Abstract
The present study stems from our previous observations that the brains of adult estrogen receptor β knockout (ERβ−/−) mice show regional neuronal hypocellularity especially in the cerebral cortex. We now show that ERβ is necessary for late embryonic development of the brain and is involved in both neuronal migration and apoptosis. At embryonic day (E)18.5, ERβ−/− mouse brains were smaller than those of the wild-type (WT) littermates, and there were fewer neurons in the cortex. There were no differences in size or cellularity at E14.5. When proliferating cells were labeled with 5′-bromodeoxyuridine (BrdUrd) on E12.5, a time when cortical neurogenesis in mice begins, and examined on E14.5, there was no difference between WT and ERβ−/− mice in the number of labeled cells in the cortex. However, when BrdUrd was administered between E14.5 and E16.5, a time when postmitotic neurons migrate to layers of the cortex, there were fewer BrdUrd-labeled cells in the superficial cortical layers by E18.5 and postnatal day 14 in mice lacking ERβ. At E18.5, there were more apoptotic cells in the ventricular zone of mice lacking ERβ. In addition, the processes of the cortical radial glia, which are essential for guiding the migrating neurons, were fragmented. These findings suggest that by influencing migration and neuronal survival, ERβ has an important role in brain development.
In the CNS of developing and adult mammals, estrogens have actions that extend far beyond the control of reproduction (1–3). Through its neurotrophic and differentiation-promoting effects, 17β-estradiol acting via estrogen receptors (ERs) α and β is crucial for the sexual differentiation of CNS structures and functions during a “critical period” of brain development that extends from the late prenatal period until the first 2 weeks after birth (1). 17β-Estradiol also stimulates neural differentiation and modulates neural survival both in vivo and in vitro (1, 4, 5) and promotes synapse formation and extension and branching of neurites of cortical neurons (6, 7).
The estrogen-synthesizing enzyme aromatase (8–10) as well as both nuclear ERα and ERβ are expressed in many areas of the developing brain of several species (11–14). In the developing mammalian brain, neurons destined to form the ordered layers of the cortex are generated in the ventricular and subventricular zones, lining the lateral ventricles, and must migrate along processes of the radial glia to their final destination. Cortical development begins with the formation of the preplate followed by the appearance of the cortical plate (CP), which is the precursor of most of the cortex (15). The period of neurogenesis in cortex in mice is between embryonic day (E)11 and E17 (16, 17), and during this period the majority of CP neurons are generated. The CP increases in thickness by the addition of neurons migrating radially from the ventricular zones. Radial migrations then establish the neuronal layers with neurons migrating beyond previously established layers to settle at progressively more superficial levels (18, 19). Thus, deep cortical layers V and VI are generated early, whereas progressively younger neurons form cortical layers IV, III, and II. This “inside-out corticogenetic gradient” is a general feature of the mammalian cortex (20).
After neuronal migration is completed, radial glial cells transform into stellate astrocytes. These cells are easily recognizable on immunohistochemical staining by their high content of the glial fibrillary acidic protein (GFAP). This highly organized pattern of migration of neurons in the CNS is controlled by a plethora of signal exchanges between glial cells and neurons during the course of nervous-system development. In has been known for some time that glial cells are indispensable for the development of the CNS (21), but recently their role has been shown to be even more complex with the evidence that radial glial cells can also generate neurons (22).
Because we observed an increased number of GFAP-immunopositive astrocytes in 2-month-old ERβ knockout (ERβ−/−) mice (23) and radial glial cells are the precursor of astrocytes, we speculated that ERβ might be important in the function of radial glial cells during brain ontogeny.
In this study we show evidence that ERβ is involved in migration of cortical neurons and thus controls cortical formation at a late stage in embryonic development.
Materials and Methods
Animals and Tissue Preparation.
ERβ−/− mice were generated as described (24). Heterozygous mice were used for breeding. ERβ+/− female mice were mated overnight with ERβ+/− males and inspected at 9:00 a.m. on the following day for the presence of vaginal plug. Noon of this day was assumed to correspond to E0.5. All animals were housed in the animal-care facility with a 12-h light/12-h dark photoperiod and given free access to tap water and rodent chow. To obtain embryos, pregnant mice were anesthetized deeply with CO2 and perfused with PBS followed by 4% paraformaldehyde (in 0.1 M PBS, pH 7.4). Embryos were taken out and put on ice, and heads or brains were dissected and postfixed in the same fixative overnight at 4°C. For the postnatal day (P)14 group, pups were perfused individually with PBS followed by 4% paraformaldehyde, and brains then were removed and postfixed overnight. Sex was determined after direct visual inspection of the gonads with a dissecting microscope, and the tail and limbs were removed from each embryo for genotyping. After fixation, brains were processed for either paraffin (6 μm) or frozen (30 μm) sections.
BrdUrd Labeling and Analysis.
Pregnant females were injected i.p. with 100 mg/kg BrdUrd in PBS (0.1 M, pH 7.4). For early developmental stage studies, BrdUrd was given at E12.5, and embryos were removed at E14.5. For late developmental stage studies, pregnant mice were injected with BrdUrd at E14.5 and E15.5 or E15.5 and E16.5 and killed at either E17.5 or E18.5. Two of the females were allowed to deliver pups, and pups were killed at P14. The paraffin-embedded brain sections were dewaxed in xylene, rehydrated, processed for antigen retrieval with 10 mM citrate buffer (pH 6.0), and then incubated in 2 M HCl for 10 min at room temperature. This was followed by neutralization in 0.05 M borate buffer (pH 8.5) for 15 min and blocking of endogenous peroxidase with 1% H2O2 for 30 min. Sections then were immunostained with an anti-BrdUrd monoclonal antibody (1:100, Becton Dickinson) overnight at 4°C followed by biotinylated goat anti-mouse secondary antibody (1:200, Vector Laboratories) and avidin-biotin peroxidase complex (1:100, Vector Laboratories) for 2 h at room temperature. After sections were washed in PBS, BrdUrd immunostaining was revealed by using 3,3-diaminobenzidine peroxidase reaction. The quantitative distribution of BrdUrd-positive cells in the cerebral cortex was determined from photomicrographs covering the superficial cortical layers of the somatosensory area. The number of BrdUrd-positive cells was counted on images in an area of 50 × 50 μm in the upper layer of the cerebral cortex in coronal sections (three animals per condition, three images each). All pictures were location-matched between ERβ−/− mice and their littermate controls. Statistical analysis was performed with Student's t test. To control embryonic day precisely, comparison was made between littermates. Both WT and heterozygous mice were used as controls, because no significant difference was found between them.
Histology and Immunohistochemistry.
In this study we used Nissl and hematoxylin/eosin staining to examine the histology of brains with light microscopy. Brain sections were blocked for 30 min with 1% H2O2 followed by 10% normal serum, rinsed three times with PBS, and incubated overnight with the antibodies microtubule-associated protein (MAP)-2B (1:250, Becton Dickinson), rat-401 monoclonal antibody (which recognizes nestin, 1:15, Development Studies Hybridoma Bank, Iowa City, IA), rabbit anti-GFAP (1:500, Santa Cruz Biotechnology), and calbindin D-28K (1:3,000, Swant, Bellinzona, Switzerland). These sections were processed further with biotinylated secondary antibodies, the avidin-biotin peroxidase complex, and diaminobenzidine as used in BrdUrd staining.
Detection of Apoptotic Cells.
The terminal deoxynucleotidyltransferase-mediated dUTP end-labeling (TUNEL) assay was used to label cells that are undergoing apoptosis. After antigen retrieval, brain sections were stained with TUNEL reaction mixture according to the manual (in situ cell death-detection kit, Roche Molecular Biochemicals). The sections were incubated at 37°C for 1 h, rinsed three times in PBS, and then incubated in bisbenzimide (1 μg/ml, Sigma) for 1 min at room temperature.
Apoptotic cells were identified by their TUNEL reactivity as well as their fragmented and condensed nuclei stained with bisbenzimide.
Results
Gross Anatomical and Histological Changes in Brains of ERβ−/− Mouse Embryos.
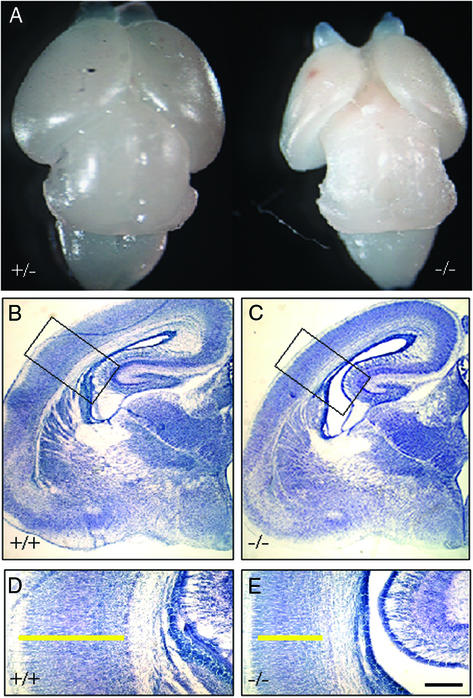
At E14.5, there were no discernable differences between the brains of ERβ−/− mice and their WT littermates in either size or gross morphology. Nissl staining of coronal brain sections showed that the cytoarchitecture was similar also (Fig. 1 A–D). However, at E18.5 the brains of ERβ−/− mice were clearly smaller than those of littermate controls (Fig. 2A). The retarded development was especially obvious in the cerebral cortex. Nissl staining showed clear evidence of a thinner cortex in the ERβ−/− mouse brains in both male and female mice. As shown in Fig. 2 B–E, no morphological differences were evident in the hippocampus, thalamus, and hypothalamus when ERβ−/− and WT mice were compared.
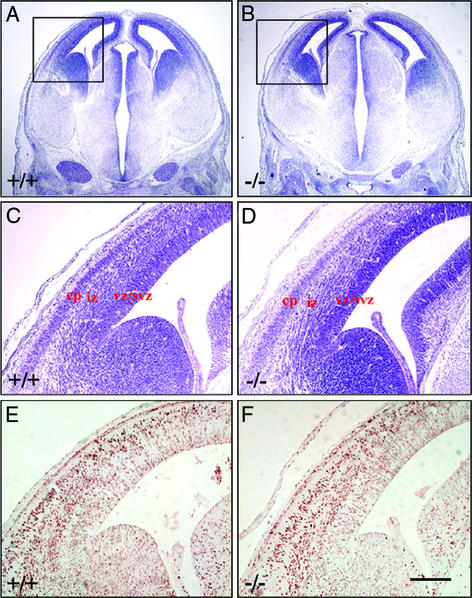
Figure 1.
Comparison of morphology and BrdUrd-labeling pattern in the brains of ERβ−/− mice and their WT littermates at E14.5. Embryos received BrdUrd injection at E12.5 and were examined at E14.5. Comparable coronal brain sections (6 μm, paraffin) were processed for Nissl (A–D) and BrdUrd (E and F) staining. At E14.5, the morphology is similar in the ERβ−/− (B and D) and control (A and C) brains. (C and D) Higher-power views of the boxed areas in A and B, respectively. vz/svz, ventricular zone/subventricular zone; iz, intermediate zone. At E14.5, BrdUrd-labeling patterns are also similar in the telencephalon of the ERβ−/− (F) and control (E) mice. (Scale bars: A and B, 12.5 μm; C–F, 50 μm.)
Figure 2.
Gross anatomy of the cerebral hemispheres in a male ERβ−/− mouse and a +/− littermate at E18.5. Note the retarded development of cerebral cortex in the ERβ−/− brain (A). Comparable coronal sections (30 μm, frozen) from females were processed for Nissl staining (B–E). (D and E) Higher-power views of the boxed areas in B and C, respectively. Note that the structures of the hippocampus, thalamus, and hypothalamus are similar in ERβ−/− and WT brain, but there are differences in the cortex. The width of the cerebral cortex in the ERβ−/− mouse (C and E) is clearly reduced when compared with that of WT mice (B and D). The yellow bars in D and E indicate the thickness of cerebral cortex. (Scale bars: B and C, 50 μm; D and E, 25 μm.)
When BrdUrd was administered on E12.5 and embryos were examined on E14.5, there was no difference between WT and ERβ−/− mice in the number or pattern of labeled neurons in the cortex (Fig. 1 E and F). This indicates that early cortical development is not affected by lack of ERβ. At E14.5 no distinguishable layers could be seen in the cortex, but it was easy to distinguish the laminar organization of the cerebral hemisphere as ventricular zones/subventricular zones, intermediate zones, and CP.
The Role of ERβ in Migration of Cortical Neurons.
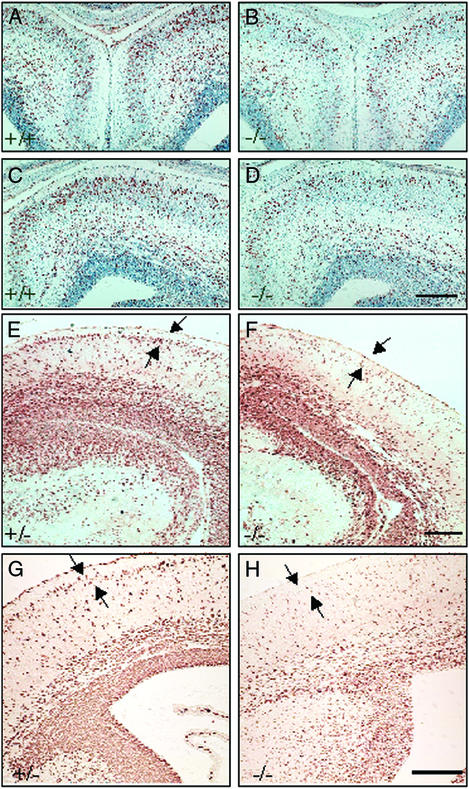
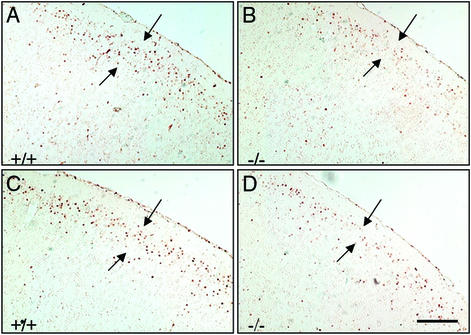
In the mouse, late-generated neurons destined to cortical layers II–III become postmitotic at E15–E17 (20, 25, 26). As shown by BrdUrd immunostaining, in WT mice many neurons generated at E14.5 and E15.5 have already settled down in the upper layers of the cortex by E17.5 and are distinguishable as a band of BrdUrd-labeled cells (Fig. 3 A and C). In contrast, in ERβ−/− mice most of the neurons labeled on E14.5 and E15.5 are still in the deep layers, and few BrdUrd-labeled cells are seen in the upper layers (Fig. 3 B and D). The same is true for neurons generated at E15.5 and E16.5 and observed at E18.5 (Fig. 3 E–H). To confirm further the fate of these neurons, the distribution of BrdUrd immunoreactivity was studied at P14. In both WT and ERβ−/− mice, almost all the BrdUrd-labeled cells had migrated to layers II–III. However, significantly fewer neurons, generated at E15.5 and E16.5, were found in the cortex of ERβ−/− mice. This was true for both male (Fig. 4 A and B) and female (Fig. 4 C and D) mice.
Figure 3.
Proliferation and migration of neurons in brains of ERβ−/− mice and their WT littermates. (A–D) Coronal sections (6 μm, paraffin) of the anterior telencephalon from male littermates were stained with anti-BrdUrd antibody and counterstained with hematoxylin. Cortical neurons generated at E14.5 and E15.5 and labeled with BrdUrd were analyzed at E17.5. Note fewer BrdUrd-labeled neurons in the ERβ−/− brain (B and D), especially the superficial layer of the cerebral cortex, compared with littermate control (A and C). (Scale bar, 50 μm.) Comparable coronal sections (6 μm, paraffin) from male (F) and female (H) ERβ−/− mice and their +/− littermates (E and G) are shown. Cortical neurons generated at E15.5 and E16.5 and labeled with BrdUrd were analyzed at E18.5. Note that the BrdUrd-labeled neurons in the ERβ−/− brain (F and H) are distributed diffusely in the cerebral cortex and are not organized into a well defined layer (arrows) at the superficial part in the cortex as in the control brains (E and G). (Scale bar, 50 μm.)
Figure 4.
Fate in the postnatal brain of neurons generated at E15.5 and E16.5. Neurons generated and labeled at E15.5 and E16.5 were analyzed at P14. Comparable coronal sections (6 μm, paraffin) from male (A and B) and female (C and D) littermates were stained with anti-BrdUrd antibody. Note the decreased numbers of BrdUrd-labeled neurons distributed in the lamina II–III of cortex (arrows) in ERβ−/− brains (B and D) compared with littermate controls (A and C). (Scale bar, 50 μm.)
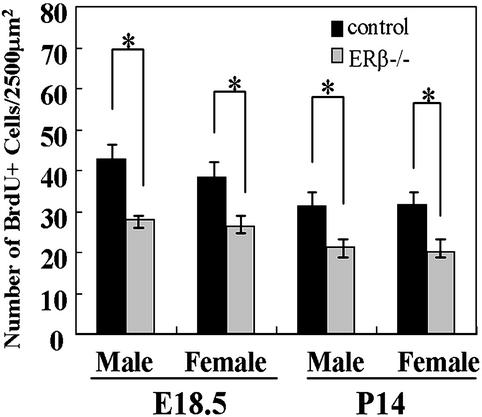
Quantitative comparison of the number of BrdUrd-immunopositive cells in multiple comparable sections showed that there were significantly fewer neurons in the upper layers (shown with arrows in Figs. 3 and 4) of the cerebral cortex of ERβ−/− than in littermate controls at E18.5 and P14 (Fig. 5).
Figure 5.
Numbers of BrdUrd (BrdU+)-labeled neurons. Quantitative analysis reveals that there is a significantly decreased number of neurons in the upper layer of cerebral cortex labeled by BrdUrd at E15.5 and E16.5 and analyzed at E18.5 and P14 in ERβ−/− mice compared with their littermate controls in both sexes. *, P < 0.01.
The architecture and organization of the radial glial scaffold was also examined in coronal sections of ERβ−/− and WT mouse cortex at developmental stage E18.5 (Fig. 6). Radial glia cells provide the structural framework that establishes neuronal patterning in the mammalian forebrain. At around the time of birth, these glial cells transform into astrocytes and can be identified by the expression of GFAP. Radial glia cells in the ERβ−/− mouse cortex at E18.5 were organized in an approximately radial pattern across the thickness of the cortex. The pattern of the radial scaffold emanating from them appeared to be misaligned and disorganized by the presence of astroglia-like cells. These cells stained positively for nestin but not GFAP. Their processes appeared as many short fragments interspersed among the radial glial processes (Fig. 6 B, D, and E).
Figure 6.
Abnormalities in radial glia in ERβ−/− mice. Antinestin antibodies were used to visualize radial glial cells. The radial scaffold formed by processes of radial glia are apparently truncated and appear as many short fragments, with more abundant end feet (*) in layer I in ERβ−/− brains (B and D) compared with their WT littermates (A and C). (C and D) Higher-power views of the boxed areas in A and B, respectively. More astroglial-like nestin-immunoreactive cells (arrow) appeared in ERβ−/− brains (E). (Scale bars: A and B, 50 μm; C and D, 25 μm; E, 5 μm.)
Another noticeable difference was in the neurons in layer I close to the pial surface. In ERβ−/− mice, the radial glial fibers formed more abundant end feet on these neurons (Fig. 6D). With an antibody against the calcium-binding protein calretinin, a marker for Cajal–Retzius cells, no differences in either the number or morphology of Cajal–Retzius cells between ERβ−/− and WT mice was evident (data not shown).
The Role of ERβ in Differentiation.
Microtubules play a crucial role in the development and structure of nerve cells. MAPs are important for the assembly and stability of microtubules during neurite outgrowth and for the morphology of neuronal processes such as dendrites. MAP-2B, a 280-kDa protein, is a neuronal antigen expressed throughout brain during development (27). At E18.5, most cortical neurons were MAP-2B-positive and immunoreactivity was more prominent in layer I. In the cortex of WT mice, neurons in the upper layers displayed in a clear laminar and radial formation (Fig. 7A), whereas in ERβ−/− mice, cortical neurons appeared to be orientated randomly and the upper layers were disorganized (Fig. 7C). In ERβ+/− mice, the structure was more similar to that seen in WT mice (Fig. 7B).
Figure 7.
Comparison of neuronal differentiation in ERβ−/− mice, ERβ+/− mice, and WT littermates. Anti-MAP-2B antibodies were used to visualize neurons in coronal sections (6 μm, paraffin). In WT mice (A), cortical neurons are tightly clustered in a radial pattern, whereas the marginal zone is less cellular and displays intense MAP-2B immunoreactivity. The structure of the ERβ+/− mice (B) is similar to that of WT mice. However, in ERβ−/− mice (C), the cortical neurons appear smaller and disorganized with no clear radial pattern formed. (Scale bar, 50 μm.)
The Role of ERβ in Apoptosis.
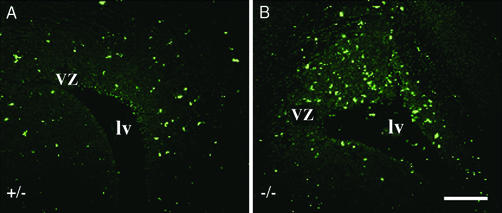
Although abnormalities in the processes of radial glia would be expected to result in the number of late-generated cortical neurons as is observed in the ERβ−/− mouse brain, other factors such as a decrease in cell proliferation and/or an increase in apoptosis could also contribute to this neuronal deficit. When the number of apoptotic cells was assessed by TUNEL assay at E18.5, there were clearly many more apoptotic cells in ventricular zone in ERβ−/− mice than in control littermates (Fig. 8). However, in both ERβ−/− and control mice there were few apoptotic cells in the cerebral cortex. These results indicate that fewer neurons, destined to reside in the cerebral cortex, reach their goal because of increased apoptosis in the ventricular zone in ERβ−/− mice.
Figure 8.
Comparison of apoptosis in ERβ−/− and littermate control brains. Comparable coronal sections (6 μm, paraffin) of ERβ−/− (B) and littermate (A) control brain at E18.5 were processed for the TUNEL reaction. Note that more apoptotic cells were detected in the ventricular zone but not cortex (not shown) in ERβ−/− brains. vz, ventricular zone; lv, lateral ventricle. (Scale bar, 50 μm.)
Discussion
Our previous study showed that in the cerebral cortex of brains of mice lacking ERβ, there is a significant neuronal deficit and an overabundance of astroglia cells (23). To understand the mechanism of this neuronal deficit further, we examined the ERβ−/− mouse brains for defects in neural development. Cortical neurogenesis in mice begins at ≈E11 and continues through E17. During early neurogenesis, very few progenitor cells in the neuroepithelium exit the cell cycle to become postmitotic neurons, whereas the vast majority of progenitor cells reenter the cell cycle after mitosis, resulting in a steady expansion of the neural progenitor population. As neurogenesis proceeds, a fraction of neural progenitor cells exit the cell cycle, differentiate into postmitotic neurons, and migrate to form the developing neocortex. Therefore, a reduction in the number of neurons in the cortex could occur via several possible mechanisms: decreased proliferation, increased apoptotic cell death, abnormal differentiation, and migration. Because the abnormalities in ERβ−/− mouse brains appear quite early, we speculated that there could be either exaggerated neuronal death and/or defective migration of neurons during development. In mammals, E12.5 marks the beginning of the formation of the CP. BrdUrd injected at E12.5 should label cortical neurons that are generated early in the developmental program. According to the “inside-outside” orchestrated layering, these neurons should be destined to a location in a deep layer of the cortex. Because the neuronal loss in the adult ERβ−/− mouse cortex was more pronounced in superficial layers than deeper layers (23), we speculated that neurons in the deep layer would be affected little by the lack of ERβ. In the present study, we examined this possibility.
Although ERβ is not required for survival of mice, at E18.5 the brains of ERβ−/− mice were smaller than those of their WT and heterozygous littermates. Histological analysis of E18.5 ERβ−/− mouse brains revealed an obvious defect in the cerebral cortex, which was characterized by a reduction in cortical thickness with no compensatory increase in cell density. Moreover, because at E14.5 there was no cortical defect, the retardation of development mainly occurs at later stages of corticogenesis.
Neuronal labeling with BrdUrd pinpointed the defect to the late stage of corticogenesis. In the cortex of ERβ−/− mice, neurons that were generated early migrated to the correct position as evidenced by formation of the clear CP indistinguishable from that in WT mice. However, neurons generated later and destined to layers II–IV were delayed in their migration in mice lacking ERβ. As the mice aged, the neurons labeled at E15.5 and E16.5 eventually did migrate to the correct position in the cortex, but there were far fewer of these neurons than were seen in control mice.
Recently, it was reported that ERβ mRNA was detected from E10.5 to E16.5 in mouse embryos, whereas the earliest detection of ERα mRNA was on E16.5 (28). These results indicate that ERβ could be the more important ER during early embryogenesis. A previous study (29) showed that in the developing cerebral cortex of rats, using an oligodeoxyribonucleotide probe encoding a sequence of the estrogen-binding domain of rat ERα cDNA, ERα mRNA was found to be expressed extensively in the ventricular zone, primitive plexiform layer, and immature CP at least as early as E16 (equal to mouse E14.5). During the first 3 postnatal weeks, cortical ΕRα mRNA expression was increasingly restricted to the upper third of the cerebral cortex and to the neurons of the cortical subplate (layer VIb/VII), and it decreased to low levels by P28. This pattern of ERα expression is quite in line with its expression in late generated cortical neurons. Autoradiographic studies with 11β-methoxy-16α-[125I]iodoestradiol also demonstrated the presence of estrogen target cells in postnatal mouse cerebral cortex and showed that an increase occurs first in the deep and later in the superficial laminae of the cortex between birth and P25 (30). Analysis of [3H]moxestrol uptake by radioautography and by cell nuclear isolation and counting of radioactivity revealed a marked increase in the number of ERs in the brains of mice during late fetal and early postnatal development (31). Although the distribution pattern of ERβ in the cerebral cortex during brain development is still unclear, previous data together with our BrdUrd-labeling study strongly suggest that ERβ might play an important role in regulation of migration of the upper laminae cortical neurons during perinatal life.
Radial glial cells have a function in neuronal migration and laminar patterning of the cortex. During the perinatal period they are transformed into astroglia (18, 19, 21, 32–34). We examined ERβ−/− and WT mouse brains at a late developmental stage for the presence of these two cell types. Few astroglial cells were seen in either ERβ−/− or WT mouse brains. Thus, a lack of ERβ did not hasten the maturation of glial cells into astroglia, and one can conclude safely that the increase in the number of astroglia in the adult ERβ−/− mouse brain occurs postnatally.
Although their maturation was not hastened by the lack of ERβ, the radial glial cells in cerebral cortex in ERβ−/− mice are morphologically different from those in WT mice. When stained for nestin, an intermediate filament shared by radial glial cells and neuronal precursors (35), the processes of the radial glial cells appear to be truncated or less organized into radial formations. Such abnormal processes might not be able to provide guidance for migrating neurons. The radial glia also have more branched end feet at the marginal zone, and this might result in abnormal contact with Cajal–Retzius cells. These cells are involved directly in the regulation of the radial glial phenotype (36) and are well known as the cells that express reelin (37, 38). When the reelin gene is disrupted (reeler mouse), there is abnormal migration and positioning of migrating neurons in the cerebral cortex (25, 39). Although the Cajal–Retzius cells themselves appear normal in ERβ−/− mice, their function in neuronal guidance in the absence of ERβ needs to be examined.
It will also be necessary to consider whether the recently reported role for radial glia in neurogenesis (22) is a factor in the cortical deficit in ERβ−/− mice. It remains possible that some of the neurons that die or end up in the wrong place could actually be derived from radial glial cells.
Structural malformation of the cortex was evident also when neurons were stained for MAP-2B, a marker specific for postmitotic neurons (40). In ERβ−/− mice, neurons stained positively for MAP-2B, indicating that the differentiation was normal. However, when neurons were visualized with this marker, it was clear that the cytoarchitecture of the cortex was disorganized. This disorganization might be closely related to the abnormality of the radial glia processes described above.
We also assessed by the TUNEL assay whether the decreased number of late-generated neurons in the ERβ−/− cortex was caused by neuronal death. Many more apoptotic cells were found in the proliferative zones in ERβ−/− mice, indicating that the lack of ERβ promotes apoptosis. Interestingly, in contrast to our data, an in vitro study showed that ERα is neuroprotective, whereas ERβ mediates the induction of apoptosis in neuronal cells (4). These apparently contradictory results might be due to many differences between the two experimental systems. Because no neuronal deficits have been reported in ERα−/− mice and ERα does not compensate for the loss of ERβ in CNS development, it seems that ERα does not have the same functions in the developing CNS as does ERβ.
Taken together, the results of our study have confirmed that there is a neuronal deficit in the cerebral cortex of adult ERβ−/− mice and this deficit is at least in part due to disturbances during development. The observed deficit in corticogenesis at the late developmental stage is due to abnormal neuronal migration and increased level of apoptotic neuronal death.
Acknowledgments
This work was supported by Loo och Hans Ostermans Stiftelse, AFA Sjukförsäkingsaktiebolags Jubileums Stiftelse, Fredrik och Ingrid Thurings Stiftelse, KaroBio AB Sweden, and the Swedish Cancer Fund.
Abbreviations
- ER
estrogen receptor
- CP
cortical plate
- En
embryonic day n
- Pn
postnatal day n
- GFAP
glial fibrillary acidic protein
- MAP
microtubule-associated protein
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP end labeling
References
- 1.Beyer C. Anat Embryol. 1999;199:379–390. doi: 10.1007/s004290050236. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B S, Alves S E. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Segura L M, Azcoitia I, DonCarlos L L. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 4.Nilsen J, Mor G, Naftolin F. J Neurobiol. 2000;43:64–78. doi: 10.1002/(sici)1097-4695(200004)43:1<64::aid-neu6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Meda C, Vegeto E, Pollio G, Ciana P, Patrone C, Pellicciari C, Maggi A. J Neuroendocrinol. 2000;12:1051–1059. doi: 10.1046/j.1365-2826.2000.00541.x. [DOI] [PubMed] [Google Scholar]
- 6.Brinton R D, Tran J, Proffitt P, Montoya M. Neurochem Res. 1997;22:1339–1351. doi: 10.1023/a:1022015005508. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Chang Y H, Barker J L, Hu Q, Maric D, Li B S, Rubinow D R. Neurosci Lett. 2000;281:57–60. doi: 10.1016/s0304-3940(99)00942-8. [DOI] [PubMed] [Google Scholar]
- 8.MacLusky N J, Walters M J, Clark A S, Toran-Allerand C D. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- 9.Lephart E D. Brain Res Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 10.Balthazart J, Ball G F. Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- 11.Keefer D, Holderegger C. Brain Res. 1985;351:183–194. doi: 10.1016/0165-3806(85)90190-7. [DOI] [PubMed] [Google Scholar]
- 12.Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H. J Clin Endocrinol Metab. 2001;86:2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- 13.Zsarnovszky A, Belcher S M. Brain Res Dev Brain Res. 2001;129:39–46. doi: 10.1016/s0165-3806(01)00180-8. [DOI] [PubMed] [Google Scholar]
- 14.Brandenberger A W, Tee M K, Lee J Y, Chao V, Jaffe R B. J Clin Endocrinol Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 15.Marin-Padilla M. Anat Embryol. 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, Nowakowski R S, Caviness V S., Jr J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyama S, Takahashi T, Nowakowski R S, Caviness V S., Jr Cereb Cortex. 1997;7:678–689. doi: 10.1093/cercor/7.7.678. [DOI] [PubMed] [Google Scholar]
- 18.Rikic P. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 19.Hatten M E. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 20.Angevine J B, Sidman R L. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 21.Lemke G. Annu Rev Neurosci. 2001;24:87–105. doi: 10.1146/annurev.neuro.24.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Gotz M, Hartfuss E, Malatesta P. Brain Res Bull. 2002;57:777–788. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Andersson S, Warner M, Gustafsson J A. Proc Natl Acad Sci USA. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J A, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caviness V S J. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 26.Smart I H, Smart M. J Anat. 1982;134:273–298. [PMC free article] [PubMed] [Google Scholar]
- 27.Kalcheva N, Albala J S, Binder L I, Shafit-Zagardo B. J Neurochem. 1994;63:2336–2341. doi: 10.1046/j.1471-4159.1994.63062336.x. [DOI] [PubMed] [Google Scholar]
- 28.Lemmen J G, Broekhof J L, Kuiper G G, Gustafsson J A, van der Saag P T, van der Burg B. Mech Dev. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- 29.Miranda R C, Toran-Allerand C D. Cereb Cortex. 1992;2:1–15. doi: 10.1093/cercor/2.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Shughrue P J, Stumpf W E, MacLusky N J, Zielinski J E, Hochberg R B. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- 31.Gerlach J L, McEwen B S, Toran-Allerand C D, Friedman W J. Brain Res. 1983;313:7–18. doi: 10.1016/0165-3806(83)90197-9. [DOI] [PubMed] [Google Scholar]
- 32.Voigt T. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- 33.Culican S M, Baumrind N L, Yamamoto M, Pearlman A L. J Neurosci. 1990;10:684–692. doi: 10.1523/JNEUROSCI.10-02-00684.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Rourke N A, Sullivan D P, Kaznowski C E, Jacobs A A, McConnell S K. Development (Cambridge, UK) 1995;121:2165–2176. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- 35.Frederiksen K, McKay R D. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Super H, Del Rio J A, Martinez A, Perez-Sust P, Soriano E. Cereb Cortex. 2000;10:602–613. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 38.Soriano E, Alvarado-Mallart R M, Dumesnil N, Del Rio J A, Sotelo C. Neuron. 1997;18:563–577. doi: 10.1016/s0896-6273(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 39.Caviness V S J, Sidman R L. J Comp Neurol. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 40.Crandall J E, Jacobson M, Kosik K S. Brain Res. 1986;393:127–133. doi: 10.1016/0165-3806(86)90072-6. [DOI] [PubMed] [Google Scholar]