Abstract
The early interaction of Lawsonia intracellularis with host cells was examined with the use of porcine ileum models. Two conventional swine were anesthetized, and ligated ileum loops were prepared during abdominal surgery. The loops were inoculated with 108 L. intracellularis or saline. After 60 min, samples of each loop were processed for routine histologic and electron microscopic study. Histologic and ultrathin sections of all the loops appeared normal, with no apposition of bacteria and host cells or bacterial entry events in any loop. Portions of ileum from a single gnotobiotic piglet were introduced as xenografts into the subcutis of each flank of 5 weaned mice with severe combined immunodeficiency disease. After 4 wk, 108 L. intracellularis were inoculated into each of 4 viable xenografts with a sterile needle; the other 3 viable xenografts received saline. Histologic and ultrathin sections of all the xenografts 3 wk after inoculation showed relatively normal porcine intestinal architecture, with normal crypts, crypt cell differentiation, and low villous structures; the xenografts treated with the bacteria also showed intracytoplasmic L. intracellularis within crypt and villous epithelial cells. Thus, entry of L. intracellularis into target epithelial cells and multiplication may not be sufficient alone to directly cause cell proliferation. A proliferative response may require active division of crypt cells and differentiation in conjunction with L. intracellularis growth.
Résumé
Les premières interactions entre Lawsonia intracellularis et les cellules hôtes ont été examinées à l’aide de modèles d’iléon porcin. Deux porcs conventionnels ont été anesthésiés et des anses ligaturées d’iléon ont été préparées lors d’une chirurgie abdominale. Les anses ont été inoculées avec 108 cellules de L. intracellularis ou de la saline. Après 60 minutes, des échantillons de chaque anse ont été traitées pour examen histologique conventionnel et par microscopie électronique. Les coupes histologiques et ultra-minces de toutes les anses paraissaient normales, sans évidence d’apposition de bactéries et de cellules hôtes ou d’entrée de bactéries dans aucune des anses. Des portions d’iléon provenant d’un porcelet gnotobiotique ont été introduites en tant que xénogreffes dans le tissu sous-cutané de chaque flanc de 5 souris sevrées avec une déficience immunitaire combinée sévère. Quatre semaines après la greffe, 108 cellules de L. intracellularis ont été inoculées dans chacune de 4 xénogreffes viables avec une aiguille stérile, et les 3 autres xénogreffes inoculées avec de la saline. Trois semaines suivant les inoculations, des coupes histologiques et ultra-minces de toutes les xénogreffes ont montré une architecture intestinale porcine relativement normale, avec des cryptes normales, une différenciation des cellules des cryptes et des structures à villosités peu élevées; les xénogreffes traitées avec les bactéries ont également montré des cellules de L. intracellularis intracytoplasmiques à l’intérieur des cryptes et des cellules épithéliales avec villosités. Ainsi, l’entrée de L. intracellularis dans les cellules épithéliales cibles et sa multiplication pourrait ne pas être suffisant pour causer directement la prolifération cellulaire. Une réponse proliférative pourrait nécessiter une division active des cellules des cryptes et une différentiation conjointement à la croissance de L. intracellularis.
(Traduit par Docteur Serge Messier)
Proliferative enteropathy (PE) is a common enteric disease of weaned pigs and other animals (particularly horses and hamsters) caused in all species by the obligately intracellular bacterium Lawsonia intracellularis (1,2). The characteristic pathological feature in all species is marked proliferation of immature epithelial cells in the crypts of the ileum or colon, or both, which leads to thickening and branching of the crypts and gross mucosal thickening (2,3). The bacteria are invariably present in the apical cytoplasm of the proliferative enterocytes (2,3). The clinical signalment in affected weaned animals can include diarrhea, weight loss, and melena (4). Infections resulting in these signs are common on swine farms, and informed estimates of the annual economic losses are generally around $100 million for the US swine industry alone (4).
Exposing susceptible pigs orally to L. intracellularis or to diseased mucosa containing these intracellular bacteria consistently reproduces PE (2,5–7). In typical studies of conventional weaned pigs (3 wk old) challenged with an oral inoculum of 108 L. intracellularis, numerous intracellular bacteria can be visualized in the developing proliferative intestines and in feces 1 to 3 wk later (5–8). The incidence of bacterial infection and associated proliferative lesions peaks 3 wk after challenge. However, cell proliferation is not observed after challenge of gnotobiotic pigs (2). Naïve conventional pigs of a wide age range (1 d old to adult) are susceptible to oral challenge (4–8). Natural or experimental infection of laboratory rodents other than hamsters (Mesocricetus auratus) has not been widely pursued as a challenge model. Studies thus far have indicated that outbred mice and rats have only limited and transient infection. Oral inoculation of hamsters with L. intracellularis derived from pigs usually causes moderate infection and lesions of PE in about 50% of the animals (8,9). After oral challenge in conventional hamster and pig models, PE develops initially as a progressive proliferation of immature epithelial cells in the ileum, once L. intracellularis has populated these cells (3,8,9). In most cases, no significant inflammatory reaction occurs, and the organisms remain in the epithelium at this stage. Oral challenge models generally lack the ability for refined study of the moments when the agent contacts the ileum cells and the related influencing factors at those moments.
Limited in vivo and in vitro studies have elucidated some of the early events in the interaction of bacteria and host cells (8,9–11). Bacteria associate with the cell membrane and enter the enterocyte via a short-lived entry vacuole. Specific adhesins, ligands, or receptors have not yet been identified. The entry of bacteria into cells appears to depend on cell, but not necessarily bacterial, viability; that is, a type of induced phagocytosis (10). Lawsonia intracellularis multiplies free (not membrane-bound) within the cytoplasm, often adjacent to mitochondria. The mechanism whereby intracellular L. intracellularis causes a monotype of infected mammalian epithelial cells to form hyperplastic features is not yet understood in any respect (12), and studies in the decade since that observation have not been illuminatory. The current in vitro cell co-culture models of L. intracellularis infections do not display any striking cytopathic effects; in particular, they lack recognizable cell proliferation (11,13). The use of cell cultures to study intestinal bacteria also does not account for natural intestinal factors such as local kinase or other growth factors or immunologic responses. Also, the intestinal or other cell lines used are already laboratory-adapted, including being immortalized, so may be considered to be already “abnormal” as a baseline for cell-analysis studies. Because the relevant bacterial ligands, cell receptors, and mechanisms of L. intracellularis survival and growth inside the cells are not understood, the role, if any, that these processes play in the associated cell proliferation is not known. This preliminary study aimed to explore possible refinement, targeted at the early interaction of host cells and L. intracellularis in the ileum, of porcine models known to be useful for studying enteric infections.
Lawsonia intracellularis isolate NCTC (National Collection of Type Cultures) 12657 was derived from the mucosal lesions of a pig affected with PE by means of purification and co-culture techniques described previously (13). Briefly, the bacteria were released from fresh, homogenized PE mucosa and partially purified by differential filtration and centrifugation. The bacterial suspensions were co-cultured in the rat intestinal epithelial cell line IEC 18 (ATCC [American Type Culture Collection] CRL1589) under microaerobic conditions at 37°C and passaged weekly onto fresh, uninfected cells. Co-cultures used for each experiment were checked for purity by routine aerobic and anaerobic bacterial culture and by the use of commercial immunoassays for Mycoplasma and Chlamydia spp. Counting of L. intracellularis within suspensions and co-cultures was performed by immunostaining of sentinel portions with an L. intracellularis-specific monoclonal antibody (14). Cohort co-cultures of this isolate had previously been established as capable of pathogenic infection of conventional swine (2,7).
Injection of L. intracellularis into ligated small intestine loops of swine was performed at United States Department of Agriculture (USDA) facilities according to policies and methods developed for Escherichia coli enterotoxin studies (15). Briefly, 7 ileum loops were prepared during abdominal surgery in 2 anesthetized 3-wk-old conventional swine by aseptically ligating each end of a terminal intestinal loop portion approximately 6 cm long, without compromising blood supply, with 3-cm interloop areas. With a sterile needle, 108 L. intracellularis in 0.2 mL of physiologic saline were injected into each of 5 intestinal loops; the other 2 loops received 0.2 mL of saline alone. The pigs remained under anesthesia, the surgical sites held in sterile conditions, at 35°C for 60 min. After euthanasia of the pigs, the fluid volume and length of each loop were recorded. Samples of each loop were then collected and processed as described later.
Methods for implanting intestinal xenograft samples into the subcutis of mice with severe combined immunodeficiency disease (SCID) were those described for studies with donor rabbit or porcine intestinal tissues (16,17). Briefly, 5 weaned SCID mice (CB-17/ Icr-scid/scid) were obtained from Jackson Laboratory, Bar Harbor, Maine, USA, housed in sterile conditions (sterile bedding, feed, and water; filtered air intake), and monitored by trained attendants. In an aseptic surgical area, the 3-wk-old SCID mice were anesthetized with a mixture of ketamine, 100 mg/kg, and xylazine, 20 mg/kg. Two superficial incisions 2 mm long and 2.5 cm apart were made in the skin of each flank. With a small alligator forceps, a 2-cmlong portion of fresh neonatal gnotobiotic porcine ileum (see later) was introduced into the subcutis of each flank by “pull-through” between each incision. The mice were allowed to recover and were housed in the same manner for a further 4 wk to allow the porcine xenografts to become established. The mice were then sedated with lighter doses of ketamine and xylazine, and 108 L. intracellularis in 0.1 mL of physiologic saline were injected into the lumen of each of 4 viable ileum portions in the subcutis with a sterile needle; the 3 remaining viable ileum portions received saline alone. The other portions were not viable, with dystrophic calcification and closure at this time. Three weeks after inoculation, the mice were euthanized, and samples of the donor implants and the mice intestines were processed (see later).
The portion of each ileum used for xenograft had been obtained immediately after euthanasia of a 2-d-old cesarean-derived gnotobiotic piglet held with cohorts in a sterile plastic “bubble” enclosure and had been prepared and maintained with routine gnotobiotic procedures. After aseptic removal from the piglet, each ileum portion was held in warm, sterile physiologic saline, and 2-cm-long portions were prepared for implantation within 20 min after euthanasia. The procedures on the piglets and mice followed approval under animal care guidelines for Tufts University.
Samples of intestine loops, xenograft donor portions, and jejunum, ileum, mesenteric lymph nodes, and colon of the xenograft recipient mice were fixed by immersion in 10% buffered formalin for routine histologic examination. Separate sections of intestine underwent indirect immunostaining for L. intracellularis (14). Separate portions of intestine loops and xenograft implant tissues were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, fixed again in 2% osmium tetroxide in the same buffer, and embedded in epoxy resin; ultrathin sections were cut and placed on copper grids, stained with uranyl acetate and lead citrate solutions, and examined in a transmission electron microscope (JEOL, Peabody, Massachusetts, USA).
The final volume per centimeter of porcine intestine loop was used as an indicator of fluid accumulation. For all loops, whether treated with L. intracellularis or placebo, the values were less than 0.05 mL/cm, and histologic and immunostained sections appeared normal. Occasional brightly stained vibrioid bacteria, typical of L. intracellularis, were noted free in the lumen in immunostained sections. Exhaustive examination of ultrathin sections by electron microscopy (EM) failed to detect clear apposition of bacteria and host cells or entry events in any loop.
All histologic and ultrathin sections of xenografts, whether treated with L. intracellularis or placebo, showed relatively normal porcine intestinal architecture, with normal crypts, crypt cell differentiation, and low villous structures (Figure 1). With immunostaining and EM, the sections treated with L. intracellularis showed numerous intracytoplasmic L. intracellularis in several crypts. The cell morphology of both infected crypt epithelial cells (Figure 2) and infected mature villous epithelial cells (Figure 3) and surrounding cells appeared normal. The bacterial numbers, morphology, intracellular location, association with numerous mitochondria, and lack of association with vacuoles were considered consistent with active intracellular growth and multiplication of L. intracellularis. There was no clear evidence of local proliferation of crypt cells or dedifferentiation of villous neck cells. Placebo-treated xenografts showed no evidence of L. intracellularis infection or cell abnormalities. Sections of the intestines and mesenteric lymph nodes of all the recipient mice appeared normal.
Figure 1.
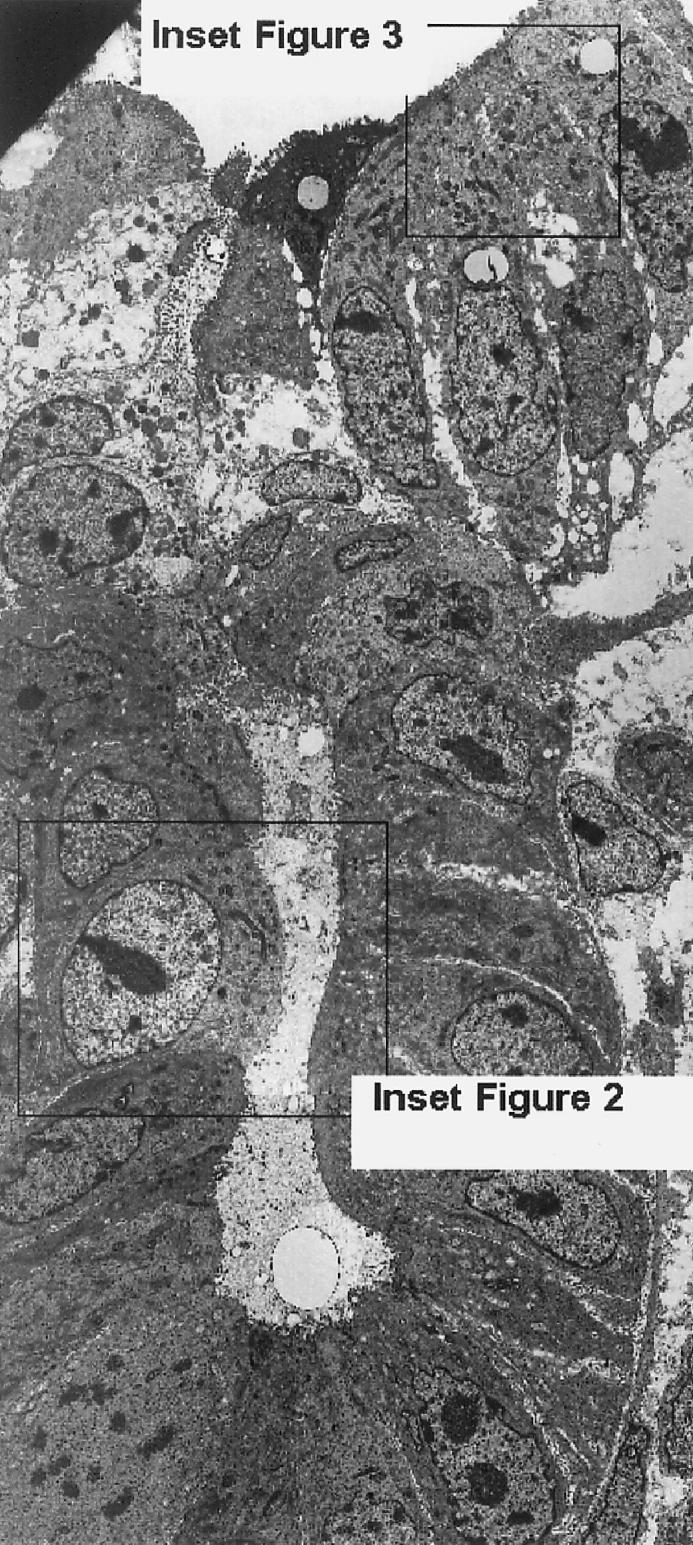
Transmission electron micrograph of ultrathin section of porcine ileum xenograft stained with uranyl acetate and lead citrate, showing normal architecture, cell types, and cell morphology of entire crypt structure, crypt neck, and lower portion of villi. Insets indicate sites of Figures 2 and 3. Magnification × 700.
Figure 2.
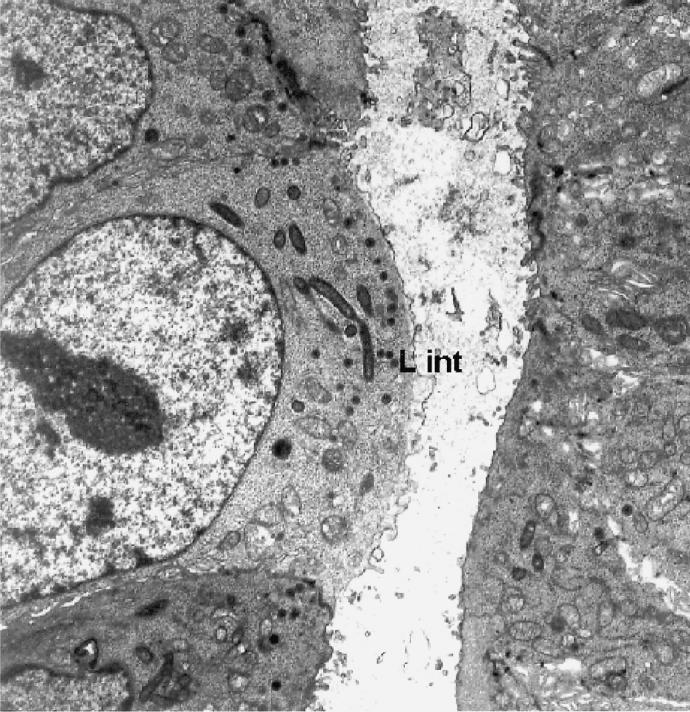
Transmission electron micrograph of ultrathin section of crypt area of porcine ileum xenograft, showing intracytoplasmic Lawsonia intracellularis (L int) and immature microvilli typical of immature crypt cells. Magnification × 1400.
Figure 3.
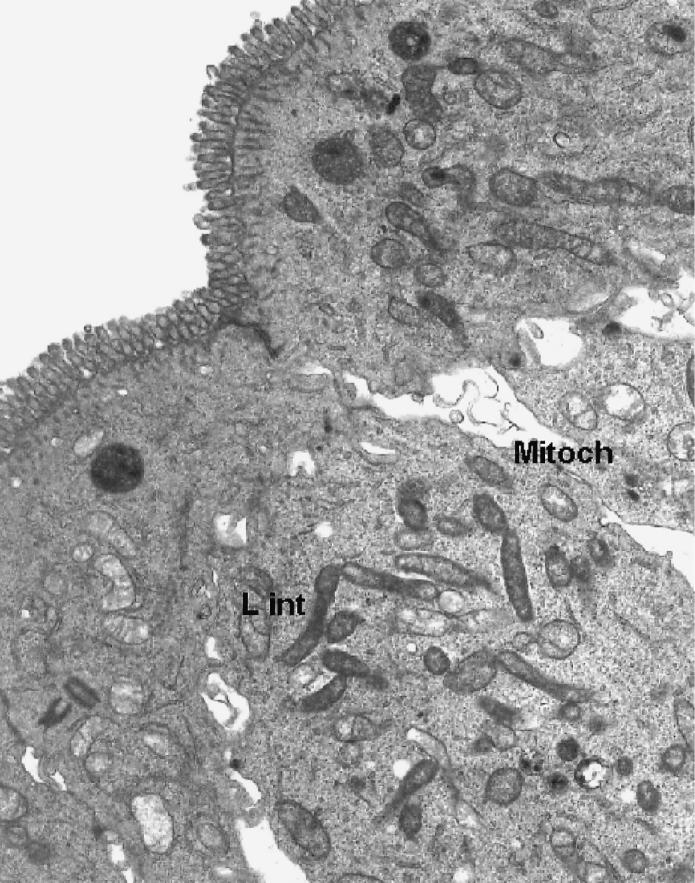
Transmission electron micrograph of ultrathin section of lower villous area of porcine ileum xenograft, showing numerous intracytoplasmic L. intracellularis (L int), adjacent mitochondria (Mitoch), and mature microvilli and other organelles typical of mature intestinal epithelial cells. Magnification × 2200.
This study explored novel models to investigate the early interactions of L. intracellularis with target host cells. The use in swine of ligated gut loops, which limit the dispersal of inoculation material and peristalsis, has provided fruitful studies of the early stages of enterotoxigenic E. coli infection (15). This method is facilitated by the fact that the reaction time for apparent fimbrial and enterotoxin activity is within the limited period suitable for gut loops to be maintained in a surgical site. The results of our study suggest that E. coli-like enterotoxin or fimbrial-like activity is not a key virulence factor for L. intracellularis. Only limited in vitro and in vivo studies have illustrated early events in the attachment to and entry of L. intracellularis into target epithelial cells (9,11). Our study did not identify the loop or xenograft models as assisting study of these early events. Sampling of xenograft loops at earlier postinoculation times might be useful.
The mitochondrial association with free intracytoplasmic L. intracellularis illustrated in our study (Figure 3) confirms previous indications (2,9) that the processes of cell entry and intracellular activity of this bacterium are similar to the early entry-vacuole release and “energy parasite” reactions of some other obligate intracellular bacteria, such as Rickettsia prowazekii, to which L. intracellularis is unrelated. This may indicate a convergent evolutionary effect in the life cycle of some intracellular bacteria. However, unlike other intracellular bacteria, L. intracellularis also possesses specific factors that result in monotypic proliferation of the target host epithelial cell (2,7). Previous oral challenge exposure studies in susceptible hamsters and pigs failed to elucidate these factors, despite a series of well-conducted sequential sampling studies, including detailed EM examination of affected ileum (2,3,8). Oral challenge studies do not readily allow correct timing of detailed epithelial sampling for analysis of possible key interactions between the bacteria and host cells in the ileum, nor do they allow the possible incorporation of local epithelial or cell modifiers at this site. In contrast, the use of the xenograft model could allow meaningful levels of modifiers, such as cell growth factors, cell division inhibitors, L. intracellularis bacterial fractions, and other potential pathogenetic markers, to be introduced within an active intestinal xenograft before or during the periods of infection and cell proliferation, which may cover some days. This extended period for intervention therefore presents an advantage over the limited period available for study of ligated loops. Intestinal xenograft models in SCID mice have been successfully used to evaluate the development of infections by other enteric pathogens, such as Shigella spp. (17) and other intracellular agents (16). The development of the donor intestines in this study (in a 2-d-old gnotobiotic piglet) was typical of that seen in other similar intestinal xenograft studies, fully developed crypts and low villi appearing 3 to 4 wk after the graft procedure (16). Portions of ileum from conventional piglets were not used because of the risks of graft-to-host reactions and deleterious mouse infections.
The bacterial numbers, morphology, and intracellular location of L. intracellularis in this xenograft study were considered consistent with active intracellular growth and multiplication of the bacteria, as noted in previous studies of natural infections and oral experimental challenge exposure studies (2,4–6). However, the morphology of both infected crypt epithelial cells (Figure 2) and infected mature villous epithelial cells (Figure 3), as well as the surrounding cells, appeared normal. This presence of viable L. intracellularis within target host intestinal epithelial cells 3 wk after inoculation, but without apparent proliferation, is a novel finding and may have implications for the key feature of monotypic proliferation of host epithelial cells in response to the presence of L. intracellularis. The results of this study provide some insights into previous explanations offered for this proliferation response (12). Neonatal yet conventional piglet intestine has been shown to be susceptible to active L. intracellularis infection, with notable associated cell proliferation (6,7). Therefore the exact epithelial cell and tissue origin used in this SCID xenograft study and the times allowed for infection and pathogenic reaction were considered sufficient for, and susceptible to, the intended proliferation reaction, but it did not occur. These findings suggest that cellular entry and subsequent multiplication and respiration of the bacteria in the target epithelial cells are not sufficient alone to cause fully evident cell proliferation. This conclusion may confirm the findings of both in vitro studies, which failed to find notable cell proliferations, even when exact target cells (porcine or rat ileal or colonic epithelial cells) were cultured and coinfected in vitro (11,13), and attempted infections of gnotobiotic piglets, which also had negative results (2). Thus, processes other than those associated directly with bacterial-cell receptors or intracellular bacterial multiplication may best explain the unique mechanisms of mammalian cell proliferation. The cell-entry mechanism of L. intracellularis was also previously described as an induced phagocytosis process, considered to be an innate feature of the bacterium, unrelated to its activity against the target cells (10).
A marked increase in proliferation and differentiation of epithelial cells occurs in the crypts of piglets and other animals at weaning (18), a reaction not seen in gnotobiotic pigs, establishing that the normal microbial flora stimulate full normal crypt and villous activity and size. In this study and in all others involving oral inoculations of L. intracellularis into completely germ-free intestines, there was no clear evidence of local proliferation of crypt cells (2). Also, L. intracellularis can enter a wide range of cell types in vitro and has a wide host species range, but the unique cell-proliferation response has only been demonstrated in the intestinal crypts of conventional hosts (4). It is therefore likely that the cell proliferation associated with L. intracellularis is restricted and specific to the rapidly dividing and differentiating crypt cell types in full-sized conventional crypts in vivo. This response could then be most likely due to a DNA-acting or similar factor operating during crypt cell division and growth (promotion factor) or crypt neck cell differentiation (inhibition factor), or both. An association between intestinal cell division and activity of an intracellular agent leading to local epithelial cell proliferation was demonstrated for Eimeria bakuensis, an intracellular agent that causes a clinically and pathologically similar PE in sheep (19). Similarly, proliferative intestinal crypt adenomatous hyperplasia was noted in animals deprived of p27 kinase, the key crypt cell differentiation factor (20). Our findings and those of other challenge studies suggest that the proliferation response peculiar to PE in swine and other animals requires crypt cell division and differentiation activity that occurs in conjunction with L. intracellularis growth. Use of the new in vivo models presented in this study may not replace genomic and oral challenge studies in the search for final understanding of the pathogenetic secrets of L. intracellularis.
Acknowledgments
This work was supported by the authors’ facilities. We thank the technical and scientific staff that assisted, particularly Chris Pearson, Art Donohue-Rolfe, and Linda Keller at Tufts University and Tom Casey and Bob Kunkle at the USDA National Animal Disease Center.
Footnotes
Dr. McOrist’s current address is University of Nottingham, School of Veterinary Medicine and Science, Sutton Bonington, Loughborough LE12 5RD, England.
References
- McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov., sp. nov. , the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820–825. doi: 10.1099/00207713-45-4-820. [DOI] [PubMed] [Google Scholar]
- McOrist S, Jasni S, Mackie RA, MacIntyre N, Neef N, Lawson GHK. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun. 1993;61:4286–4292. doi: 10.1128/iai.61.10.4286-4292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk CS, Wagner JE. Experimental hamster enteritis: an electron microscopic study. Am J Vet Res. 1977;38:1861–1868. [PubMed] [Google Scholar]
- McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames, Iowa: Iowa State University Press, 1999:521–534.
- Guedes RMC, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after oral challenge with a pathogenic isolate or an attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135–145. doi: 10.1016/s0378-1135(02)00301-2. [DOI] [PubMed] [Google Scholar]
- McOrist S, Lawson GHK. Reproduction of proliferative enteritis in gnotobiotic pigs. Res Vet Sci. 1989;46:27–33. [PubMed] [Google Scholar]
- McOrist S, Mackie RA, Neef N, Aitken I, Lawson GHK. Synergism of ileal symbiont intracellularis and gut bacteria in the reproduction of porcine proliferative enteropathy. Vet Rec. 1994;134:331–332. doi: 10.1136/vr.134.13.331. [DOI] [PubMed] [Google Scholar]
- McOrist S, Lawson GHK, Rowland AC, MacIntyre N. Early lesions of proliferative enteritis in pigs and hamsters. Vet Pathol. 1989;26:260–264. doi: 10.1177/030098588902600311. [DOI] [PubMed] [Google Scholar]
- Jasni S, McOrist S, Lawson GHK. Experimentally induced proliferative enteritis in hamsters: an ultrastructural study. Res Vet Sci. 1994;56:186–192. doi: 10.1016/0034-5288(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Lawson GHK, Mackie RA, Smith DGE, McOrist S. Infection of cultured rat enterocytes by ileal symbiont intracellularis depends on host cell function and actin polymerization. Vet Microbiol. 1995;45:339–350. doi: 10.1016/0378-1135(94)00142-j. [DOI] [PubMed] [Google Scholar]
- McOrist S, Jasni S, Mackie RA, Berschneider HM, Rowland AC, Lawson GHK. Entry of the bacterium ileal symbiont intracellularis into cultured enterocytes and its subsequent release. Res Vet Sci. 1995;59:255–260. doi: 10.1016/0034-5288(95)90013-6. [DOI] [PubMed] [Google Scholar]
- McOrist S, Roberts L, Jasni S, et al. Developed and resolving lesions in porcine proliferative enteropathy: possible pathogenetic mechanisms. J Comp Pathol. 1996;115:35–45. doi: 10.1016/s0021-9975(96)80026-0. [DOI] [PubMed] [Google Scholar]
- Lawson GHK, McOrist S, Jasni S, Mackie RA. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J Clin Microbiol. 1993;31:1136–1142. doi: 10.1128/jcm.31.5.1136-1142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOrist S, Boid R, Lawson GHK, McConnell I. Monoclonal antibodies to intracellular Campylobacter-like organisms of the porcine proliferative enteropathies. Vet Rec. 1987;121:421–422. doi: 10.1136/vr.121.18.421. [DOI] [PubMed] [Google Scholar]
- Casey TA, Herring CJ, Schneider RA, Bosworth BT, Whipp SC. Expression of heat-stable enterotoxin STb by adherent Escherichia coli is not sufficient to cause severe diarrhea in neonatal pigs. Infect Immun. 1998;66:1270–1272. doi: 10.1128/iai.66.3.1270-1272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulin JD, Kuhlenschmidt MS, Gelberg HB. Development, characterization, and utilization of an intestinal xenograft model for infectious disease research. Lab Invest. 1991;65:719–731. [PubMed] [Google Scholar]
- Zhang Z, Jin L, Champion G, Seydel K, Stanley SL. Shigella infection in a SCID mouse–human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun. 2001;69:3240–3247. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DJ. Alterations in piglet small intestinal structure at weaning. Res Vet Sci. 1986;40:32–40. [PubMed] [Google Scholar]
- Gregory MW, Catchpole J, Norton CC, Pittilo RM. Synchronized division of coccidia and their host cells in the ovine intestine. Parasitol Res. 1987;73:384–386. doi: 10.1007/BF00531095. [DOI] [PubMed] [Google Scholar]
- Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumor suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]


