Abstract
Multi-drug-resistant (MDR) Salmonella enterica serovar Newport strains are increasingly isolated from animals and food products of animal origin and have caused septicemic illness in animals and humans. The purpose of this study was to determine the occurrence and the epidemiologic, phenotypic, and genotypic characteristics of S. Newport of animal origin that may infect humans, either via the food chain or directly. During the 1993–2002 period, the Office International des Épizooties Reference Laboratory for Salmonellosis in Guelph, Ontario, received 36 841 Salmonella strains for serotyping that had been isolated from animals, environmental sources, and food of animal origin in Canada. Of these, 119 (0.3%) were S. Newport. Before 2000, none of 49 S. Newport strains was resistant to more than 3 antimicrobials. In contrast, between January 2000 and December 2002, 35 of 70 isolates, primarily of bovine origin, were resistant to at least 11 antimicrobials, including the extended-spectrum cephalosporins. The blaCMY-2, flost, strA, strB, sulII, and tetA resistance genes were located on plasmids of 80 to 90 MDa that were self-transmissible in 25% of the strains. Conserved segments of the integron 1 gene were found on the large MDR-encoding plasmids in 3 of 35 strains additionally resistant to gentamicin and spectinomycin or to spectinomycin, sulfamethoxazole– trimethoprim, and trimethoprim. Resistance to kanamycin and neomycin was encoded by the aphA-1 gene, located on small plasmids (2.3 to 6 MDa). The increase in bovine-associated MDR S. Newport infections is cause for concern since it indicates an increased risk of human acquisition of the infection via the food chain.
Résumé
Des souches multi-résistantes de Salmonella enterica serovar Newport sont de plus en plus isolées d’échantillons provenant d’animaux et de leurs produits; ces souches ont causé des septicémies chez les animaux et les humains. Le but de cette étude était de déterminer l’occurrence et les caractéristiques épidémiologiques, phénotypiques, et génotypiques des S. Newport d’origine animale pouvant infecter l’humain, via la chaîne alimentaire ou directement. Durant la période de 1993 à 2002, le Laboratoire de Référence des Salmonelles de l’Office International des Épizooties localisé à Guelph, Ontario, a reçu 36 841 souches de Salmonella pour le sérotypage. Ces souches ont été isolées d’animaux, de sources environnementales, et de viandes d’origine animale au Canada. De ces souches, 119 (0,3 %) étaient du S. Newport. Avant 2000, aucune des 49 souches de S. Newport n’était résistante à plus de 3 antimicrobiens. Par contre, entre janvier 2000 et décembre 2002, 35 des 70 isolats, majoritairement d’origine bovine, étaient résistants à au moins 11 antimicrobiens, incluant les céphalosporines à spectre étendu. Les gènes de résistance blaCMY-2, flost, strA, strB, sulII, et tetA étaient localisés sur des plasmides de 80 à 90 MDa, et ceux-ci étaient transférables chez 25 % des souches. Des segments conservés du gène de l’intégron de classe 1 ont été retrouvés sur de larges plasmides de multi-résistance chez 3 des 35 souches également résistantes à la gentamicine et à la spectinomycine ou à la spectinomycine, le sulfaméthoxazole–triméthoprime, et le triméthoprime. Les résistances à la kanamycine et à la néomycine étaient codées par le gène aphA-1, localisé sur de petits plasmides (2,3 à 6 MDa). L’augmentation des infections bovines associées aux souches de S. Newport multi-résistantes est un problème indiquant un plus grand risque dans l’acquisition de l’infection chez les humains via la chaîne alimentaire.
(Traduit par Docteur Serge Messier)
Introduction
Salmonella Newport infections increased as a proportion of reported infections by all Salmonella serovars in humans in the United States from 5% to 10% during the period 1996–2001 (1) and in Canada from 2.2% to 3.6% during the years 2002 and 2003 (National Laboratory for Enteric Pathogens, Winnipeg, Manitoba, 2003: unpublished data). The percentages of S. Newport isolates in particular but also of other Salmonella serovars and Escherichia coli isolated from humans, animals, and foods of animal origin that are resistant to the extended-spectrum cephalosporins (ESCs) are increasing (1–4). Human infections with ESC-resistant S. Newport are often foodborne (1), may result from direct exposure to infected dairy cows and calves (1), and have occurred in people handling contaminated pet treats (5). Multi-drug-resistant (MDR) S. Newport, S. Typhimurium, and other Salmonella serovars and phage types have been associated with increased morbidity and mortality in humans (1,6,7) and animals (1,2). Little is known about the occurrence and the phenotypic and genotypic characteristics of the antimicrobial resistance (AMR) of S. Newport isolates from animals and food products of animal origin in Canada. Therefore, the purpose of this study was to determine the occurrence of S. Newport among food-producing and other animals, food of animal origin, and environmental sources in Canada, to note the clinical signs and pathological findings associated with S. Newport infection of animals, and to characterize the strains by examining the phenotypic and genotypic aspects of AMR and the transferability of drug-resistance determinants.
Materials and methods
Bacterial strains
This study was based on passive laboratory-based surveillance. We examined S. Newport strains isolated and submitted for serotyping during 1993–2002 in Canada (Table I) as follows.
Table I.
Epidemiologic summary of Salmonella Newport submissions for serotyping during 1993–2002 in Canada
| Source of strain (n = 119)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Province (number of strains) | Submissions (number) | Proportion of strains (number/total) | Bovinea | Beef productsb | Chickenc | Turkeyc | Equine | Human | Other animals | Animal feed | Water | Other |
| 1993 | Ontario (4) | 3 | 0.2% (4/2 424) | 1 | 1 | 1 | 1 | ||||||
| 1994 | Ontario (3) | 3 | 0.1% (3/3 251) | 1 | 1 | 1 | |||||||
| 1995 | Ontario (1) | 1 | < 0.1% (1/1 860) | 1 | |||||||||
| 1996 | Alberta (1) | 1 | < 0.1% (1/2 015) | 1 | |||||||||
| 1997 | Ontario (4), New Brunswick (1) | 5 | 0.2% (5/2 696) | 2 | 1 | 1 | 1 | ||||||
| 1998 | Ontario (2) | 2 | 0.1% (2/3 448) | 1 | 1 | ||||||||
| 1999 | Ontario (31), Quebec (2) | 13 | 0.6% (33/5 093) | 1 | 25 | 1 | 4 | 1 | 1 | ||||
| 2000 | Ontario (17), Alberta (4) | 15 | 0.5% (21/4 523) | 5 | 10 | 1 | 1 | 3 | 1 | ||||
| 2001 | Ontario (26), New Brunswick (1), Quebec (1) | 25 | 0.5% (28/5 766) | 7 | 5 | 2 | 8 | 1 | 1 | 4 | |||
| 2002 | Ontario (19), Alberta (1), New Brunswick (1) | 18 | 0.4% (21/5 765) | 5 | 2 | 5 | 9 | ||||||
| Total | 119 | 86 | 0.3% (119/36 841) | 21 | 42 | 12 | 23 | 3 | 2 | 5 | 7 | 3 | 1 |
Samples from intestines, feces, and lungs
Samples from fresh and frozen beef, a milk filter, hamburger, and hamburger patties for dogs
Samples from feces, intestines, eggs, carcass rinse, fluff, and environmental swabs
Antimicrobial susceptibility testing
We determined the antimicrobial susceptibility of S. Newport strains and their transformants and transconjugants at resistant and, for certain antimicrobials, at intermediate and susceptible breakpoint levels, as specified by standards M31-A (8) and M100-S12 (9) of the National Committee for Clinical Laboratory Standards, with the agar dilution test, using Mueller–Hinton (MH) agar (Table II). To determine resistance to florfenicol, Aquaflor (Schering Plough Animal Health, Pointe Claire, Quebec), containing 50% florfenicol, was dissolved in dimethylformamide (11).
Table II.
| Antimicrobial | Abbreviation | Resistance breakpoint level (μg/mL)a |
|---|---|---|
| Amikacin | Amk | 64 |
| Ampicillin | Amp | 32 |
| Amoxicillin/clavulanic acid | Amc | 32/8b |
| Apramycin | Apr | 32c |
| Carbadox | Crb | 30d |
| Cephalothin | Cef | 32 |
| Ceftiofur | Ctf | 8 |
| Ceftriaxone | Cro | 64e |
| Cefoxitin | Fox | 32 |
| Chloramphenicol | Chl | 32 |
| Ciprofloxacin | Cip | 4f |
| Florfenicol | Fen | 16g |
| Gentamicin | Gen | 16 |
| Kanamycin | Kan | 64 |
| Nalidixicacid | Nal | 32 |
| Neomycin | Neo | 16c |
| Nitrofurantoin | Nit | 64h |
| Spectinomycin | Spt | 64c |
| Streptomycin | Str | 32c |
| Sulfisoxazole | Sul | 512 |
| Sulfamethoxazole–trimethoprim | Sxt | 76/4 |
| Tetracycline | Tet | 16 |
| Tobramycin | Tob | 8 |
| Trimethoprim | Tmp | 16 |
Susceptible breakpoint levels were also examined for amikacin at 16 μg/mL, ceftriaxone at 8 μg/mL, and ciprofloxacin at 0.125 and 1 μg/mL
Strains were considered resistant when growing on agar plates with this concentration
There are no interpretive standards from the National Committee for Clinical Laboratory Standards for these antimicrobials; strains were considered resistant at these concentrations
Strains were considered resistant to this concentration of this veterinary growth promoter for pigs
Strains that grew on Mueller-Hinton agar with 8 μg/mL were also tested at intermediate-resistance levels of 16 and 32 μg/mL
A 0.125 μg/mL concentration determines reduced susceptibility (10)
Strains were considered resistant at this concentration (11)
Strains were considered resistant at this concentration; however, isolates from the human urinary tract are considered resistant at 128 μg/mL (9)
Plasmids
Plasmid DNA was prepared by the alkaline lysis method (12) and then underwent electrophoresis, staining, and visualization as described previously (13). We used the following plasmids as molecular mass standards: pDT285, 96 MDa; pDT369, 23 MDa (13); pSA971028, an S. Typhimurium virulence-associated 62-MDa plasmid (11); and the 8 plasmids of E. coli V517 with molecular masses of 1.4 to 35.8 MDa (14).
Strain conjugation
To determine if plasmids were self-transmissible and transferred resistance genes, we conjugated strains as described by Provence and Curtiss (15) with i) E. coli C600, ii) a lactose-fermenting, antimicrobial-susceptible S. Newport, iii) S. Typhimurium LB5000, and iv) S. Typhimurium LB5010 (16), strains that had been made resistant to nalidixic acid. To eliminate restriction proficiency, the recipient bacteria were grown for 15 to 20 min at 45°C before mating (15).
Strain transformation
The prepared plasmid DNA was used to transform E. coli DH10B (Gibco BRL, Burlington, Ontario) by electroporation (17) to determine if plasmids that could not be transferred by conjugation, and were therefore likely to not be self-transmissible, encoded drugresistance determinants. Standard conditions (2.5 kV, 200 Ω, and 25 μF) were used (18). The transformants were plated on selective agar to identify isolates that contained plasmids encoding AMR. Plasmid profiles and antimicrobial susceptibility profiles of the transformants were determined.
Amplification, sequencing, and hybridization of gene sequences
Table III shows the primers, amplification product sizes, and GenBank accession numbers of the AMR genes used to examine the parent S. Newport strains, their transformants, and their transconjugants. Other genes that were examined by polymerase chain reaction (PCR) but did not yield a product included aac(3)-IV, blaPSE-1, sulIII, tetB, tetC, tetD, and tetY, which encode resistance to apramycin and other aminoglycosides, penicillins and carbenicillin, sulfonamides, and tetracyclines. All primers had previously been shown to produce amplicons in other antimicrobial-resistant isolates of Salmonella and other gram-negative pathogens and commensals and were surmised to function similarly in the S. Newport isolates. The AMR genes, resistances encoded, and mechanisms of resistance (19–28) are summarized in Table III. Primers for the 5’ and 3’ conserved segments of class 1 integrons were used to identify the integrons and the associated AMR genes (25). A touchdown thermal cycling program was used, with an annealing temperature of 60°C initially; for 15 cycles, the temperature was decreased 1°C per cycle to 46°C, and then there were 40 standard PCR cycles, of 95°C for 15 s, 51°C for 30 s, 72°C for 2 min, and final extension at 72°C for 7 min. Plasmid DNA preparations of parents, transconjugants, and transformants underwent electrophoresis and Southern blotting. Digoxigenin (DIG)-labelled probes were generated by means of the PCR DIG Probe Synthesis Kit (Roche), and hybridizations were performed as described previously (29). The PCR reaction mixtures were cleaned prior to DNA sequencing with the MinElute PCR Purification Kit (Qiagen, Mississauga, Ontario). The DNA sequences were determined with the DYEnamic ET terminator cycle sequencing kit and a MegaBACE 500 sequencing system (Amersham Pharmacia, Piscataway, New Jersey, USA).
Table III.
Primers, GenBank accession numbers, and mechanisms of resistance of antimicrobial-resistance (AMR) genes used to examine the parent S. Newport strains
| Gene | 5′ and 3′ primer sequences | Accession number (and position) | Mechanism of resistance | Encodes resistance to |
|---|---|---|---|---|
| ant(3”)-Ia (aadA) | F: GTGGATGGCGGCCTGAAGCC
R: ATTGCCCAGTCGGCAGCG |
X02340 (514–1040) | Aminoglycoside adenyltransferase (19) | Spt, Str |
| aph(3”)-Ib (strA) | F: TGACTGGTTGCCTGTCAGAG
R: CGGTAAGAAGTCGGGATTGA |
NC_001740 (101–506) | Aminoglycoside phosphoryltransferase (19) | Str |
| aph(6)-Id (strB) | F: ATCGCTTTGCAGCTTTGTTT
R: CGTTGCTCCTCTTCTCCATC |
NC_001740 (1248–1650) | Aminoglycoside phosphoryltransferase (19) | Str |
| ant(2”)-Ia (aadB) | F: TCCAGAACCTTGACCGAAC
R: GCAAGACCTCAACCTTTTCC |
X04555 (1098–1797) | Aminoglycoside adenyltransferase (20) | Gen, Kan, Tob |
| aphA-1 | F: TTATGCCTCTTCCGACCATC
R: GAGAAAACTCACCGAGGCAG |
U63147 (417–905) | Aminoglycoside phosphoryltransferase (20) | Kan, Neo |
| blaCMY-2 | F: ATAACCACCCAGTCACGC
R: CAGTAGCGAGACTGCGCA |
U77414 (209–839) | Extended-spectrum β-lactamase (cephamycinase) (21) | Oxyimino-cephalosporins (ceftazidime, cefotaxime, Cro), 7-α-methoxy-cephalosporins (Fox), and β-lactamase inhibitors (clavulanate) |
| blaTEM-1 | F: CAGCGGTAAGATCCTTGAGA
R: ACTCCCCGTCGTGTAGATAA |
AF309824 (267–910) | Beta-lactamase (22) | Amp, Cef |
| dhfrI | F: TGGCTGTTGGTTGGACGC
R: TCACCTTCCGGCTCGATGTC |
X17477 (710–966) | Dihydrofolate reductase inhibitor (23) | Tmp |
| flost | F: ACCCGCCCTCTGGATCAAGTCAAG
R: CAAATCACGGGCCACGCTGTATC |
AF097407 (577–1124) | Efflux (24) | Chl, Fen |
| int1 | F: GGCATCCAAGCAGCAAGC
R: AAGCAGACTTGACCTGAT |
X12870 (1193–2201) VRa | Encoded by genes in integron 1 (25) | Aminoglycosides, Tets, Chl, Fen, Amp, and other drugs if resistance genes inserted into integron 1 |
| qacEΔI-sulI | F: ATCGCAATAGTTGGCGAAGT
R: GCAAGGCGGAAACCCGCGCC |
X12870 (2278–3075) | Encoded by genes in integron 1 (25) | Quaternary ammonium compounds, sulfonamides |
| sulI | F: GCGCGGCGTGGGCTACCT
R: GATTTCCGCGACACCGAGACCAA |
U49101 (2113–2469) | Dihydropteroate synthase inhibitor (26) | Sulfonamides |
| sulII | F: CGGCATCGTCAACATAACC
R: GTGTGCGGATGAAGTCAG |
M36657 (338–1059) | Dihydropteroate synthase inhibitor (27) | Sulfonamides |
| tetA | F: GTGAAACCCAACATACCCC
R: GAAGGCAAGCAGGATGTAG |
X00006 (1328–2215) | Efflux (28) | Tets |
| tetG | F: GCTCGGTGGTATCTCTGCTC
R: AGCAACAGAATCGGGAACAC |
Y19117 (1–468) | Efflux (28) | Tets |
VR — variable region, the size of which depends on the number and size(s) of inserted gene cassette(s)
Molecular analysis of genetic relatedness
To determine the genetic relatedness of the isolates, we analyzed the patterns produced by pulsed-field gel electrophoresis (PFGE) after digestion with XbaI and BlnI. Whole-cell DNA was prepared, digested, and separated as described previously by the Centers for Disease Control and Prevention (30). Whole-cell DNA of S. Newport am01144 restricted with XbaI was used as a molecular size marker. The PFGE patterns were determined as described by Liebisch and Schwarz (31). We analyzed the results with BioNumerics (Applied Maths, Austin, Texas), using the Dice similarity coefficient (optimization 1.0%, position tolerance 1.0%).
Results
Epidemiologic observations (Table I)
During the 1993–2002 period, 36 841 Salmonella strains, isolated from animals, the environment, food of animal origin, and other sources in Canada, were submitted to our Office International des Épizooties (OIE) Reference Laboratory for Salmonellosis in Guelph, Ontario, for serotyping. Of the strains, 119 (0.3%) were S. Newport isolates, from 86 submissions; the proportion increased from the 1993–1998 period (< 0.1% to 0.2%) to the 1999–2002 period (0.4% to 0.6%). In 1993–1998, there were 1 to 5 S. Newport submissions annually, each from a different location. In 1999–2002, the number of submissions was 13 to 25 per annum, from 40 locations. Reisolation of S. Newport strains from the same premises occurred on several occasions. Recrudescence of suspected septicemic salmonellosis occurred particularly in veal calves at veal calf operations and in calves and cows at dairy farms. In 1993–2002, 107 S. Newport strains were isolated from premises in Ontario, 6 from Alberta, 3 from New Brunswick, and 3 from Quebec. Two of the Ontario strains were isolated from veal calves within days of importation from New York State. In 2000–2002, 90% of the S. Newport strains but only 44% to 51% of all Salmonella isolates were from Ontario sources. Of all 119 S. Newport isolates in 1993–2002, 63 were of bovine origin, and 56 were from turkeys (n = 23), chickens (n = 12), horses (n = 3), and other sources (n = 18). Among the 63 bovine isolates, 21 were clinical isolates from feces, the intestines, and other tissues, and the other 42 were nonclinical isolates: 27 from frozen beef, 9 from fresh beef, 5 from hamburger, and 1 from a milk filter.
Clinical and pathological observations
Clinical signs and pathological findings in animals from which S. Newport was isolated were often severe, but transient colonization (bovine) was also identified in association with outbreaks of MDR S. Newport in Ontario. In Holstein cows, fever, diarrhea (watery, bloody, or both), dehydration, and diminished milk production were seen. Fever, diarrhea, dehydration, enteritis, enlarged mesenteric lymph nodes, fibrinous pneumonia, and emaciation occurred in red and white veal calves. In turkey poults, diarrhea, yolk sacculitis, fibrinous enteritis, fibrinous pericarditis, splenomegaly, and enlarged ceca were seen, and the mortality rate was increased. Fever, diarrhea, ulcerative enteritis, colic, and pneumonia were observed in horses.
Antimicrobial susceptibility
Of the 49 S. Newport strains isolated before 2000, 32 were susceptible to all 24 antimicrobials in the testing panel, 14 were resistant only to sulfisoxazole, and 3 showed resistance to 3 or fewer antimicrobials (Amp and Spt; Spt, Str, and Sul; and Kan, Neo, and Tet). Among the 70 strains isolated during the January 2000 to December 2002 period, 32 of 33 from a bovine source, 1 of 18 from turkeys, and 2 of 10 from chickens were resistant to β-lactams, including the 1st-generation cephalosporin cephalothin, showed reduced susceptibility to the ESC ceftriaxone, and were resistant to the ESCs ceftiofur and cefoxitin (a cephamycin), to the amoxicillin/clavulanic acid combination, and to Chl, Fen, Str, Sul, and Tet (Table IV). Some of the ESC-resistant strains were additionally resistant to Kan and Neo (n = 10), Gen, Kan, Neo, and Spt (n = 2), or Spt, Sxt, and Tmp (n = 1). On 2 Ontario farms, veal calves from which resistant S. Newport was isolated had been treated with ceftiofur and florfenicol, respectively.
Table IV.
Genetic and plasmid profiles of the 35 S. Newport strains that were resistant to at least 11 antimicrobials, including the extended-spectrum cephalosporins
| Strain number(s) | AMRs of parent strains | AMR genes identified in transformants or transconjugants or both | Plasmids (MDa) |
|---|---|---|---|
| 00-3183 B | AAcCfCrCtFxCFStSuT | blaCMY-2, flost, strA, strB, sulII, tetA | 90 |
| 00-4272 B | AAcCfCrCtFxCFStSuT | blaCMY-2, flost, strA, strB, sulII, tetA | 80, 2.3 |
| 00-3051 B, a 00-3800 B, 00-4056 B, 00-4235 B, a 00-4263 B, a 00-4264 B, 00-4265 B, 00-4266 B, 01-0606 B, 01-1809 B, 01-4636 B, 01-6249 B | AAcCfCrCtFxCFStSuT | blaCMY-2, flost, strA, strB, sulII, tetA | 90, 2.3 |
| 00-3050 B, 00-3798 B | AAcCfCrCtFxCFStSuT | blaCMY-2, flost, strA, strB, sulII, tetA | 90, 40, 2.3 |
| 01-0991 B, b 01-3221 B, b 01-3233 B, 02-5850 C, b 02-5851 C | AAcCfCrCtFxCFStSuT | blaCMY-2, flost, strA, strB, sulII, tetA | 90, 72, 2.3 |
| 00-4273 B | AAcCfCrCtFxCFStSuT | blaCMY-2, flost, strA, strB, sulI, sulII, tetA | 90, 2.3 |
| 01-5180 B, 02-5483 B, 02-5758 B, 02-5858 B | AAcCfCrCtFxCFStSuT + KN | blaCMY-2, flost, strA, strB, sulII, tetA, aphA-1 | 90, 3.7 |
| 01-3783 B, 01-3784 B | AAcCfCrCtFxCFStSuT + KN | blaCMY-2, flost, strA, strB, sulII, tetA, aphA-1 | 80, 6, 2.3 |
| 01-4980 B | AAcCfCrCtFxCFStSuT + KN | blaCMY-2, flost, strA, strB, sulII, tetA, aphA-1 | 90, 2.3 |
| 00-2987 B, 01-3987 T | AAcCfCrCtFxCFStSuT + KN | blaCMY-2, flost, strA, strB, sulII, tetA, aphA-1 | 90, 5 |
| 02-3908 B | AAcCfCrCtFxCFStSuT + KN | blaCMY-2, flost, strA, strB, sulI, sulII, tetA, tetG, aphA-1 | 90, 3.7 |
| 02-4818 B | AAcCfCrCtFxCFStSuT + SpSxtTm | blaCMY-2, flost, sulII, tetA, aadA, dhfr1, int1, qacEΔI-sulI | 90, 3.7, 1.6 |
| 00-4923 B | AAcCfCrCtFxCFStSuT + GSp + KN | blaCMY-2, flost, sulII, tetA, aadA, aadB, int1, qacEΔI-sulI, aphA-1 | 90, 5 |
| 02-1652 B | AAcCfCrCtFxCFStSuT + GSp + KN | blaCMY-2, flost, sulII, tetA, aadA, aadB, int1, qacEΔI-sulI, aphA-1 | 90, 5, 3.2, 2.8 |
B — bovine; C — chicken; T— turkey; AAcCfCrCtFxCFStSuT — resistant to Amp, Amc, Cef, Cro, Ctf, Fox, Chl, Fen, Str, Sul, and Tet, respectively; Cro — reduced susceptibility to ceftriaxone at 8 μg/mL; K — Kan; N — Neo; Sp — Spt; Tm — Tmp; G — Gen
The transconjugants, obtained by conjugation at 28°C, contained the 90-MDa plasmid with its associated AMRs
The transconjugants, obtained by conjugation at 37°C, contained 90- and 72-MDa plasmids, the larger plasmid encoding the associated resistances
Plasmid profiles
All 35 strains isolated in 2000–2002 that were resistant to Amp, Amc, Ctf, Cro, Cef, Fox, Chl, Fen, Str, Sul, and Tet, including those additionally resistant to Gen and Spt or to Spt, Sxt, and Tmp, harbored a large (80 to 90 MDa) plasmid (Table IV). In contrast, such large plasmids were found in only 3 of 84 strains (4%) that were pansusceptible or were resistant to only 1 to 3 antimicrobials.
Conjugation
Conjugation with each recipient strain was attempted with 24 S. Newport strains that were derived from different sources, had different plasmid profiles, or had different AMRs, or some combination. Plasmids of 6 strains produced transconjugants only with the NalR E. coli C600 recipient strain. Three strains possessed plasmids of 90 and 2.3 MDa that cotransferred at 28°C. An additional 3 strains possessed plasmids of 90 and 72 MDa that cotransferred at 37°C. The 90-MDa plasmid transferred resistance to Amp, Amc, Ctf, Cro, Cef, Fox, Chl, Fen, Str, Sul, and Tet to the transconjugants (Table IV).
Transformation
Transformation by electroporation of E. coli DH10B with plasmid DNA of each of the 35 ESC-resistant strains revealed that the large plasmid of all strains was transferable and conferred resistance to Amp, Amc, Ctf, Cro, Cef, Fox, Chl, Fen, Str, Sul, and Tet (Table IV). When the parent strains were additionally resistant to Gen and Spt or to Spt, Sxt, and Tmp, the transformants were resistant to these antimicrobials as well. Similarly, examination of transformants selected on MH agar containing Kan showed that all strains additionally resistant to Kan and Neo transferred these resistances to E. coli DH10B. The recipient strains contained the corresponding plasmid of 6, 5, 3.7, or 2.3 MDa that encoded these resistances.
Amplification, sequencing, and hybridization of gene sequences
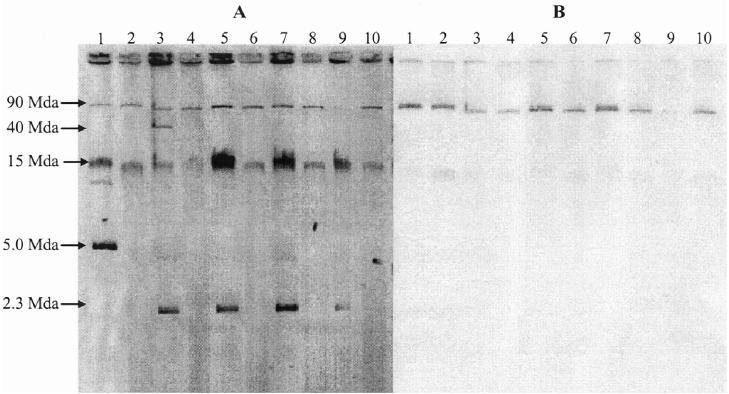
The presence of the blaCMY-2 gene was confirmed by sequencing PCR products from each of the 35 strains that were resistant to Amp, Amc, Cef, Ctf, and Fox and that showed reduced susceptibility to Cro at 8 μg/mL (Table IV). Similarly, all strains resistant to Chl and Fen harbored the flost gene, strains resistant to Str but not Spt harbored the strA and strB genes, strains resistant to Sul contained the sulII or the sulI and sulII genes, and strains resistant to Tet harbored the tetA gene or the tetA and tetG genes. Each of the 35 corresponding transformants and the 6 transconjugants also possessed the same resistance genes. Hybridization of Southern blots of plasmid DNA with the blaCMY-2 probe showed that the gene was present in each of the transformants on a large plasmid (Figure 1).
Figure 1.
Plasmid profiles (A) and Southern blot hybridization (B) with a digoxigenin-labelled blaCMY-2 probe of parent and transformant strains of Salmonella Newport. Lane 1, SA00-2987; lane 2, SA00-2987T (Escherichia coli DH10B transformed with plasmid DNA of the parent strain); lane 3, SA00-3050; lane 4, SA00-3050T; lane 5, SA00-4056; lane 6, SA00-4056T; lane 7, SA01-4636; lane 8, SA01-4636T; lane 9, SA00-3800; lane 10, SA00-3800T.
Three of the 35 parent strains and their respective transformants produced an amplicon of 1009 base pairs (bp) with the 5’ and 3’ conserved segment primers of the int1 integron. Upon sequencing, int1 was shown to contain the aminoglycoside-resistant gene cassette aadA, which confers resistance to Str and Spt (Table IV). The 3 strains also contained the int1-associated qacEΔI-sulI genes. Southern blot hybridization of plasmid DNA showed that int1 was located on a large plasmid. Two of these strains (00-4923 and 02-1652) were additionally resistant to Gen, Kan, Neo, and Spt and had been isolated twice over 2 y from veal calves at the same location. Resistance to Gen and Spt was encoded by the aadB and aadA genes, respectively, on the transferable 90-MDa plasmid along with resistance to Amp, Amc, Ctf, Cro, Cef, Fox, Chl, Fen, Str, Sul, and Tet. Strain 02-4818 and its transformant were additionally resistant to Spt, Sxt, and Tmp; resistance to Tmp was encoded by the dhfr1 gene, on the same 90-MDa plasmid that also contained blaCMY-2, other resistance genes, and int1.
In the 12 strains resistant to Kan and Neo, resistance was encoded by the aphA-1 gene, carried on a plasmid of 6, 5, 3.7, or 2.3 MDa.
Genetic relatedness
Analysis by PFGE of the 35 ESC-resistant S. Newport isolates digested with XbaI resulted in 7 distinct patterns, 21 (60%) of the isolates having the same pattern. Digestion with BlnI resulted in 6 patterns, 22 (63%) of the isolates having the same pattern. Analysis of the composite data revealed 8 patterns, 20 (57%) of the isolates displaying the same pattern. The same combination of AMR pattern, plasmid profile, and PFGE pattern was seen in 13 (65%) of 20 isolates from 12 sources. A high degree of relatedness was observed in multiple isolates from some locations on different sampling dates, including locations B (96%), C, D, and E (100%), and F (98%). The PFGE patterns of the isolates from location A were dissimilar (73% relatedness) (Figure 2).
Figure 2.

Genetic relatedness of the strains resistant to extended- spectrum cephalosporins, determined from patterns generated by pulsed- gel electrophoresis after digestion with XbaI and BlnI. The phage types were determined by the use of phages and a preliminary phage typing scheme at the National Microbiology Laboratory in Winnipeg, Manitoba. - — other geographic sources.
Discussion
This study revealed that a high percentage (53%) of the S. Newport isolates submitted were derived from dairy cattle or animal food products of bovine origin. The isolation of S. Newport from frozen and fresh beef and from an inline milk filter at a dairy farm suggests that humans might acquire S. Newport via the food chain (1,6); those consuming unpasteurized milk might be at increased risk of acquiring salmonellosis by this route (32). The clinical and pathological findings in animals infected with S. Newport suggest that S. Newport causes severe septicemic salmonellosis in susceptible animals and that infection with this pathogen is associated with significant morbidity and mortality in dairy cattle and calves, turkeys, horses, and possibly other animal species. Measures should be taken to prevent human infection with MDR S. Newport via the food chain or by direct contact with infected animals. Such measures might include prohibiting the sale of overtly infected calves and cows, isolating and treating infected animals, providing education on the risks of consuming unpasteurized milk, and promoting the prudent use of antimicrobials by veterinarians and farmers.
The epidemiologic observations were based on passive laboratory- based Salmonella surveillance. Such a surveillance system has inherent biases. Many factors, including intensity of surveillance, severity of disease, and association with recognized outbreaks, affect the number of isolates submitted. However, the data do permit broad comparisons and identification of trends.
Of the 70 S. Newport strains isolated during 2000–2002, 50% were resistant to amoxicillin/clavulanate, ampicillin, cefoxitin, ceftiofur, ceftriaxone, cephalothin, chloramphenicol, florfenicol, streptomycin, sulfisoxazole, and tetracycline. Resistance to amoxicillin/clavulanate, ampicillin, cefoxitin, ceftiofur, and cephalothin and reduced susceptibility to ceftriaxone are typical of strains producing the AmpC β-lactamase encoded by the blaCMY-2 gene. Such strains are resistant to the penicillins, cephalothin, and the 3rd-generation cephalosporins, including the cephamycins. The β-lactamases produced by these strains are not bound by β-lactamase inhibitors such as clavulanic acid and sulbactam (21,29,33). Each of the 35 MDR S. Newport strains and their transformants harbored a large plasmid (approximately 80 to 90 MDa, or 120 to 135 Kb) that hybridized with the blaCMY-2 DNA probe. The sequence of the amplicon was completely homologous with that of the plasmid-borne blaCMY-2 gene in Klebsiella pneumoniae (33) and in S. Typhimurium DT104, S. Bredeney, and S. Ohio isolates (29).
Remarkably, all 35 strains carried not only the blaCMY-2 gene but also the flost gene (34) on the large plasmid. Previously, we found the plasmidic flost gene in only 1 of 90 MDR S. Typhimurium DT104 strains, whereas in the other 89 strains, the flost and other AMR genes (excluding blaCMY-2) were chromosomally located within int1 (11). In the present study, int1 was found on a 90-MDa plasmid in 3 of the 35 MDR S. Newport strains, with additional resistance to gentamicin and spectinomycin or to spectinomycin, sulfamethoxazole– trimethoprim, and trimethoprim. In the previous study (11), all but 1 of the MDR S. Typhimurium DT104 strains produced amplicons of 1009 or 1133 bp (25), or both, with the int1 primers. In contrast, the 3 S. Newport strains in the current study with the additional resistances produced only the 1009-bp amplicon. This suggests that the 3 strains had only 1 plasmidic type-1 integron, containing the aadA gene and conferring resistance to spectinomycin and streptomycin, and not another plasmidic integron harboring the blaPSE-1 gene (25) and conferring resistance to the penicillins and carbenicillin (22). These findings are reminiscent of those of Rankin and colleagues (2), who identified the int1 gene, the blaCMY-2 gene, and other AMR genes on a large plasmid in 16 of 42 MDR S. Newport isolates.
There were 4 AMR patterns and 6 AMR-gene profiles for the 35 resistant strains studied. This suggests that the large plasmids encoding the AMR differ in composition and size. Previously, Carattoli and associates (35) reported that the blaCMY-2 gene was encoded on plasmids with 3 different backbone structures (types A, B, and C) in Salmonella strains isolated from several sources in the United States, whereas Giles and coworkers (36) found only the type C plasmid in 23 S. Newport isolates with 3 AMR patterns.
Additional resistance to kanamycin and neomycin was present in 12 of the 35 MDR S. Newport strains and was encoded in all cases by the aphA-1 gene, located on a small plasmid (2.3 to 6 MDa). These findings are similar to those observed with S. Typhimurium DT104 (11): resistance to kanamycin and neomycin was present in 50 of 51 strains and was also encoded by the aphA-1 gene on a small plasmid (2.0 MDa).
Conjugation is likely an important mechanism by which resistance to ESCs and other antimicrobials spreads among S. Newport, other Salmonella serovars, and other members of the Enterobacteriaceae. The 90-MDa plasmid was transferred by conjugation to the recipient from 6 of 24 strains (25%); 3 strains possessed 90- and 2.3-MDa plasmids that cotransferred at 28°C, and the other 3 strains possessed 90- and 72-MDa plasmids that cotransferred at 37°C. We have not examined the presence of tra and finO genes (37) on these large plasmids and do not know if these genes would also reside on the 72-MDa plasmid. Further evidence of the importance of conjugation in transferring AMR, including resistance to the ESCs, was shown in a recent study: a conjugative plasmid containing the blaCMY-2 and other resistance genes transferred readily from a commensal E. coli to S. Newport and other commensal E. coli in the intestinal tract of turkey poults (38). These findings support the resistance-gene reservoir hypothesis (39) that bacteria in transit or temporarily present in the ceca and colon acquire or transfer resistance genes by conjugation to the resident intestinal microflora. Therefore, it is tempting to speculate that bacteria such as MDR S. Newport may play a role as resistance-gene “traffickers” by exchanging resistance genes with other intestinal bacteria and thereby contributing to the bacterial gene reservoir in the intestines.
Of the 35 MDR S. Newport isolates, 20 had the same PFGE profile when digested with XbaI and BlnI, which indicated genetic relatedness. The same combination of AMR pattern, plasmid profile, and PFGE pattern was seen in 13 of 20 strains that had been isolated from 12 sources. However, differences in PFGE pattern, AMR pattern, and plasmid profile were found among isolates from location A, a veal-calf operation that received multiple entries of calves from a variety of sources (Figure 2). This suggests that methods to prevent the spread of S. Newport and other infections on dairy, veal-calf, cow-calf, and other cattle farms would be to limit the entry of other animals into the herd and to segregate new arrivals from the existing herd until they are shown to be free of deleterious infections.
This study showed that 50% of the S. Newport strains isolated during the latter part of the review period were resistant to the ESCs and other antimicrobials. Some isolates were additionally resistant to gentamicin and trimethoprim and, because they harbored int1 genes on the same plasmid as genes encoding drug resistances, they could acquire additional resistance genes through insertion of gene cassettes. The plasmids encoding the drug resistances were selftransmissible in 25% of the strains. Treatment options would be very limited upon infection of the animal or human host with such MDR pathogens capable of causing serious illness. Every effort should be made to prevent the spread of significant zoonotic pathogens such as ESC-resistant S. Newport.
Acknowledgments
We thank Linda Cole and Betty Wilkie of the OIE Reference Laboratory for Salmonellosis for serotyping the isolates, Kristen Rekker for carrying out the AMR studies, and Sara Hahn, Nathan Larson, Alyssa Loughborough, and Kristen Brown, co-op students at the University of Guelph, for their assistance with the molecular studies.
References
- Gupta A, Fontana J, Crowe C, et al. for the National Antimicrobial Resistance Monitoring System PulseNet Working Group. Emergence of multidrug-resistant Salmonella enterica Newport infections resistant to expanded-spectrum cephalosporins in the United States. J Infect Dis. 2003;188:1707–1716. doi: 10.1086/379668. [DOI] [PubMed] [Google Scholar]
- Rankin SC, Aceto H, Cassidy J, et al. Molecular characterization of cephalosporin-resistant Salmonella enterica serotype Newport isolates from animals in Pennsylvania. J Clin Microbiol. 2002;40:4679–4684. doi: 10.1128/JCM.40.12.4679-4684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, White DG, McDermott PF, et al. Identification and expression of cephamycinase blaCMY-2 genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob Agents Chemother. 2001;45:3647–3650. doi: 10.1128/AAC.45.12.3647-3650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur PL, Vonstein DL, Hoffman LJ, Uhlenhopp EK, Doern GV. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob Agents Chemother. 2001;45:2716–2722. doi: 10.1128/AAC.45.10.2716-2722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitout JDD, Reisbig MD, Mulvey M, et al. Association between handling of pet treats and infection with Salmonella enterica serotype Newport expressing the AmpC β-lactamase, CMY-2. J Clin Microbiol. 2003;41:4578–4582. doi: 10.1128/JCM.41.10.4578-4582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spika JS, Waterman SH, Soo Hoo GW, et al. Chloramphenicolresistant Salmonella Newport traced through hamburger to dairy farms: a major persisting source of human salmonellosis in California. N Engl J Med. 1987;316:565–570. doi: 10.1056/NEJM198703053161001. [DOI] [PubMed] [Google Scholar]
- Helms M, Vastrup P, Gerner-Smidt P, Mølbak K. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg Infect Dis. 2002;8:490–495. doi: 10.3201/eid0805.010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard [NCCLS Document M31-A]. Villanova, Pennsylvania: NCCLS, 1999.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; Eighth Informational Supplement [NCCLS Document M100-S12]. Villanova, Pennsylvania: NCCLS, 2002.
- Poppe C, Ayroud M, Ollis G, et al. Trends in antimicrobial resistance of Salmonella isolated from animals, foods of animal origin, and the environment of animal production in Canada, 1994–1997. Microbial Drug Resist. 2001;7:197–212. doi: 10.1089/10766290152045084. [DOI] [PubMed] [Google Scholar]
- Poppe C, Ziebell K, Martin L, Allen K. Diversity in antimicrobial resistance and other characteristics among Salmonella Typhimurium DT104 isolates. Microbial Drug Resist. 2002;8:107–122. doi: 10.1089/107662902760190653. [DOI] [PubMed] [Google Scholar]
- Croza JH, Falkow S. Plasmids. In: Gerhardt P, Murray RGE, Costilow RN, et al, eds. Manual of Methods for General Bacteriology. Washington, DC: American Society for Microbiology, 1981:266–282.
- Poppe C, McFadden KA, Demczuk WHB. Drug resistance, plasmids, biotypes and susceptibility to bacteriophages of Salmonella isolated from poultry in Canada. Int J Food Protect. 1996;29:325–344. doi: 10.1016/0168-1605(96)00960-9. [DOI] [PubMed] [Google Scholar]
- Macrina FL, Kopecko KJ, Jones KR, Ayers DJ, McCowen SM. A multiple plasmid-containing E. coli strain: convenient source of size reference plasmid molecules. Plasmid. 1987;1:417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Provence DA, Curtiss R III. Gene transfer in gram-negative bacteria. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, eds. Methods for General and Molecular Biology. Washington, DC: American Society for Microbiology, 1994:317–347.
- Bullas LR, Ryu J-I. Salmonella typhimurium LT2 strains which are r− m+ for all 3 chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y-L, Mancino V, Birren B. Transformation of Escherichia coli with large DNA molecules by electroporation. Nucleic Acids Res. 1995;23:1990–1996. doi: 10.1093/nar/23.11.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan D, Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990;223:156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- Madsen L, Aarestrup FM, Olson JE. Characterisation of streptomycin resistance determinants in Danish isolates of Salmonella Typhimurium. Vet Microbiol. 2000;75:73–82. doi: 10.1016/s0378-1135(00)00207-8. [DOI] [PubMed] [Google Scholar]
- Prescott JF. Aminoglycosides and aminocyclitols. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial Therapy in Veterinary Medicine. Ames, Iowa: Iowa State University Press, 2000: 191–228.
- Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpCtype β-lactamases. Antimicrob Agents Chemother. 2002;46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott JF. Sulfonamides, diaminopyrimidines and their combinations. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial Therapy in Veterinary Medicine. Ames, Iowa: Iowa State University Press, 2000:290–314.
- Meunier D, Baucheron S, Chaslus-Dancla E, Martel J-L, Cloeckaert A. Florfenicol resistance in Salmonella enterica serovar Newport mediated by a plasmid related to R55 from Klebsiella pneumoniae. J Antimicrob Chemother. 2003;51:1007–1009. doi: 10.1093/jac/dkg141. [DOI] [PubMed] [Google Scholar]
- Sandvang D, Aarestrup FM, Jensen JB. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol Lett. 1998;160:37–41. doi: 10.1111/j.1574-6968.1998.tb12887.x. [DOI] [PubMed] [Google Scholar]
- Bissonnette L, Roy PH. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radström P, Swedberg G. RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob Agents Chemother. 1988;32:1684–1692. doi: 10.1128/aac.32.11.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KJ, Poppe C. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by β-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can J Vet Res. 2002;66:137–144. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Standardized Molecular Subtyping of Foodborne Bacterial Pathogens by Pulsed-Field Gel Electrophoresis. Atlanta, Georgia: CDC, 1999.
- Liebisch B, Schwarz S. Molecular typing of Salmonella enterica subsp. enterica serovar Enteritidis isolates. J Med Microbiol. 1996;44:52–59. doi: 10.1099/00222615-44-1-52. [DOI] [PubMed] [Google Scholar]
- West AM, Martin SW, McEwen SA, Clarke RC, Tamblyn SE. Factors associated with the presence of Salmonella spp. in dairy farm families in Southwestern Ontario. Can J Public Health. 1988;79:119–123. [PubMed] [Google Scholar]
- Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmidic β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother. 1996;40:221–224. doi: 10.1128/aac.40.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn AA, Nawaz MS, Kahn SA, Cerniglia CE. Detection of multidrug-resistant Salmonella typhimurium DT104 by multiplex polymerase chain reaction. FEMS Microbiol Lett. 2000;182:355–360. doi: 10.1111/j.1574-6968.2000.tb08921.x. [DOI] [PubMed] [Google Scholar]
- Carattoli A, Tosini F, Giles WP, et al. Characterization of plasmids carrying CMY-2 from expanded-spectrum cephalosporin- resistant Salmonella strains isolated in the United States between 1996 and 1998. Antimicrob Agents Chemother. 2002;46:1269–1272. doi: 10.1128/AAC.46.5.1269-1272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles WP, Benson AK, Olson ME, et al. DNA sequence analysis of regions surrounding blaCMY-2 from multiple Salmonella plasmid backbones. Antimicrob Agents Chemother. 2004;48:2845–2852. doi: 10.1128/AAC.48.8.2845-2852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd EF, Hartl DL. Recent horizontal transmission of plasmids between natural populations of Escherichia coli and Salmonella enterica. J Bacteriol. 1997;179:1622–1627. doi: 10.1128/jb.179.5.1622-1627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Martin LC, Gyles CL, et al. Acquisition of resistance to extended-spectrum cephalosporins by Salmonella enterica subsp. enterica serovar Newport and Escherichia coli in the turkey poult intestinal tract. Appl Environ Microbiol. 2005;71:1184–1192. doi: 10.1128/AEM.71.3.1184-1192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, Gupta A, Wang Y. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 2004;12:412–416. doi: 10.1016/j.tim.2004.07.004. [DOI] [PubMed] [Google Scholar]