Abstract
Young neurons born in the medial ganglionic eminence (MGE) migrate a long distance dorsally, giving rise to several types of interneurons in neocortex. The mechanisms that facilitate selective dorsal dispersion of MGE cells while restricting their movement ventrally into neighboring regions are not known. Using microtransplantation into fetal brain slices and onto dissociated substrate cells on floating filters (spot assay), we demonstrate that ventral forebrain regions neighboring the MGE are nonpermissive for MGE cell migration, whereas the dorsal regions leading to the neocortex are increasingly permissive. Spot assay experiments using filters with different pore sizes indicate that the permissive factors are not diffusible. We also show that MGE cells respond to chemoattractive and inhibitory factors diffusing from the neocortex and ventromedial forebrain, respectively. We propose that the final extent and regional specificity of MGE cell dispersion is largely dictated by contact guidance through a selectively permissive environment, flanked by nonpermissive tissues. In addition, we propose that chemotactic guidance cues superimposed over the permissive corridor facilitate efficient dorsal migration of MGE cells.
Young neurons born in the developing or adult brain have to migrate along precise pathways to find the correct sites for their final differentiation. This process is essential for proper brain development and function. Some young neurons travel long distances tangentially and settle in brain regions far from their birthplaces. The medial and lateral ganglionic eminences (MGE and LGE) are the best established sources of tangentially migrating neurons in the developing forebrain. Early in development, LGE cells migrate ventrally and anteriorly, giving rise to medium spiny neurons of the dorsal and ventral striatum (including the nucleus accumbens and olfactory tubercle; refs. 1–5) and to interneurons in the olfactory bulb (2, 3, 6). Proliferating neural stem cells derived from the LGE remain in the subventricular zone (SVZ) of the postnatal lateral ventricle (2), where they establish a germinal region, which persists into adulthood (7, 8). Young neurons born in the postnatal SVZ migrate rostrally to the olfactory bulb where they differentiate into granule and periglomerular interneurons (9, 10). It has been suggested that migration of LGE and SVZ cells is guided by repulsive factors of the Slit family secreted from the ventricular zone of the LGE in the embryo (11) and from the caudal septum (12) and choroid plexus (13) in the postnatal brain. Alternatively, it has been proposed that Slit factors normally inhibit SVZ cell migration and that this inhibitory effect is neutralized by factor(s) secreted from astrocytes along the migratory route (14).
In contrast to LGE cells, which migrate preferentially ventrally or anteriorly, MGE cells migrate dorsally and spread across most of the dorsal forebrain. Their main target is the developing neocortex (2, 15–18), but they also populate the dorsal striatum (19), amygdala, globus pallidus (2), and hippocampus (20). Interestingly, although MGE cells disperse across a relatively large region within the dorsal brain, they do not migrate into neighboring ventral regions (hypothalamus, preoptic area, septum, or olfactory bulb; ref. 2). The mechanisms restricting and guiding MGE cell migration into neocortex are not well understood. Recently, it has been proposed that MGE cells expressing low levels of semaphorin receptor neuropilin settle preferentially in the striatum (21). In addition, it has been reported that TAG-1 adhesion molecule expressed on corticofugal axons serves as a guidance cue for migrating MGE cells (22). However, many MGE cells migrate within the neocortical lower intermediate (or subventricular) zone, which is poor on corticofugal axons (2), suggesting that additional mechanisms might be involved in the guidance of MGE cells.
Here, we demonstrate that ventral forebrain regions neighboring the MGE are nonpermissive for MGE cell migration, whereas the dorsal regions leading to the neocortex are increasingly permissive. We propose that the extent of MGE cell dispersion is dictated by the pattern of permissive and nonpermissive areas in the developing forebrain. Moreover, we show that MGE cell migration is influenced by inhibitory factors secreted from the ventromedial forebrain and chemoattractive factors produced in the developing neocortex. We suggest that this chemotactic gradient might provide the directional information for the dorsal migration within the permissive corridor.
Materials and Methods
Transplantation in Slice Cultures.
Brains were dissected from 14.5-day-old (E14.5) mouse embryos in L-15 medium (GIBCO). Forebrains were cut transversally into 300- to 400-μm-thick sections. Slices containing hypothalamus, MGE, LGE, and cortex were placed on polycarbonate filters (0.4-μm pore size; Nuclepore) floating on NB medium [neurobasal medium supplemented with 2 mM l-glutamine, penicillin/streptomycin, and B27 supplement (all from GIBCO)]. Germinal zones of MGE were dissected, dissociated, and labeled with PKH26 fluorescent dye (Sigma) as described (2). Labeled MGE cells were collected by centrifugation, and pellets were cut into smaller explants (≈200 μm in diameter). MGE explants were inserted into different regions of embryonic brain slices on floating filters. Slices were cultured for 48 h and fixed in 3% paraformaldehyde, and the distribution of migrating MGE cells was analyzed.
Spot Assay.
Selected brain regions were dissected from E14.5 embryos, collected in Pipes buffer (20 mM Pipes, pH 7.4/120 mM NaCl/5 mM KCl/25 mM glucose), and triturated by repeated pipetting (25–35 times) using P200 pipette tips. Undissociated pieces were left to settle in the bottom of the tube, and dissociated cells were transferred into a new Eppendorf tube and collected by centrifugation (5 min, 800 × g). Cells were washed in NB medium supplemented with 5% horse serum and 10 μg/ml DNase I (Sigma), centrifuged and resuspended at a final concentration of 1–5 × 105 cells per μl in L-15 medium with DNase I. Cell suspension was spotted onto polycarbonate filters (≈1 μl per spot) floating on NB medium. PKH26-labeled MGE aggregates were placed in the center of each spot, and filters were cultured for 48 h, fixed, and photographed, and the extent of MGE cell migration was analyzed by using nih image v1.62 software. For flipped filter assays, cortical and hypothalamic cell suspensions were spotted onto 0.1-μm and 0.8-μm pore size filters (Nuclepore) and cultured for 7 days on the surface of NB medium supplemented with 5% horse serum. Filters were lifted and gently placed face down in a new 35-mm petri dish, 300 μl of fresh NB medium was added to the dishes, and PKH26-labeled MGE reaggregates were deposited on filters opposite to cultured tissue. As a control, some filters were maintained face up and MGE aggregates were placed directly on spots of cultured cells.
Coculture Assays.
Tested brain regions were dissected from E14.5 mouse embryos and cut into small explants (≈300–500 μm in diameter). MGE germinal zones were dissociated, reaggregated, and cut into explants as described above. MGE explants were embedded in a drop of collagen type I gel (Vitrogen; Cohesion Technologies, Palo Alto, CA) in the proximity (≈200–300 μm) of tested explants. Explants were cocultured for 24–30 h in NB medium, fixed with 3% paraformaldehyde, and photographed, and cell migration was analyzed by using the nih image software and its macro programming language. (Briefly, the software determined the center of MGE explant and drew through the center two perpendicular lines in such a way that they divided the MGE explant and migrating cells into four quadrants: proximal quadrant facing the tested tissue, distal quadrant facing away, and left and right quadrants. Finally, the software determined the number of MGE cells in each quadrant; calculated the percentage difference between the proximal and distal quadrants and the total number of migrating cells; and exported results to Microsoft excel for further statistical analysis). For some experiments, human embryonic kidney cells (HEK293) were transiently transfected with xSlit2 or Slit1 expression vectors (12). Transfected cells were pelleted by centrifugation, and pellets were allowed to reaggregate in culture overnight. Aggregates of HEK293 cells were cut into small explants and used in cocultures with MGE explants as described above.
In Situ Hybridization.
In situ hybridization, using digoxigenin-labeled riboprobes, was performed on 100-μm vibratome sections of E14.5 mouse embryos as described (23). Rat cDNAs encoding slit1 and -2 (24) were used as templates for riboprobe synthesis.
Results
Neocortex Is Selectively Permissive for MGE Cell Migration.
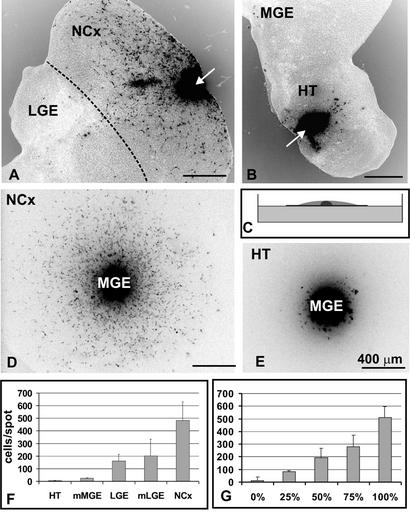
Using embryonic brain slices (17) as well as in utero fate mapping (2), we have previously reported that MGE cells migrate to the developing neocortex but avoid the ventromedial forebrain (for the purpose of this study we use the term ventromedial forebrain to describe regions into which MGE cells do not migrate; these regions include hypothalamus, preoptic area, septum, and olfactory bulb). Several mechanisms could explain these observations: (i) the ventral forebrain may be nonpermissive for MGE cell migration, (ii) there may be a nonpermissive boundary between the medial ganglionic eminence and the ventral forebrain, or (iii) ventromedial forebrain is permissive, but directional guidance cues instruct MGE cells to migrate dorsally. To distinguish between these three possibilities, we transplanted labeled E14 MGE cells ectopically into the neocortex and ventromedial forebrain in embryonic brain slices in vitro. After 2 days in vitro, large numbers of labeled cells (≈230 cells per slice) were observed dispersing from MGE grafts placed into the host neocortex, but only rarely did these cells migrate ventrally into the LGE (Fig. 1A). Graft-derived cells were bipolar, with a long, often bifurcated, leading process tipped with a prominent growth cone, a structure typical of tangentially migrating neuronal precursors (25, 26). In contrast, when MGE cells were transplanted into the host hypothalamus, thalamus or septum, none or only few cells migrated into the host tissue (fewer than five cells per slice; Fig. 1B). Occasionally, we observed short processes extending from MGE grafts, suggesting that MGE cells survived within the graft but were unable to migrate into ventromedial forebrain tissue. These results suggested that the developing diencephalon is largely nonpermissive for MGE cell migration.
Figure 1.
Different regions of the developing brain are differentially permissive for MGE cell migration. (A) PKH26-labeled MGE cells (black) were transplanted into neocortex in brain slice cultures (arrow). Forty-eight hours later, many MGE cells had dispersed throughout the neocortex. Note that only a few cells crossed the boundary back to the LGE (broken line). (B) MGE cells grafted into the hypothalamus remained at the graft site (arrow), unable to penetrate into the host brain tissue. (C) Diagram of the spot assay. Dissociated cells (gray) were spotted on polycarbonate membrane floating on the surface of culture medium in a petri dish, and a reaggregate of labeled MGE cells (black) was placed in the center of the spot. Cells were cultured for 48 h. (D) Labeled MGE cells (black) readily disperse through neocortical cells. (E) MGE cells do not migrate into a spot of hypothalamic cells. (F) Quantification of MGE cell migration through spots of different brain regions (number of cells per spot ± SD). (G) Migration of MGE cells through spots containing mixed neocortical and hypothalamic cells. The percentage indicates the amount of neocortical cells in a particular spot (number of cells per spot ± SD). NCx, neocortex; HT, hypothalamus; mMGE, mantle zone of the MGE; mLGE, mantle zone of the LGE. Scale bars = 400 μm.
Increasingly Permissive Pathway Connects MGE with Neocortex.
Microtransplantation experiments suggested that different embryonic brain regions have different capacities to support MGE cell dispersion. However, it was difficult to analyze MGE cell migration quantitatively after microtransplantation, because the different tested regions had different shapes and uneven cellular compositions. To assess the capacity of various embryonic brain regions to support migration of MGE cells, we developed a simple and easily quantifiable spot migration assay (Fig. 1 C–E). Briefly, the substrate neural tissue was dissected and dissociated, and high-density cell suspension was spotted onto floating filters in culture dishes. Labeled MGE cells were placed in the center of each spot, and the number of MGE cells migrating into the tested tissue was quantified after 48 h in culture. All results obtained from spot assays were confirmed by direct transplantation into brain slices, implying that spot assays can be used as quantitative indicators of permissiveness for MGE cell migration. Of all tested areas, the embryonic neocortex stands out as the best substrate for MGE cell migration (≈500 migrating MGE cells per spot; Fig. 1 D and F). Intermediate migratory support (150–300 cells per spot) was provided by the hippocampus, cerebellum (rhombic lip), LGE, and the mantle region of the LGE (mLGE, which consists mainly of the paleocortex; Fig. 1F, data not shown). The mantle region of the MGE (mMGE, which consists mainly of the ventral striatum), the entire hypothalamus, thalamus, septum, and midbrain did not support migration of MGE cells (<50 cells per spot; Fig. 1 E and F, data not shown). These results are consistent with the hypothesis that forebrain regions neighboring the ventral MGE are nonpermissive for MGE cell migration. In contrast, the region between the MGE and the cortex contains cells that provide an increasingly permissive migratory support for MGE cells.
Because we observed a graded capacity of different brain tissues to support MGE cell migration we wanted to assess whether different concentrations of permissive and nonpermissive factors could account for this result. We mixed cortical and hypothalamic cells in different ratios and measured the extent of MGE cell migration through the mixed spots. We found that, depending on the ratio of cortical to hypothalamic cells in the mixture, we could gradually change the extent of MGE cell migration, suggesting that neither one of the two environments dominated over the other (Fig. 1G). Thus, differential permissiveness of forebrain tissues could be achieved by changes in the concentration of permissive and/or nonpermissive factors in different brain regions.
Neocortical Permissive Factors Are Not Diffusible.
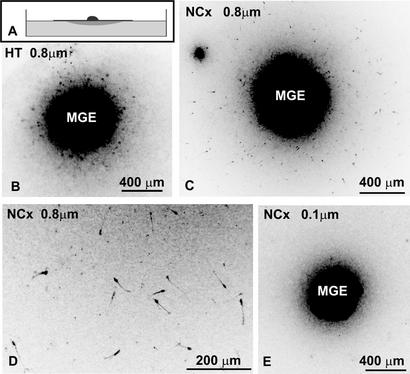
We wondered whether neocortical factors that permitted the migration of MGE cells are diffusible or associated with neocortical cells. We modified the spot migration assay by culturing neocortical and hypothalamic cells on polycarbonate membranes with either 0.8-μm pores (which allow direct cell–cell contact across the filter) or 0.1-μm pores (which allow only diffusion of secreted molecules). Cells were cultured for 7 days in vitro to allow deposition of extracellular molecules or extension of cellular processes through the pores in filters to the side facing culture medium. After this period, we flipped some of the filters and placed labeled MGE cells on the membrane opposite to the spots (Fig. 2A). As a control, we kept some filters right side up and placed MGE cells directly on the spots of neocortical or hypothalamic cells. Cultured neocortex and hypothalamus retained their specificity with respect to MGE cell migration. MGE cells dispersed through the cultured neocortical cells (130 ± 40 cells per spot) whereas almost no cells migrated through the hypothalamic cells (fewer than five cells per spot). On the flipped filters, there were no MGE cells migrating either on polycarbonate filters alone or on sides opposite to the hypothalamus regardless of the pore size (Fig. 2B). When MGE cells were placed on flipped 0.8-μm filters with cultured neocortical cells, MGE cells migrated throughout the area opposite to the neocortical spots (67 ± 34 cells per spot; Fig. 2 C and D). In contrast, no MGE cell migration was observed on the opposite sides of neocortical tissue cultured on the 0.1-μm filter (Fig. 2E). We concluded that MGE cells migrating through the neocortical tissue use migratory substrates associated with the surface of neocortical cells.
Figure 2.
The permissive nature of the neocortex is not mediated by diffusible molecules. (A) Diagram of the flipped spot assay. Dissociated cells were spotted on floating polycarbonate membranes and cultured for 7 days. Membranes were flipped upside down, and reaggregate of MGE cells was placed on the opposite side to the cultured spot of tissue. Cells were cultured for an additional 48 h. (B) PKH26-labeled MGE cells (black) do not migrate when placed opposite to hypothalamic tissue cultured on membranes with 0.8-μm pores. (C) Labeled MGE cells (black) disperse when placed opposite to neocortical tissue on membranes with 0.8-μm pores, which allow cell–cell contact. (D) Higher magnification of cells migrating opposite neocortical spot on 0.8-μm membrane. (E) MGE cells do not migrate when placed opposite to neocortical tissue on 0.1-μm membranes, which do not allow cell–cell contact. NCx, neocortex; HT, hypothalamus.
Chemotactic Guidance of MGE Cells.
To test whether long range diffusible factors were involved in the guidance of MGE cells through the permissive corridor, we cocultured MGE explants with pieces of developing neocortex (the target tissue where MGE cells migrate to) and hypothalamus (the neighboring tissue into which MGE cells do not migrate) embedded in collagen gels. To avoid the inherent asymmetry of MGE explants (which is caused by the asymmetrically located ventricular zone cells within explants), we dissociated and reaggregated MGE cells. Aggregates were cut into small explants and embedded next to the explants of tested tissue.
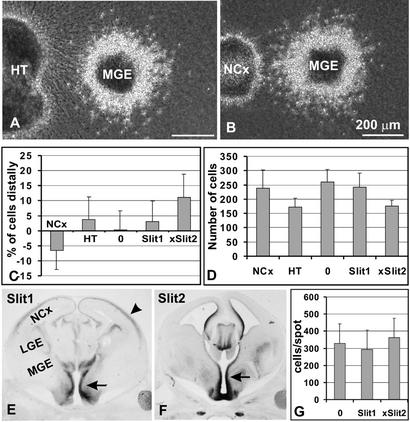
The dorsal hypothalamus and lateral neocortex were microdissected into explants that contained mainly the germinal zones, and explants that contained mainly the mantle regions. When MGE aggregates were cultured in the proximity (≈200 μm) of the germinal zones from the dorsal hypothalamus, significantly more cells were found in the distal quadrant (53.6%) than in the proximal (46.4%; n = 17, t test P = 0.03; Fig. 3 A and C). To determine whether factors diffusing from the hypothalamus are chemorepulsive or inhibitory, we compared the absolute numbers of migrating MGE cells. The total number of neurons emerging from MGE aggregates cultured in the proximity of hypothalamic germinal zones was significantly lower than the number of MGE cells migrating alone in collagen gel or cocultured next to HEK293 cells (human embryonic kidney cell line used as a heterologous cell type, which did not affect MGE cell migration in the coculture assay; P < 0.01, t test; Fig. 3D), suggesting that hypothalamic explants reduce the motility of MGE cells. Moreover, the number of MGE cells emerging at the distal quadrant was significantly lower (44 ± 14) than the number of distal MGE cells cocultured with HEK293 cells (62 ± 12; P < 0.01, t test). These findings allow us to conclude that factors secreted from the hypothalamus act as inhibitors of MGE cell motility rather than as chemorepulsive factors (in which case more MGE cells would be observed in the distal quadrant as compared with control explants and the total number of migrating cells would be unchanged).
Figure 3.
Diffusible molecules influence MGE cell migration. (A) Coculture of MGE reaggregate with hypothalamic explant. More MGE cells migrate distally than proximally to the explant. (B) Coculture of MGE reaggregate with neocortical explant. More MGE cells migrate proximally than distally. (C) Quantification of the difference in the number of cells found in the distal and proximal quadrant (percentage difference ± SD). (D) Quantification of the total number of MGE cells migrating out of the explant (number of migrating cells ± SD). (E) In situ hybridization reveals strong expression of Slit1 in the hypothalamic region (arrow) next to the MGE. Slit1 is also weakly expressed in cortical plate (arrowhead), the target area of MGE cell migration. (F) In situ hybridization reveals strong expression of Slit2 in the hypothalamic region (arrow). (G) Mixing of neocortical cells with HEK293 cells expressing Slit1 or Slit2 does not result in suppression of MGE cell migration in the spot assay (the inhibitory activity of Slit-expressing cells was confirmed in coculture assays) (number of cells per spot ± SD). NCx, neocortex; HT, hypothalamus.
When MGE was cultured adjacent to germinal zones of the neocortex, significantly more cells were detected in the proximal (56.6%), compared with the distal, quadrant (43.4%; n = 15, P = 0.02, t test; Fig. 3 B and C). The total number of cells emerging from MGE aggregates cultured close to the neocortical germinal zones (239 ± 62) was not significantly different from the number of cells emerging from control aggregates (259 ± 45, P = 0.7, t test; Fig. 3D). Because the distal quadrant contained fewer cells (50 ± 13) than the control distal quadrant (62 ± 12), while the proximal quadrant contained more cells (67 ± 21) as compared with the control proximal quadrant (61 ± 13), we concluded that the neocortex releases a chemoattractive factor.
Slit Factors Are Potential Inhibitory Cues Secreted from the Ventromedial Forebrain.
Slit molecules were previously identified as potent chemorepulsive factors guiding axonal outgrowth (23, 27–29) and migration of postnatal SVZ and embryonic LGE cells (11–13). Moreover, it has been shown that Slit factors inhibit postnatal SVZ cell motility (14). We therefore decided to analyze the expression pattern of Slit factors in the developing hypothalamus. It has been reported that both Slit1 and Slit2 are strongly expressed in the embryonic septum (29), preoptic area (23), thalamus, and epithalamus (30, 31), where they act as guidance cues for the olfactory bulb and retinal ganglion cell efferent axons. To complement these studies, we analyzed the expression of Slit molecules in hypothalamic regions directly adjacent to the MGE. In situ hybridization revealed a strong expression of both Slit1 and Slit2 in the periventricular regions in the hypothalamus neighboring the MGE (Fig. 3 E and F). Therefore, we decided to directly test whether MGE cell migration is directional in a gradient of Slit factors.
Mouse Slit1 (Slit1) and Xenopus Slit (xSlit2) [which is homologous to mouse Slit2 (12)] were transiently expressed in HEK293 cells. When MGE cells were placed next to reaggregates of mock-transfected HEK293 cells, MGE cell migration was symmetrical (Fig. 3C). In contrast, MGE cells cocultured next to cells transfected with either Slit1 or xSlit2 migrated asymmetrically (Fig. 3C). This effect was more pronounced with xSlit2 (n = 17, t test P < 0.001). The total number of cells migrating from MGE reaggregates was significantly decreased in cocultures with cells expressing xSlit2 as compared with mock-transfected HEK293 cells (P < 0.01; Fig. 3D). Similarly, like in cocultures with hypothalamic germinal zones, there were fewer cells in the distal quadrant as compared with controls. These findings demonstrate that MGE cells can respond to a gradient of Slit factors similarly as they respond to hypothalamic germinal zone explants in vitro, indicating that Slit factors secreted from the hypothalamus, preoptic area or septum might provide guidance for MGE cells in vivo.
xSlit2 Is Not Sufficient To Create a Nonpermissive Environment.
The ability of Slit molecules to reduce MGE cell motility prompted us to examine whether Slit factors might be responsible for the nonpermissive nature of the ventromedial forebrain. We analyzed the ability of xSlit2 to block MGE migration within cortical cells by using the spot migration assays. When neocortical cells were mixed with mSlit1-transfected, xSlit2-transfected, or mock transfected HEK293 cells (3:1) and spotted on floating filters, there was no significant difference in the extent of MGE cell migration (Fig. 3G). In contrast, migration of MGE cells in neocortical tissue, which is mixed 3:1 with hypothalamic cells, is reduced by ≈49% (Fig. 1G). These findings indicate that Slit factors alone are not sufficient to generate the nonpermissive environment encountered in the ventromedial forebrain.
Discussion
The minimum requirement for cell migration is the presence of a permissive environment that allows random dispersion of migratory cells. The overall extent and shape of cell dispersal can be influenced by boundaries between permissive and nonpermissive environments. Directional migration can be achieved by two general principles: contact guidance and diffusible gradients (32). Contact guidance relies on an increasingly permissive environment connecting the source of migrating cells with the target area. Directional migration within regions of uniform permissiveness can be achieved by superposition of gradients of diffusible guidance cues over the permissive region.
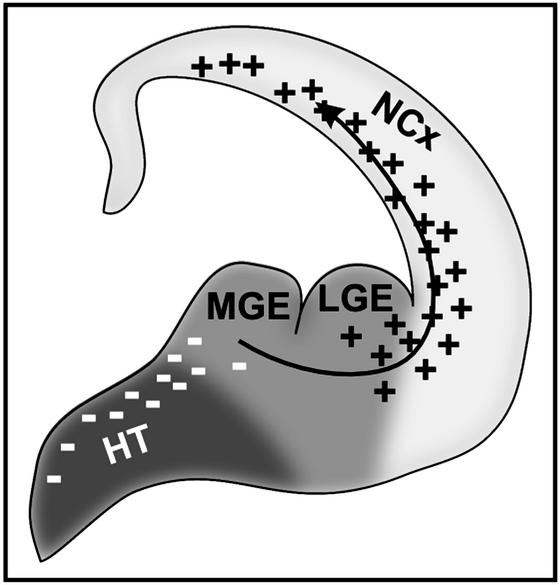
In contrast to the relatively extensive body of work focusing on diffusible molecules involved in the guidance of cell migration, the distribution and molecular nature of permissive and nonpermissive substrates in the developing central nervous system remain poorly understood. Here, we have developed a simple assay that facilitated mapping of permissive substrates for MGE cell migration in the developing forebrain. We demonstrated that all major forebrain areas into which MGE cells normally do not migrate are nonpermissive for MGE cells. Moreover, an increasingly permissive corridor leads from MGE to the neocortex. Therefore, permissive and nonpermissive environments might play a primary role in determining the regional specificity and overall direction of MGE cell migration (Fig. 4).
Figure 4.
Model of the guidance of MGE cells to the neocortex. MGE is ventromedially surrounded with a nonpermissive tissue (dark gray). In contrast, neocortex is the most permissive tissue in the central nervous system for MGE cell migration (light gray). Inhibitory factors are secreted from the ventromedial forebrain (including Slit1 and Slit2), which suppress MGE cell migration in that direction. Neocortex secretes a factor(s) that seems to act as a chemoattractant for migrating MGE cells. NCx, neocortex; HT, hypothalamus.
The molecular nature of the permissive and nonpermissive substrates has not been determined. However, we show that the permissive substrate used by migrating MGE cells is associated with the surface of neocortical cells. Previous reports suggest that MGE cells use TAG-1 adhesion molecules expressed on the surface of corticofugal axons as a substrate for their migration (22). Recently, we reported that the majority of MGE cells enter the neocortex via a region adjacent to the cortical ventricular zone, but only relatively few cells migrate through the axon-rich upper intermediate zone (2). When labeled MGE cells were cocultured with neocortical explants extending multiple neurites, MGE cells selectively migrated into cell rich neocortical tissue avoiding axonal bundles (H.W. and A.A.-B., unpublished observations). In vitro experiments demonstrated that MGE cells are capable of long distance migration in a complex extracellular substrate (matrigel) as well as within simple collagen type I substrate (17). Therefore, it is likely that, besides TAG-1 adhesion molecule, there are other permissive substrates, possibly produced by cortical subventricular or ventricular zone cells, that are used by tangentially migrating MGE cells.
In addition to a selectively permissive environment, directional migration of MGE cells might be facilitated by chemotactic gradients. We present evidence that the neocortex secretes chemoattractive factor(s) and that ventromedial forebrain secretes factor(s) inhibiting cell motility (Fig. 4). We do not know the molecular nature of the chemoattractive factor secreted from the neocortex. It has been shown that neocortex produces hepatocyte growth factor (HGF), which acts as a motogen (stimulates migration) for tangentially migrating cells (33). It remains to be determined whether HGF could also act as a chemoattractant for MGE cells. Slit factors were previously shown to inhibit migration of postnatal SVZ neuronal precursors (29). Similarly, we demonstrate that Slit factors are abundantly expressed in ventromedial forebrain and that these factors mimic the inhibitory activity detected in the dorsal hypothalamus.
Besides being strongly expressed in ventromedial forebrain, Slit1 is also weakly expressed in the cortical plate, the target area of MGE cell migration (ref. 11; Fig. 3E). It is likely that either MGE cells lose their responsiveness to Slit factors (34–36), the permissive environment in the neocortex dominates over Slit inhibition, or neocortical tissue blocks Slit signaling (14). All of these possibilities are consistent with the observation that MGE cell migration is not suppressed when neocortical cells are mixed with Slit-expressing HEK293 cells. Interestingly, we observed that the addition of a high concentration of heparin (10 units/ml) to culture medium significantly increases (1.9-fold) the number of MGE cells migrating through neocortical tissue in spot assays (H.W. and A.A.-B., unpublished results). One possible explanation of this result is that heparin, which has been shown to disrupt interactions between Slit factors and heparan sulfate proteoglycan glypican-1 (37–39), interferes with Slit-mediated suppression of MGE cell migration normally occurring in the developing neocortex.
MGE cells move at high speed over long distances within the developing brain. This behavior allows them to contribute to the histogenesis of distant brain regions like neocortex. This remarkable migratory potential is restricted during development by precise boundaries between the MGE and neighboring brain regions where these cells are not allowed to invade. Boundary formation and parcelation is an important mechanism to control the navigation of both axons and neural cells (32). It will be interesting to identify permissive factors that allow MGE cell migration into the neocortex and to develop animal models in which this migration is disrupted. It has been recognized that defects in neuronal migration can result in severe brain abnormalities and intractable epilepsy (40, 41). It is tempting to speculate that disrupted tangential migration of inhibitory interneurons originating in the MGE contributes to these pathologies (42). The unique migratory potential of MGE cells may also have applications in cell replacement therapies in certain neurodegenerative diseases as MGE cells are the only primary neuronal precursors known to be able to disperse when grafted into the adult brain (17). The present results could help develop methods to steer MGE cells to specific locations after transplantation in the adult brain.
Acknowledgments
We thank Dr. Carol Mason for constructive discussion and critical reading of the manuscript. We thank Cynthia Yaschine for help with the preparation of the manuscript. We thank Dr. Yi Rao for providing us with Slit1 and xSlit2 constructs and Dr. Marc Tessier-Lavigne for providing us with probes for Slit1 and Slit2. H.W. was the recipient of a DeWitt Wallace/Reader's Digest fellowship. L.E. was the recipient of European Molecular Biology Organization and Human Frontier Science Program fellowships. This work was supported by National Institute of Child Health and Human Development Grant HD32116 (to A.A.-B.).
Abbreviations
- MGE
medial ganglionic eminence
- LGE
lateral ganglionic eminence
- SVZ
subventricular zone
- E14.5
embryo day 14.5
References
- 1.Olsson M, Campbell K, Wictorin K, Bjorklund A. Neuroscience. 1995;69:1169–1182. doi: 10.1016/0306-4522(95)00325-d. [DOI] [PubMed] [Google Scholar]
- 2.Wichterle H, Turnbull D H, Nery S, Fishell G, Alvarez-Buylla A. Development (Cambridge, UK) 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 3.Corbin J G, Gaiano N, Machold R P, Langston A, Fishell G. Development (Cambridge, UK) 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- 4.Yun K, Potter S, Rubenstein J L. Development (Cambridge, UK) 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Toresson H, Potter S S, Campbell K. Development (Cambridge, UK) 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 6.Anderson S A, Eisenstat D D, Shi L, Rubenstein J L. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 7.Altman J. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 8.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 10.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Li H, Zhou L, Wu J Y, Rao Y. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu J Y, Rao Y. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- 14.Mason H A, Ito S, Corfas G. J Neurosci. 2001;21:7654–7663. doi: 10.1523/JNEUROSCI.21-19-07654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sussel L, Marin O, Kimura S, Rubenstein J L. Development (Cambridge, UK) 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 16.Lavdas A A, Grigoriou M, Pachnis V, Parnavelas J G. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wichterle H, Garcia-Verdugo J M, Herrera D G, Alvarez-Buylla A. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 18.Anderson S A, Marin O, Horn C, Jennings K, Rubenstein J L. Development (Cambridge, UK) 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 19.Marin O, Anderson S A, Rubenstein J L. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pleasure S J, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein D H, Rubenstein J L. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 21.Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein J L. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 22.Denaxa M, Chan C H, Schachner M, Parnavelas J G, Karagogeos D. Development (Cambridge, UK) 2001;128:4635–4644. doi: 10.1242/dev.128.22.4635. [DOI] [PubMed] [Google Scholar]
- 23.Erskine L, Williams S E, Brose K, Kidd T, Rachel R A, Goodman C S, Tessier-Lavigne M, Mason C A. J Neurosci. 2000;20:4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brose K, Bland K S, Wang K H, Arnott D, Henzel W, Goodman C S, Tessier-Lavigne M, Kidd T. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 25.Kishi K. J Comp Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- 26.Wichterle H, Garcia-Verdugo J M, Alvarez-Buylla A. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 27.Brose K, Tessier-Lavigne M. Curr Opin Neurobiol. 2000;10:95–102. doi: 10.1016/s0959-4388(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 28.Kidd T, Bland K S, Goodman C S. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen Ba-Charvet K T, Brose K, Marillat V, Kidd T, Goodman C S, Tessier-Lavigne M, Sotelo C, Chedotal A. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 30.Niclou S P, Jia L, Raper J A. J Neurosci. 2000;20:4962–4974. doi: 10.1523/JNEUROSCI.20-13-04962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringstedt T, Braisted J E, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O'Leary D D. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tessier-Lavigne M, Goodman C S. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 33.Powell E M, Mars W M, Levitt P. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 34.Kramer S G, Kidd T, Simpson J H, Goodman C S. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson B J. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 36.Simpson J H, Kidd T, Bland K S, Goodman C S. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 37.Hu H. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- 38.Liang Y, Annan R S, Carr S A, Popp S, Mevissen M, Margolis R K, Margolis R U. J Biol Chem. 1999;274:17885–17892. doi: 10.1074/jbc.274.25.17885. [DOI] [PubMed] [Google Scholar]
- 39.Ronca F, Andersen J S, Paech V, Margolis R U. J Biol Chem. 2001;276:29141–29147. doi: 10.1074/jbc.M100240200. [DOI] [PubMed] [Google Scholar]
- 40.Allen K M, Walsh C A. Epilepsy Res. 1999;36:143–154. doi: 10.1016/s0920-1211(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 41.Rakic P. Adv Neurol. 2000;84:1–14. [PubMed] [Google Scholar]
- 42.Anderson S, Mione M, Yun K, Rubenstein J L. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]