Abstract
Mutations in the serine-threonine kinases WNK1 and WNK4 [with no lysine (K) at a key catalytic residue] cause pseudohypoaldosteronism type II (PHAII), a Mendelian disease featuring hypertension, hyperkalemia, hyperchloremia, and metabolic acidosis. Both kinases are expressed in the distal nephron, although the regulators and targets of WNK signaling cascades are unknown. The Cl− dependence of PHAII phenotypes, their sensitivity to thiazide diuretics, and the observation that they constitute a “mirror image” of the phenotypes resulting from loss of function mutations in the thiazide-sensitive Na–Cl cotransporter (NCCT) suggest that PHAII may result from increased NCCT activity due to altered WNK signaling. To address this possibility, we measured NCCT-mediated Na+ influx and membrane expression in the presence of wild-type and mutant WNK4 by heterologous expression in Xenopus oocytes. Wild-type WNK4 inhibits NCCT-mediated Na-influx by reducing membrane expression of the cotransporter (22Na-influx reduced 50%, P < 1 × 10−9, surface expression reduced 75%, P < 1 × 10−14 in the presence of WNK4). This inhibition depends on WNK4 kinase activity, because missense mutations that abrogate kinase function prevent this effect. PHAII-causing missense mutations, which are remote from the kinase domain, also prevent inhibition of NCCT activity, providing insight into the pathophysiology of the disorder. The specificity of this effect is indicated by the finding that WNK4 and the carboxyl terminus of NCCT coimmunoprecipitate when expressed in HEK 293T cells. Together, these findings demonstrate that WNK4 negatively regulates surface expression of NCCT and implicate loss of this regulation in the molecular pathogenesis of an inherited form of hypertension.
Keywords: protein serine-threonine kinases‖hypertension‖thiazide-sensitive Na–Cl cotransporter‖ion transport‖medical genetics
Hypertension is the most common disease in industrialized societies, affecting >20% of the adult population and contributing to morbidity and mortality from stroke, myocardial infarction, renal failure, and congestive heart failure (1). Its pathogenesis is largely unknown, resulting in empiric pharmacologic therapy. In recent years, genetic approaches investigating rare Mendelian forms of high and low blood pressure have provided fundamental insight into mechanisms that contribute to blood pressure variation (2). These have demonstrated the causal role of inherited variation in renal salt homeostasis in blood pressure variation, with mutations in many genes known to play a role in mediating or regulating renal salt reabsorption resulting in altered blood pressure.
Pseudohypoaldosteronism type II (PHAII; Online Mendelian Inheritance in Man database no. 145260) is an autosomal dominant disease featuring hypertension with hyperkalemia despite normal glomerular filtration rate; renal tubular acidosis is a variable associated finding. The clinical features of this disease are chloride dependent and are also corrected with thiazide diuretics, specific antagonists of the Na–Cl cotransporter (NCCT) of the distal convoluted tubule (3–7). We have recently demonstrated (8) that PHAII is caused by mutations in either of two serine-threonine kinases, WNK1 and WNK4 [with no lysine (K) at a key catalytic residue]. PHAII-causing mutations in WNK1 are large deletions in the first intron of the gene that appear to increase WNK1 expression. Mutations in WNK4 are missense mutations in highly conserved segments remote from the kinase domain (8). Both kinases are present in the kidney, with their expression confined to the distal convoluted tubule, connecting tubule, and collecting duct; these nephron segments are known to play a key role in the regulation of salt, K+, and pH homeostasis (8). These findings implicate WNK1 and WNK4 in a previously unrecognized signaling pathway that regulates the balance between Cl− reabsorption versus K+ and H+ secretion. Nonetheless, the upstream regulators and the downstream molecular targets of these kinases are presently unknown, leaving unresolved the question of their normal physiologic role and the mechanism by which their mutation results in the observed PHAII phenotypes.
One attractive target for the WNK kinases is the thiazide-sensitive NCCT. This cotransporter mediates the apical reabsorption of Na+ with Cl− and is expressed predominantly in the distal convoluted tubule (9, 10). Consequently, the expression of WNK4 and NCCT overlap in epithelial cells of the distal nephron. Moreover, we have previously shown that loss-of-function mutations in NCCT cause Gitelman's syndrome, a disease featuring a phenotype that is the mirror image of PHAII, with reduced blood pressure, hypokalemia, and metabolic alkalosis (11). Coupled with the exquisite sensitivity of PHAII phenotypes to thiazide diuretics, these observations suggest that PHAII could result from increased activity of the NCCT due either to loss of normal inhibition or constitutive activation by mutant WNK kinases. We now demonstrate that the wild-type WNK4 kinase is a negative regulator of the thiazide-sensitive NCCT and that WNK4 mutations found in patients with PHAII abrogate this inhibitory function. This provides an explanation by which mutations in WNK4 impart their physiologic effect and reveals aspects of a new signaling pathway involved in blood pressure and electrolyte homeostasis.
Methods
Assembly of cDNA Constructs.
The complete coding sequence of mouse WNK4 was amplified by PCR from first-strand mouse kidney cDNA in two overlapping segments of ≈2 kb. The fragments were combined by PCR to yield a full-length WNK4 cDNA that was directly cloned into pcDNA3.1− (Invitrogen) by ligation into the KpnI and EcoRV sites of the vector. A hemagglutinin A (HA) epitope tag was introduced in-frame at the carboxyl terminus of WNK4 by PCR with ligation into pcDNA3.1− to generate WNK4-HA. The function of WNK4 with and without the HA epitope was no different in effects on Na+ flux and surface expression (data not shown). Mutant WNK4-HA constructs (kinase-dead D318A and PHAII Q562E and E559K) were generated using the QuikChange site-directed mutagenesis system (Stratagene). A pSPORT1 clone containing enhanced green fluorescent protein (EGFP) (12) fused in-frame to the 5′ end of rat NCCT as described (13) was used for quantitation of NCCT expression. cRNA was transcribed in vitro from linearized plasmids by using the T7 mMESSAGE mMACHINE system (Ambion, Austin, TX) and quantitated by UV spectroscopy.
For immunoprecipitation studies, full-length mouse WNK4 was subcloned into pEF1/Myc-His A (Invitrogen), which added a Myc epitope to the carboxyl terminus of WNK4. A construct containing the intracytoplasmic carboxyl terminus of NCCT with the V5 epitope at the C terminus was prepared by amplification of amino acids 605-1021 of NCCT (GenBank accession no. X91220) from human kidney cDNA by using specific primers and cloning the product into pcDNA3.1D/V5-His (Invitrogen). All constructs were verified by sequence analysis.
Na+ Transport Measurements.
Oocytes were isolated from adult Xenopus laevis by using standard procedures (14). Stage V–VI oocytes were injected with 25 ng of NCCT cRNA alone or together with 25 ng of wild-type or mutant WNK4-HA cRNA in a total volume of 50 nl. Oocytes were incubated at 18°C for 3 days in ND96 supplemented with sodium pyruvate (2.5 mM) and gentamicin (5 mg/ml); on the fourth day oocytes were transferred to a Cl−-free ND96 medium (96 mM sodium isethionate/2.0 mM potassium gluconate/1.8 mM calcium gluconate/1.0 mM magnesium gluconate/5.0 mM Hepes/Tris, pH 7.4). 22Na+ uptake was assessed in groups of 15–20 oocytes 4 days after injection as described (15). In brief, oocytes were incubated for 30 min in a Cl−-free ND96 medium with bumetanide (0.1 mM), followed by a 60-min uptake period in a K+-free NaCl medium containing ouabain, amiloride, bumetanide, and 2.5 μCi (1 Ci = 37 GBq) of 22Na+ per ml (NEN; ref. 15). Thiazide sensitivity of 22Na influx was assessed by measuring 22Na+ uptake in paired groups of oocytes with or without metolazone (0.1 mM) in the incubation and uptake media. All experiments were performed at 32°C. At the end of the uptake period, oocytes were washed five times in ice-cold uptake solution without isotope to remove extracellular fluid tracer. After the oocytes were dissolved in 10% SDS, tracer activity was determined for each oocyte by β-scintillation counting. Flux measurements were made using 26–75 oocytes from at least two frogs in each group, with the exception of the “kinase-dead” WNK4 group, for which 10 oocytes were studied. For each injection series, the mean 22Na-influx value for NCCT alone was set at 100%, and other values were expressed as percentage of this value.
Surface Expression Measurements.
Oocytes were injected with 40 ng of EGFP-NCCT cRNA alone or together with 40 ng of wild-type or mutant WNK4-HA cRNA. Oocytes were incubated for 3–4 days at 18°C in ND96 solution supplemented with penicillin and streptomycin. Membrane surface expression of EGFP-NCCT was assayed by laser-scanning confocal microscopy with an LSM410 microscope (Zeiss) as described (13). Excitation was performed at 488 nm, and fluorescent emissions were detected through a 515- to 565-nm band-pass filter. Fluorescent images of equatorial sections of injected and uninjected oocytes were captured using a ×10 objective lens. Brightness and contrast settings were kept constant during imaging of all oocytes in each injection series, which always included an EGFP-NCCT-alone control for comparison. Total membrane fluorescence intensity was calculated for each imaged oocyte by using sigmascan pro software (Jandel, San Rafael, CA). Surface expression measurements were made using a total of 45–80 oocytes from at least two different frogs for each experimental condition. For each injection series, the mean fluorescence value for NCCT alone was set at 100%, and other values were expressed as percentage of this value.
Statistical Methods.
The significance of differences in 22Na influx and NCCT expression between groups of oocytes was assessed by two-tailed Student's t test in which a P value of 0.05 was considered significant.
Transient Transfections and Immunoprecipitation.
HEK 293T cells were cultured at 37°C under a 5% CO2/95% air atmosphere in DMEM (Life Technologies, Grand Island, NY) supplemented with 2 mM l-glutamine, streptomycin, penicillin, 1 mM sodium pyruvate, and 10% FCS. Transient transfections were performed by CaPO4 precipitation with 10–20 μg of plasmid DNA, with cells grown to 70% confluency. After transfection, cells were incubated in Ultracho media (BioWhittaker) with penicillin and streptomycin for 48 h; cells were then washed in cold PBS and lysed at 4°C in lysis buffer [10 mM Tris⋅HCl, pH 8.0/2.5 mM MgCl2/5 mM EGTA, pH 8.0/0.5% Triton X-100/1 mM Na3VO4/50 mM NaF, one tablet of protease inhibitor mixture (Roche Molecular Biochemicals) per 10 ml of buffer]. Lysates were cleared by centrifugation, and the supernatant was used for immunoprecipitation. For each immunoprecipitation, 1 μg of mouse monoclonal anti-V5 (Invitrogen), rabbit polyclonal anti-myc (Santa Cruz Biotechnology), or rabbit polyclonal anti-HA (CLONTECH) antibody was coupled to 30 μl of rec-Protein G-Sepharose (Zymed) for 1 h at 4°C. The resulting antibody-protein G-Sepharose was resuspended in PBS, added to the lysates, and incubated overnight at 4°C. Immunoprecipitates were washed with PBS, and bound protein was eluted by boiling for 5 min in 2× SDS sample buffer.
Western Blotting.
Lysates and immunoprecipitated proteins were fractionated using 4–15% SDS/PAGE gradient gel electrophoresis (Bio-Rad). Proteins were transferred to poly(vinylidene difluoride) (PVDF) membrane (Bio-Rad) at 100 V and 4°C for 2 h. The membrane was blocked in 5% nonfat dried milk in PBS. Primary antibodies were diluted in 1% milk in PBS and incubated with the membrane for 1 h at room temperature. Blots were washed in PBS with 0.1% Tween 20 and probed with an HRP-conjugated secondary antibody (Zymed) in 1% milk in PBS for 30 min at RT. The filter was then washed and chemiluminescence performed using ECL-Plus (Amersham Pharmacia), following standard protocols.
Results
The full-length mouse WNK4 cDNA was cloned as described in Methods, and the sequence has been deposited in GenBank (accession no. AY187027). The encoded protein is 86% identical to human WNK4 and is shorter than the human ortholog by 21 aa (GenBank accession no. AF390018). This cDNA structure is different from GenBank accession no. XM_109998, which was deduced from genomic sequence and indicates a coding sequence that begins within the highly conserved kinase domain.
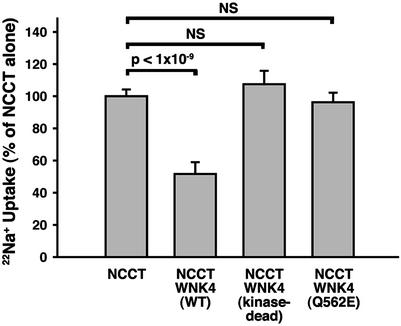
To assess the potential effect of WNK4 on Na–Cl flux mediated by NCCT, we injected Xenopus oocytes with combinations of cRNA of NCCT and wild-type or mutant WNK4 and determined the resulting 22Na influx into oocytes. Injection of NCCT cRNA alone (Fig. 1) resulted in a 7-fold increase of metolazone-sensitive 22Na influx into oocytes compared with water-injected controls. Coinjection of NCCT with wild-type WNK4 resulted in a 50% reduction of the 22Na influx seen with NCCT alone (Fig. 1). This suppression was reproducible in 74 oocytes from five different frogs and was highly statistically significant (P < 1 × 10−9). This finding demonstrates the ability of wild-type WNK4 to inhibit the activity of the cotransporter.
Figure 1.
Effect of WNK4 on 22Na influx mediated by NCCT in Xenopus oocytes. Oocytes were injected with cRNA encoding NCCT and wild-type or mutant WNK4; 22Na entry was measured as described in Methods. The injected cRNAs are indicated and the mean and standard error of 22Na influx is shown for each set of oocyte injections; for each set, the mean 22Na influx seen among oocytes injected with NCCT cRNA alone is expressed as 100%, and other injection series are expressed as a percentage of this value.
To determine whether the inhibition of NCCT by WNK4 depends on the catalytic function of the WNK4 kinase domain and to rule out trivial explanations of this inhibitory effect, we prepared a kinase-dead mutant of WNK4 in which aspartate 318 in the highly conserved kinase domain is mutated to alanine. Aspartate is conserved within the catalytic domain of virtually all serine-threonine kinases, because the carboxyl group of this amino acid is essential for Mg2+ binding and catalytic function (16). This mutant WNK4 showed no inhibition of NCCT-mediated 22Na influx, demonstrating the dependence of WNK4 suppression on kinase domain function (Fig. 1).
Is the observed effect of WNK4 on NCCT of physiologic relevance? Mutations in WNK4 that cause PHAII are clustered in a short, highly conserved segment remote from the kinase domain and raise the question as to whether WNK4 harboring these missense mutations loses the ability to inhibit NCCT-mediated 22Na influx in the oocyte. To address this question, we have tested the effect of WNK4 harboring the PHAII-causing missense mutation Q562E (corresponding to the human Q565E). This mutation is seen only in PHAII and precisely segregates with the disease in PHAII kindred 13 (8). WNK4 Q562E showed no inhibition of NCCT-mediated 22Na influx in oocytes, demonstrating the functional significance of this mutation (Fig. 1); 22Na influx was significantly higher than that seen with wild-type WNK4 and not significantly different from the result seen with the kinase-dead WNK4 or in the absence of WNK4 altogether. Other nearby WNK4 mutations that cause PHAII, such as E559K (E562K in human WNK4), also show loss of inhibition of NCCT function (data not shown).
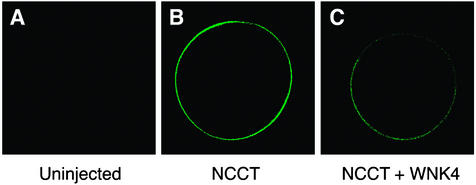
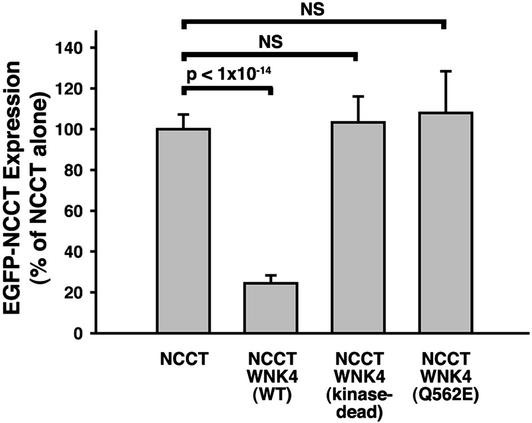
These experiments establish that wild-type WNK4 inhibits NCCT function, and that PHAII mutations eliminate this effect. To address the mechanism of this inhibition, we measured expression of EGFP-tagged NCCT (EGFP-NCCT) in Xenopus oocytes by using fluorescence confocal microscopy after injection of NCCT alone or in combination with various WNK4 constructs (Fig. 2). EGFP-NCCT is almost entirely localized to the plasma membrane (Fig. 2B). This surface expression is markedly reduced by addition of wild-type WNK4 (Fig. 2C). Quantitation of this effect indicates a 75% reduction in NCCT expression in the presence of WNK4 (Fig. 3; P < 1 × 10−14). As was found for 22Na influx, this inhibitory effect of WNK4 is abolished when kinase domain function is disrupted by the D318A mutation or when WNK4 bears the PHAII-causing Q562E missense mutation (Fig. 3). Similar results were obtained with WNK4 E559K (data not shown). These findings indicate that WNK4 inhibits NCCT function by reducing the amount of NCCT present at the cell surface.
Figure 2.
Effect of WNK4 on expression of NCCT in Xenopus oocytes. Confocal microscopy of Xenopus ooctyes expressing EGFP-tagged NCCT was performed in the presence and absence of expression of wild-type WNK4 as described in Methods. Representative examples of fluorescence seen in the absence of EGFP-NCCT (A), after expression of EGFP-NCCT alone (B), and after expression of EGFP-NCCT with wild-type WNK4 (C) are shown. The results demonstrate reduced expression of EGFP-NCCT at the cell surface after expression of WNK4.
Figure 3.
Quantitation of expression of EGFP-NCCT in response to wild-type and mutant WNK4. Oocytes were injected with cRNA encoding EGFP-tagged NCCT and wild-type or mutant WNK4; green fluorescence was quantitated by confocal microscopy of oocytes as described in Methods. The injected cRNAs are indicated, and the mean and standard error of fluorescence is shown for each set of oocyte injections; as in Fig. 1, for each set of injections, the mean fluorescence seen among oocytes injected with NCCT cRNA alone is expressed as 100%, and other injection series are expressed as a percentage of this value.
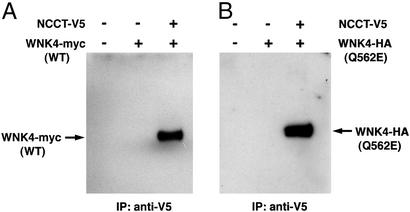
To address the specificity of the effect of WNK4 on NCCT, we have asked whether these proteins can be found in a complex in mammalian cells. We transfected expression plasmids encoding full-length WNK4 tagged with the myc epitope and the cytoplasmic C terminus of NCCT tagged with the V5 epitope into HEK 293T cells. Immunoprecipitation of NCCT with a monoclonal antibody directed against the V5 epitope was performed, and the precipitated protein complex was fractionated by SDS/PAGE. Staining with anti-myc antibody revealed a protein in the immunoprecipitate the size of the myc-tagged WNK4 construct (Fig. 4A). Detection of this protein depended on transfection of both plasmids (Fig. 4A) and was not detected in cells coexpressing V5 coupled to other peptides and tagged WNK4 (data not shown). Similarly, in the converse experiment, immunoprecipitation of myc-tagged WNK4 coprecipitated V5-tagged NCCT (data not shown). Finally, to determine whether the PHAII-causing mutation Q562E imparts its effect by preventing WNK4 from binding to the NCCT-containing complex, we repeated immunoprecipitation of V5-tagged NCCT in cells expressing tagged WNK4 harboring the Q562E mutation. This mutant WNK4 is still coprecipitated with NCCT (Fig. 4B), indicating that this mutation does not prevent the entry of WNK4 into a complex with NCCT.
Figure 4.
Coimmunoprecipitation of WNK4 and C terminus of NCCT. (A) Full-length wild-type WNK4 tagged with the myc epitope and the C terminus of NCCT tagged with the V5 epitope were expressed in HEK 293T cells as described in Methods. Cellular extracts were prepared, and immunoprecipitation was performed with anti-V5 antibodies as described in Methods. The precipitated protein was subjected to SDS/PAGE on 4–15% gradient gels, transferred to membrane, and stained with anti-myc antibody. A 170-kDa protein corresponding to myc-tagged WNK4 is detected in immunoprecipitates from cells expressing both NCCT and WNK4, but not in cells transfected with only one or neither tagged protein. (B) The experimental protocol is as in A, except that the Q562E mutation found in PHAII kindred 13 has been introduced into WNK4; this mutant WNK4 is tagged with the HA epitope rather than myc, and staining is with anti-HA antibody. Mutant WNK4 is coprecipitated by anti-V5 antibody only when both tagged NCCT and WNK4 are transfected.
Discussion
These studies establish that one function of wild-type WNK4 is to inhibit activity of the NCCT via reduced cell surface expression of the cotransporter. Na+-influx studies and measurement of membrane expression in all cases yield concordant results and are consistent with all of the inhibitory effects of WNK4 being attributable to loss of NCCT from the cell surface. This inhibitory effect of WNK4 depends on its kinase activity, because inhibition is lost in kinase-dead WNK4. The coimmunoprecipitation of the C terminus of NCCT and WNK4 indicates that these proteins can exist together in a complex in mammalian cells, consistent with the physiologic relevance of WNK4-mediated inhibition of NCCT; nonetheless, establishing the precise sites required for this interaction, whether this interaction is direct or indirect, and whether WNK4 directly phosphorylates NCCT will require further investigation. Importantly, the presence of both NCCT and WNK4 in epithelia of the mammalian distal nephron is consistent with the relevance of this interaction and inhibitory effect of WNK4 (see below). Whether WNK4 reduces NCCT surface expression by increasing removal of NCCT from the cell surface or by decreasing its delivery to the cell surface is presently uncertain. Preliminary experiments using dominant-negative dynamin (17) or dominant-negative amphiphysin 1 (18), agents that arrest endocytosis of cell surface proteins via clathrin-coated pits, demonstrate that down-regulation of NCCT by WNK4 is unaltered, suggesting that this pathway is not involved in the mechanism (data not shown).
Mutations in WNK4 that cause PHAII are located outside the kinase domain and are clustered within a short, well conserved segment (8). WNK4 bearing these missense mutations distal to the first coiled-coil domain loses the ability to inhibit NCCT expression. This finding establishes a specific biochemical consequence of these disease-causing mutations and implicates unrestrained activity of NCCT in the pathogenesis of PHAII. This inference is strongly supported by the clinical phenotypes of PHAII, which can potentially all be explained by increased NCCT activity (8). Increased NCCT activity is anticipated to increase net renal salt reabsorption, thereby expanding plasma volume and raising cardiac output resulting in hypertension; this pathophysiologic sequence is shared by other Mendelian forms of hypertension (2). The increased reabsorption of Na+ with Cl− by this cotransporter could reduce the amount of Na+ reabsorption via the electrogenic epithelial Na+ channel (ENaC) in the distal nephron, impairing development of the lumen-negative potential that is required for normal secretion of K+ and H+ in the distal nephron. Furthermore, the exquisite sensitivity of PHAII phenotypes to thiazide diuretics, specific antagonists of the NCCT, and the chloride-dependence of these phenotypes are also consistent with increased NCCT activity playing an important role in the pathophysiology of PHAII.
This physiologic explanation for PHAII raises several questions. First, is NCCT the sole target of WNK4? WNK4 is expressed in the distal convoluted tubule, the domain of NCCT expression, but is more strongly expressed in the collecting duct (8), suggesting that there may be additional targets. Moreover, WNK4 appears to localize predominantly in the tight junction complex, raising the question as to whether tight junction components might also be targets (8). Other possible targets include regulators or mediators of electrolyte flux such as paracellular Cl− flux mediators, the Na+ channel ENaC, the K+ channel ROMK (involved in distal K+ secretion), or subunits of the apical H+ ATPase (involved in distal H+ secretion). The possibility of additional targets is further supported by the unusual distribution of PHAII-causing mutations. To date, four different WNK4 mutations have been reported that cosegregate with PHAII. Three of these cluster within a 4-aa sequence that is embedded within a highly conserved 10-aa segment distal to the first coil domain of the protein; the fourth occurs in a similarly conserved segment just distal to the second coil domain (8). This highly clustered distribution of mutations is not what one would expect for general loss of function mutations, which would be expected to include missense mutations distributed throughout essential domains of the protein, as well as premature-termination, frameshift, and splice-site mutations. One possible resolution of this apparent paradox is that NCCT is only one of several WNK4 targets. In this case, mutations that knock out WNK4 function entirely could result in a broader phenotype than PHAII, and the mutations observed in PHAII kindreds might be among the small group that can selectively lose function at NCCT but preserve activity at other targets. Further investigation to establish the biochemical mechanism of these PHAII mutations will be required.
A companion question is whether increased NCCT function alone could effectively impair K+ and H+ secretion. Patients with PHAII eventually achieve salt balance, excreting the daily absorbed salt load, and in this state are delivering a normal salt load to the collecting duct; this is expected to permit normal K+ and H+ secretion. One possible explanation for the observed impaired K+ and H+ secretion is suppressed secretion of renin due to the expanded plasma volume, with the result that aldosterone secretion is not as high as it would normally be in the setting of hyperkalemia. A second possibility is raised by the observation that NCCT can be expressed beyond the distal convoluted tubule (19), suggesting that in PHAII, the domains of NCCT and ENaC expression could overlap. In this case, NCCT activity could directly attenuate the amount of Na+ reabsorbed by ENaC, thereby directly impairing K+ and H+ secretion.
Mutations in the related kinase WNK1 also cause PHAII. In contrast to the missense mutations in WNK4 that cause disease, mutations in WNK1 appear to be gain-of-function resulting from increased expression (8). This observation suggests that WNK1 and WNK4 likely have distinct biochemical mechanisms; however, the indistinguishable phenotype resulting from mutation in these two genes suggests that both act in the same pathway. Comparable experiments in the oocyte system with WNK1 will consequently be of interest.
Finally, these observations raise the question as to what the upstream regulators of WNK4 might be. One obvious possibility is aldosterone, which is known to increase NCCT expression in rats (20). Part of the mechanism of aldosterone action is increased transcription of the gene encoding the serine-threonine kinase SGK (21); interestingly, there are consensus SGK phosphorylation sites in WNK4, suggesting that phosphorylation of WNK4 by SGK might inhibit its activity, thereby resulting in increased NCCT expression. A second possibility is insulin signaling via the downstream serine-threonine kinase akt, which, interestingly, has the same substrate specificity and can share targets with SGK (22). These possibilities suggest that PHAII mutations may define a specific downstream branch shared by multiple signaling pathways.
Acknowledgments
We gratefully acknowledge Qiang Leng, Gordon MacGregor, Tony O'Connell, Ignacio Giménez, and members of the laboratory of Walter Boron for harvest of Xenopus oocytes; and Norma Vázquez for technical assistance. This work was supported in part by a National Institutes of Health Specialized Center of Research in Hypertension grant (to R.P.L.) and National Institutes of Health Grant DK36803 (to S.C.H. and G.G.). K.T.K. is the recipient of a Howard Hughes Medical Institute Medical Student Research Fellowship. R.P.L. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- PHAII
pseudohypoaldosteronism type II
- WNK
with no lysine (K)
- NCCT
Na–Cl cotransporter
- EGFP
enhanced GFP
- HA
hemagglutinin A
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY187027).
References
- 1.Mosterd A, D'Agostino R, Silbershatz H, Sytkowski P, Kannel W, Grobbee D, Levy D. N Engl J Med. 1999;340:1221–1227. doi: 10.1056/NEJM199904223401601. [DOI] [PubMed] [Google Scholar]
- 2.Lifton R, Gharavi A, Geller D. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 3.Paver W, Pauline G. Med J Aust. 1964;2:305–306. doi: 10.5694/j.1326-5377.1964.tb115766.x. [DOI] [PubMed] [Google Scholar]
- 4.Gordon R, Klemm S, Tunny T, Stowasser M. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 2111–2123. [Google Scholar]
- 5.Schambelan M, Sebastian F, Rector F., Jr Kidney Int. 1981;19:716–727. doi: 10.1038/ki.1981.72. [DOI] [PubMed] [Google Scholar]
- 6.Take C, Ikeda K, Kurasawa T, Kurokawa K. N Engl J Med. 1991;324:472–476. doi: 10.1056/NEJM199102143240707. [DOI] [PubMed] [Google Scholar]
- 7.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. J Clin Endocrinol Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 8.Wilson F, Disse-Nicodeme S, Choate K, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford D, Lipkin G, Achard J, et al. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 9.Ellison D, Velazquez H, Wright F. Am J Physiol. 1987;253:F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 10.Plotkin M, Kaplan M, Verlander J, Lee W, Brown D, Poch E, Gullans S, Hebert S. Kidney Int. 1996;50:174–183. doi: 10.1038/ki.1996.300. [DOI] [PubMed] [Google Scholar]
- 11.Simon D, Nelson-Williams C, Bia M, Ellison D, Karet F, Molina A, Vaara I, Iwata F, Cushner H, Koolen M, et al. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 12.Cormack B, Valdivia R, Falkow S. Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 13. Hoover, R., Poch, E., Monroy, A., Vázquez, N., Nishio, T., Gamba, G. & Hebert, S. (2003) J. Am. Soc. Nephrol., in press. [DOI] [PubMed]
- 14.Gamba G, Saltzberg S, Lombardi M, Miyanoshita A, Lytton J, Hediger M, Brenner B, Hebert S. Proc Natl Acad Sci USA. 1993;90:2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee W, Hediger M, Hebert S. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 16.Hanks S, Hunter T. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 17.Damke H, Baba T, Warnock D, Schimid S. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slepnev V, Ochoa G, Butler M, De Camilli P. J Biol Chem. 1999;275:17583–17589. doi: 10.1074/jbc.M910430199. [DOI] [PubMed] [Google Scholar]
- 19.Terada Y, Knepper M. Am J Physiol. 1990;259:F519–F528. doi: 10.1152/ajprenal.1990.259.3.F519. [DOI] [PubMed] [Google Scholar]
- 20.Kim G, Masilamani S, Turner R, Mitchell C, Wade J, Knepper M. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naray-Fejes-Toth A, Canessa C, Cleaveland E, Aldrich G, Fejes-Toth G. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Tang E, Zhu T, Greenberg M, Vojtek A, Guan K. J Biol Chem. 2001;276:31620–31626. doi: 10.1074/jbc.M102808200. [DOI] [PubMed] [Google Scholar]