg/kg, the TTO and thrombus weight of rdLMWH-1-treated group became comparable to those observed for heparin at 0.5 mg/kg. rdLMWH-2 was also an extremely potent inhibitor of arterial thrombosis formation, more so than heparin or enoxaparin but less so than rdLMWH-1, as expected from its lower anti-IIa activity in vitro and in vivo compared with rdLMWH-1. Taken together, the results represented here confirm the notion that a higher anti-IIa activity is associated with more potent inhibition of arterial thrombosis formation. Furthermore, to our knowledge, rdLMWH-1 and -2 represent the first LMWHs that can efficiently inhibit this process, more so than even heparin itself.
rdLMWH-1 and -2 Are Efficiently Neutralized by Protamine.
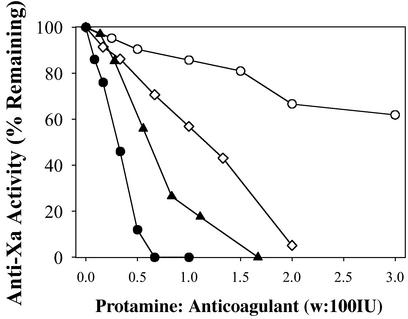
In addition to their lack of significant anti-IIa activity, another drawback of LMWHs, like enoxaparin, is that they are not easily neutralized through the addition of polycationic peptides, like protamine (30, 34). Thus, the potent anticoagulant activity of LMWHs cannot be easily reversed in the event of bleeding complications or for its safe use in other indications. This inability has been attributed, at least in part, to the polydispersity and length of LMWHs like enoxaparin and dalteparin. Therefore, we reasoned that rdLMWH-1 and -2, because of their lower polydispersity and optimal length, might be more effectively reversed by agents like protamine. To test this hypothesis, equivalent anti-Xa activity doses (i.e., 600 IU) of heparin, enoxaparin, rdLMWH-1, and rdLMWH-2 in plasma were titrated with increasing amounts of protamine in plasma (Fig. 6). We found that rdLMWH-1 and -2 are more efficiently neutralized with protamine than is heparin. In addition, the activity of enoxaparin is not efficiently neutralized at any level of protamine used in this study, consistent with clinical observations (12). Taken together, these results indicate that not only do rdLMWH-1, and -2 demonstrate superior efficacy in vitro and in vivo, but also the effects of these molecules are readily reversed by the addition of protamine.
Figure 6.
In vitro neutralization of anticoagulant heparin (⋄), enoxaparin (○), rdLMWH-1 (●), or rdLMWH-2 (▴) with protamine (w is in milligrams).
rdLMWH-1 and -2 Are Potent Anticoagulants.
Anticoagulation has been the primary clinical application for heparin for 65 years. Because of its erratic pharmacokinetics after s.c. administration, heparin has been administered instead by i.v. injection (35). Additionally, the application of heparin as an anticoagulant has been hampered by the many side effects associated with nonspecific plasma protein binding to it (35). Therefore, it is important to develop an LMWH that retains the anticoagulant activity of heparin but has reduced side effects. Previously, because of the lack of a structural correlate to the anticoagulant function of heparin, it was not possible to create molecularly tailored LMWHs. Herein, we demonstrate that quantification of a specific tetrasaccharide signature can be an accurate predictor of anticoagulant function and that this discovery enables the creation of potent LMWHs. The rationally designed LMWHs reported here couple the strengths of heparin with those of the LMWHs, like enoxaparin and dalteparin. First, while rdLMWH-1 and -2 have a lower molecular weight than heparin and thus are in the traditional molecular weight range for LMWHs, these molecules possess high anti-Xa activity and enriched anti-IIa activity, two to three times that of heparin on a mass basis. As a result of the enriched anti-Xa and IIa activity for rdLMWH-1 and -2, these molecules are more effective anticoagulants than are conventional LMWHs. In addition, because of their enriched activity and lower polydispersity, rdLMWH-1 and -2 do not suffer from some of the limitations of currently available LMWHs, including reduced susceptibility to protamine neutralization. Importantly, these second-generation LMWHs appear clinically attractive because they are potent inhibitors of both arterial and venous thrombosis. In addition, because they provide a multifocal inhibition of the coagulation cascade, rdLMWH-1 and -2 may hold more clinical promise than molecules, such as direct antithrombins or Xa-only inhibitors, that inhibit only one arm of the coagulation cascade.
Supplementary Material
Acknowledgments
We thank V. Sasisekharan for valuable comments and suggestions. We acknowledge financial assistance to R.S. from the Burroughs Wellcome Foundation, the Arnold and Mabel Beckman Foundation, the CapCure Foundation, and National Institutes of Health Grants GM 57073, CA 90940, and HL 59966. M.S., Y.Q., Z.S., D.L., G.V., R.L., and R.S. hold equity in Momenta Pharmaceuticals, which possesses certain patents in this area.
Abbreviations
- AT-III
antithrombin III
- LMWH
low-molecular weight heparin
- TFPI
tissue factor pathway inhibitor
- TTO
time to total hexosamine occlusion
- rdLMWH-1
-2, second-generation LMWH-1, -2
- IU
international unit(s)
- H
hexosamine
- I
α-l-iduronic acid
- G
β-d-glucuronic acid
- ΔU
a Δ4,5-uronic acid
- 2S
3S, and 6S, 2-O, 3-O, and 6-O sulfation, respectively
- NS and NAc
N-sulfation and N-acetylation of the glucosamine
References
- 1.Hirsh J, Lee A Y. Blood. 2002;99:3102–3110. doi: 10.1182/blood.v99.9.3102. [DOI] [PubMed] [Google Scholar]
- 2.Jin L, Abrahams J P, Skinner R, Petitou M, Pike R N, Carrell R W. Proc Natl Acad Sci USA. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shriver Z, Raman R, Venkataraman G, Drummond K, Turnbull J, Toida T, Linhardt R, Biemann K, Sasisekharan R. Proc Natl Acad Sci USA. 2000;97:10359–10364. doi: 10.1073/pnas.97.19.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petitou M, Imberty A, Duchaussoy P, Driguez P A, Ceccato M L, Gourvenec F, Sizun P, Herault J P, Perez S, Herbert J M. Chemistry. 2001;7:858–873. doi: 10.1002/1521-3765(20010216)7:4<858::aid-chem858>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Casu B, Torri G. Semin Thromb Hemostasis. 1999;25, Suppl. 3:17–25. [PubMed] [Google Scholar]
- 6.Bendetowicz A V, Beguin S, Caplain H, Hemker H C. Thromb Haemostasis. 1994;71:305–313. [PubMed] [Google Scholar]
- 7.Mureebe L, Silver D. Vasc Endovascular Surg. 2002;36:163–170. doi: 10.1177/153857440203600302. [DOI] [PubMed] [Google Scholar]
- 8.Lubenow N, Kempf R, Eichner A, Eichler P, Carlsson L E, Greinacher A. Chest. 2002;122:37–42. doi: 10.1378/chest.122.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Samama M M, Gerotziafas G T. Semin Thromb Hemostasis. 2000;26, Suppl. 1:31–38. doi: 10.1055/s-2000-9497. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser B, Hoppensteadt D A, Fareed J. Expert Opin Investig Drugs. 2001;10:1925–1935. doi: 10.1517/13543784.10.11.1925. [DOI] [PubMed] [Google Scholar]
- 11.Coccheri S. Haemostasis. 1990;20, Suppl. 1:74–80. doi: 10.1159/000216163. [DOI] [PubMed] [Google Scholar]
- 12.Makris M, Hough R E, Kitchen S. Br J Haematol. 2000;108:884–885. doi: 10.1111/j.1365-2141.2000.1902_2.x. [DOI] [PubMed] [Google Scholar]
- 13.Rusconi C P, Scardino E, Layzer J, Pitoc G A, Ortel T L, Monroe D, Sullenger B A. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- 14.Pojasek K, Shriver Z, Hu Y, Sasisekharan R. Biochemistry. 2000;39:4012–4019. doi: 10.1021/bi992514k. [DOI] [PubMed] [Google Scholar]
- 15.Ernst S, Langer R, Cooney C L, Sasisekharan R. Crit Rev Biochem Mol Biol. 1995;30:387–444. doi: 10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]
- 16.Ernst S, Venkataraman G, Winkler S, Godavarti R, Langer R, Cooney C L, Sasisekharan R. Biochem J. 1996;315:589–597. doi: 10.1042/bj3150589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataraman G, Shriver Z, Raman R, Sasisekharan R. Science. 1999;286:537–542. doi: 10.1126/science.286.5439.537. [DOI] [PubMed] [Google Scholar]
- 18.Ampofo S A, Wang H M, Linhardt R J. Anal Biochem. 1991;199:249–255. doi: 10.1016/0003-2697(91)90098-e. [DOI] [PubMed] [Google Scholar]
- 19.Shriver Z, Sundaram M, Venkataraman G, Fareed J, Linhardt R, Biemann K, Sasisekharan R. Proc Natl Acad Sci USA. 2000;97:10365–10370. doi: 10.1073/pnas.97.19.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knobloch J E, Shaklee P N. Anal Biochem. 1997;245:231–241. doi: 10.1006/abio.1996.9984. [DOI] [PubMed] [Google Scholar]
- 21.Teien A N, Lie M, Abildgaard U. Thromb Res. 1976;8:413–416. doi: 10.1016/0049-3848(76)90034-7. [DOI] [PubMed] [Google Scholar]
- 22.Teien A N, Lie M. Thromb Res. 1977;10:399–410. doi: 10.1016/0049-3848(77)90150-5. [DOI] [PubMed] [Google Scholar]
- 23.Rapaport S I. Thromb Haemostasis. 1991;66:6–15. [PubMed] [Google Scholar]
- 24.Hansen J B, Svensson B, Olsen R, Ezban M, Osterud B, Paulssen R H. Thromb Haemostasis. 2000;83:937–943. [PubMed] [Google Scholar]
- 25.Aronson D L, Thomas D P. Thromb Haemostasis. 1985;54:866–870. [PubMed] [Google Scholar]
- 26.Wessler S. Cardiovasc Clin. 1971;3:1–16. [PubMed] [Google Scholar]
- 27.Kurz K D, Main B W, Sandusky G E. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- 28.Neises B, Broersma R J, Tarnus C, Piriou F, Remy J M, Lintz C, Heminger E F, Kutcher L W. Bioorg Med Chem. 1995;3:1049–1061. doi: 10.1016/0968-0896(95)00097-z. [DOI] [PubMed] [Google Scholar]
- 29.Van Ryn-McKenna J, Cai L, Ofosu F A, Hirsh J, Buchanan M R. Thromb Haemostasis. 1990;63:271–274. [PubMed] [Google Scholar]
- 30.Racanelli A, Hoppensteadt D A, Fareed J. Semin Thromb Hemostasis. 1989;15:386–389. doi: 10.1055/s-2007-1002735. [DOI] [PubMed] [Google Scholar]
- 31.Ragni M, Golino P, Cirillo P, Scognamiglio A, Piro O, Esposito N, Battaglia C, Botticella F, Ponticelli P, Ramunno L, Chiariello M. Circulation. 2000;102:113–117. doi: 10.1161/01.cir.102.1.113. [DOI] [PubMed] [Google Scholar]
- 32.Hansen J B, Sandset P M, Huseby K R, Huseby N E, Bendz B, Ostergaard P, Nordoy A. Br J Haematol. 1998;101:638–646. doi: 10.1046/j.1365-2141.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 33.Bendz B, Andersen T O, Sandset P M. Blood Coagul Fibrinolysis. 2000;11:343–348. doi: 10.1097/00001721-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Fareed J, Walenga J M, Hoppensteadt D, Huan X, Racanelli A. Haemostasis. 1988;18, Suppl. 3:3–15. doi: 10.1159/000215861. [DOI] [PubMed] [Google Scholar]
- 35.Breddin H K. Semin Thromb Hemostasis. 1999;25, Suppl. 3:83–89. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.