Abstract
The initial recognition and binding of adenovirus vector to the host cell surface is mediated by interaction between the adenovirus fiber knob protein and its receptor, the coxsackievirus and adenovirus receptor (CAR). This natural tropism of adenovirus vector needs to be ablated in order to achieve targeted gene transfer. To this end, we noted that adenovirus serotype 40 (Ad40) contains two distinct long and short fibers; the short fiber is unable to recognize CAR, while the long fiber binds CAR. We generated adenovirus serotype 5-based mutants with chimeric Ad40-derived fibers, which were composed of either long or short shafts together with CAR binding or nonbinding knobs. The capacity of these adenovirus mutants for in vitro and in vivo gene transfer to liver cells was examined. In the case of primary human hepatocytes displaying a high expression level of CAR and αv integrin, both CAR binding ability and fiber shaft length played important roles in efficient transduction. Most significantly, the high transduction efficiency observed in the liver and spleen following intravenous administration of adenovirus vector was dramatically reduced by both ablation of fiber-CAR interaction and the use of replaceable short fiber. In other tissues displaying a low level of transduction, no significant differences in transduction efficiency were observed among adenovirus vector mutants. Furthermore, incorporation of a 7-lysine-residue motif at the C-terminal end of CAR-nonbinding short fiber efficiently achieved transduction of target cells via the heparan-containing receptor. Our results demonstrated that the natural tropism of adenovirus in vivo is influenced not only by fiber-CAR interaction but also by fiber shaft length. Furthermore, our strategy may be useful for retargeting adenovirus to particular tumors and tissue types with specific receptors.
Because adenovirus vectors are capable of efficiently delivering genes to a variety of cell types, they have been used in a number of gene therapy approaches (1,4, 42). The initial recognition and binding of adenovirus vector to the host cell surface is mediated by interaction between the adenovirus fiber knob protein and the coxsackievirus and adenovirus receptor (CAR) (3, 32, 40). However, the natural tropism of adenovirus vector for CAR makes it impossible to restrict gene delivery to specific cells in an efficient and safe manner. This natural tropism may limit widespread application of adenovirus vectors. Therefore, several studies have focused on ablation of adenovirus vector tropism as a first step to redirection of adenovirus vectors to specific cell and tissue targets (2, 4, 10, 18, 19, 20, 33).
A number of strategies to alter adenovirus vector tropism have been based on modification of viral capsid protein, in particular fiber protein (48). Chimeric vectors containing adenovirus serotype 3 (Ad3) (38), Ad7 (12), Ad11 (37), Ad17 (6), or Ad35 (35, 36) fibers instead of Ad5 fiber, which recognize receptors other than CAR, resulted in infection through CAR-independent pathways. Furthermore, it was noted that the natural tropism of Ad5 was removed by altering specific amino acid residues in the fiber knob that are involved in CAR binding (2, 4, 18, 19, 20, 33).
It is well known that intravenous administration of an adenovirus vector results mostly in hepatocyte transduction (2, 10, 16, 20). This liver tropism prevents adenovirus vectors from being used to target other cell types by intravenous injection. Nevertheless, intravenously administered CAR interaction-deficient adenovirus vectors primarily localized to the liver, similar to the wild-type Ad5 vector (2, 20). Other factors apart from CAR interaction may play a significant role in the liver tropism displayed by adenovirus vectors. In this context, it has recently been reported that removal of both CAR and integrin interaction leads to effective reduction of liver tropism (10).
We noted that Ad40 contains two distinct long and short fibers (7, 17, 22, 32, 39). The shaft domain from the Ad40 short fiber contains 12 β sheets, while its knob region was hypothesized not to bind to any receptor, including CAR. On the other hand, the shaft from the Ad40 long fiber contains 21 β sheets, while its equivalent knob region can bind CAR (32). Interestingly, the N-terminal tail regions of the Ad40 short and long fibers, which are involved in incorporating the fiber into the penton base (7, 8), are more analogous to the corresponding amino acid sequence regions of the Ad5 fiber than to other adenovirus serotypes (7). As shown in Fig. 5, the adenoviral fiber could be incorporated into the penton base in Ad5 capsid. In addition, the CAR-binding knob from both Ad5 and Ad40 long fibers begin with a TLWT hinge sequence, whereas the CAR-nonbinding knob from the Ad40 short fiber begins with a TIWS hinge sequence. These data provide evidence to support the hypothesis that the Ad40 short fiber has evolved from the Ad5 fiber (17), suggesting that the Ad40 short fiber may be a natural fiber mutant.
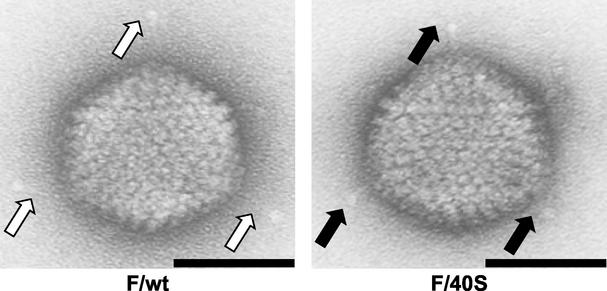
FIG. 5.
Cyto-electron micrographs of F/wt and chimeric F/40S. White arrows mark the wild-type long fiber of Ad5, while black arrows indicate the short fiber of Ad40. Bars, 50 nm.
In this study, we generated fiber mutant Ad5 carrying an Ad40 short fiber instead of the Ad5 wild-type fiber. We demonstrate here that this genetic modification reduced the native tropism of Ad5 both in vitro and in vivo. Next, in order to test whether a ligand incorporated into the Ad40 short fiber could act on its target receptor, we chose a positively charged seven-lysine-residue (K7) motif which binds to the heparan-containing receptor (20, 46, 47). Our results showed that the reduced infectivity could be restored by incorporation of the K7 motif at the C-terminal end of the Ad40 short fiber. Modification of the adenovirus capsid protein via use of the Ad40 short fiber ablated CAR binding and introduced a novel tropism. The finding that the capsid-modified adenovirus, i.e., with tumor-specific ligands, can efficiently infect tumor cells via its target receptor may have significant implications for the development of this vector for gene therapy.
MATERIALS AND METHODS
Cells and animals.
Human embryonic kidney 293 (HEK-293) and U373MG cell lines were purchased from the American Type Culture Collection (Rockville, Md.). The U251 cell line was obtained from the Tumor Registry, Division of Cancer Treatment, National Cancer Institute (Frederick, Md.). Adult human primary hepatocytes were purchased from the Applied Cell Biology Research Institute (Kirkland, Wash.).
All cells except the primary hepatocytes were maintained in Dulbecco's modified Eagle's medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum, 0.2% sodium bicarbonate, 2 mM glutamine, 100 U of penicillin per ml, and 100 mg of streptomycin per ml. The primary hepatocytes were maintained as recommended by the manufacturer (Applied Cell Biology Research Institute).
Six-week-old female C57BL/6 mice were purchased from Japan Charles River (Yokohama, Japan). All mice were fed ad libitum and received humane care in compliance with institutional guidelines for the care and use of laboratory animals in research.
Construction and purification of recombinant fiber mutant adenovirus vector.
The plasmid encoding the human Ad40 fiber was obtained from Riken Gene Bank (RDB no. 1257; Tukuba, Japan). This plasmid was digested with PacI and EcoRI, with the generated Ad40-encoding fragment being blunt ended and ligated with MluI and SalI linkers, respectively. The MluI/SalI fragment was subcloned into the corresponding sites of pSKII+6.7R-2/T (26), resulting in plasmid pSKII+6.7R-2/T/40F. The XbaI/BamHI fragment of pWE6.7R-F/wt-2 (26) was replaced with the XbaI/BamHI fragment of pSKII+6.7R-2/T/40F, resulting in pWE6.7R F/40SL.
The corresponding DNA sequence encoding the 3′ portion of the Ad40 short fiber knob was generated by PCR amplification, with pSKII+6.7R-2/T/40F as the template, with the primers 5′-CCG GAA TTC GGT ACC GGC CTC CAA TTT GAC AAT AAC GGA CGC ATT-3′ and 5′-CGC GGA TCC TTA TTG TTC AGT TAT GTA GCA AAA TAC-3′. The BstXI- and BamHI-digested PCR product was subcloned into pSKII+6.7R-2/T/40F at the corresponding restriction sites, resulting in pSKII+6.7R-2/T/40S. Subsequently, the insert-containing XbaI/BamHI fragment of pSKII+6.7R-2/T/40S was subcloned into pWE6.7R-F/wt-2 at the corresponding restriction sites, resulting in pWE6.7R F/40S.
The corresponding DNA sequence encoding the chimeric fiber of F/540S was generated by PCR amplification, with pSKII+6.7R-2/T and pSKII+6.7R-2/T/40F as templates, with the primer pairs 5′-GGC CTT TAC TTG TTT ACA GC-3′ and 5′-GAT AGA CCA TAT GGT TAG CTT ATC ATT ATT TTT-3′ plus 5′-TCA CTA CCA TAT GGT CTA TCT CGC CTA CGC-3′ and 5′-CGC GGA TCC TTA TTG TTC AGT TAT GTA GCA AAA TAC-3′. The generated PCR products were digested with HindIII and NdeI and NdeI and BamHI, respectively, followed by subcloning into the HindIII and BamHI sites of pSKII+6.7R-2/T by three-part ligation, resulting in pSKII+6.7R-2/T/540S. The insert-containing XbaI/BamHI fragment of pSKII+6.7R-2/T/540S was subcloned into pWE6.7R-F/wt-2 at the corresponding restriction sites, resulting in pWE6.7R F/540S.
The corresponding DNA sequence encoding the chimeric fiber of F/405 was generated by PCR amplification, with pSKII+6.7R-2/T as the template, with the primers 5′-CAG GCC TCC AAT TTG ACA ATA ACG GAC GCA TTA CCA TTA GTA ATC GCA TCC AGA CTC GAG GTG TAA CAT CCC TCA CTA CTT TGT GGA CCA CAC CAG CTC C-3′ and 5′-ATA ACA CAA ACG ATT CTT TA-3′. The StuI- and BamHI-digested PCR product was subcloned into pSKII+6.7R-2/T/40S at the corresponding restriction sites, resulting in pWE6.7R F/405.
The 3′ end of the Ad40 short fiber coding region was followed by a linker segment plus a seven-lysine-residue oligopeptide motif. The 3′ portion of the Ad40 short fiber coding sequence, minus its native stop codon, was generated by PCR amplification with pSKII+6.7R-2/T as the template and primers 5′-CCG GAA TTC GGT ACC GGC CTC CAA TTT GAC AAT AAC GGA CGC ATT-3′ and 5′-CGC GGA TCC TTG TTC AGT TAT GTA GCA AAA TAC AGC-3′. BstXI- and BamHI-digested PCR product was subcloned into pSKII+6.7R-2/T/40F as described above, resulting in pSKII+6.7R-2/T/40S-minus.
The DNA sequence encoding the linker region and K7 motif was generated by PCR amplification with pWE6.7R F/wt-2 as the template and primers 5′-CGA GAT CTG GTT CAG GCA GTG GAT CCG GAT CTA AAA AGA AGA AAA AGA AGA AGT AAG TCG ACA AGA ATA AAG AAT CG-3′ and 5′-GTT GAG CAA CCG CAA GTT GGA CAG-3′. The generated PCR product was digested with BamHI and AflII and subcloned into the XbaI and AflII sites of pWE6.7R F/40S by three-part ligation, together with the XbaI- and BamHI-digested fragment of pSKII+6.7R-2/T/40S-minus, resulting in pWE6.7R F/40S-K7. The predicted amino acid sequence of the C terminus of the F/40S-K7 fiber is P377TAVFCYITEQ387PSASASASAPGSGS401KKKKKKK408, whereas the N-terminal 387 amino acids are from the Ad40 short fiber, followed by the linker region and the K7 motif.
The 25-kbp EcoRI fragment of pAxKM (26), including Ad5 genomic DNA, was subcloned into the EcoRI site of pWE6.7R-F/40SL, -F/40S, -F/540S, -F/405 and -F/40-K7, resulting in the cosmids pWEAxKM-F/40SL, -F/40S, -F/540S, -F/405, and -F/40-K7, respectively. F/wt (26) is human adenovirus type 5 in which the E1A and E1B genes have been replaced with the lacZ gene under the control of a CAG promoter (28). Corresponding fiber mutant adenoviruses expressing the lacZ gene, F/40SL, F/40S, F/540S, F/405, and F/40-K7, were generated with DNA from the above-mentioned cosmids as described previously (26).
The resulting recombinant adenoviruses were propagated within the 293 cells and purified by CsCl equilibrium centrifugation as described previously (15). Purified virion preparations were dialyzed against 10 mM phosphate-buffered saline (PBS)-10% glycerol and finally stored at −80°C. Viral particle numbers (particles per milliliter) were calculated from measurements of optical density at 260 nm (OD260) (23); infectious titers (PFU per milliliter) were determined by plaque assay with 293 cells (15).
Recombinant proteins.
The generation of the recombinant fiber knob and penton base was performed as described previously (26). Each recombinant baculovirus was generated by cotransfection of baculovirus DNA and either pAcHLT-C/Fwt (26) or pAcHLT-A/PB (26) into Sf9 cells, according to the protocol provided by the manufacturer (Pharmingen, San Diego, Calif.). Recombinant Ad5 fiber knob and Ad2 penton base proteins were expressed and purified as described previously (26). Briefly, His-tagged recombinant proteins were produced in Sf9 cells infected with baculovirus, followed by affinity purification with Ni-nitrilotriacetic acid-agarose according to the recommendations of the manufacturer (Qiagen).
Monoclonal antibodies.
Anti-CAR monoclonal antibody RmcB (3) was obtained from J. M. Bergelson (The Children's Hospital of Philadelphia, Philadelphia, Pa.). Anti-αvβ3 integrin monoclonal antibody LM609 and anti-αvβ5 integrin monoclonal antibody P1F6 were purchased from Chemicon International, Inc. (Temecula, Calif.) and Gibco-BRL (Gaithersburg, Md.), respectively. Control mouse immunoglobulin G (IgG) and fluorescein isothiocyanate-conjugated goat anti-mouse IgG were purchased from Sigma and Santa Cruz, respectively.
Flow cytometric analysis.
Cells grown in 10-cm dishes were washed twice with PBS and resuspended in ice-cold PBS containing 2% fetal bovine serum at a concentration of 105 cells/ml. The cells were then incubated for 30 min on ice with a 1:1,000 final dilution of primary antibody ascites RmcB, 7 μg/ml each of primary antibody LM609 or P1F6, or normal murine IgG (negative control). Subsequently, the cells were washed with 2% fetal bovine serum-PBS and incubated for an additional 30 min with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (2.5 μg/ml). After washing with 2% fetal bovine serum-PBS, the cells were analyzed by flow cytometry with a FACScan system with CellQuest software according to the manufacturer's instructions (Becton Dickinson, San Jose, Calif.).
Adenovirus vector infection analysis.
In experiments comparing the transduction efficiencies of Ad5-based mutants with chimeric fibers, 5 × 104 cells were incubated for 30 or 60 min at 37°C with the adenovirus vector and washed with PBS to aspirate the unbound virus, followed by addition of culture medium. After incubation at 37°C for 30 h, the cells were lysed, and β-galactosidase activity was determined with a chemiluminescent reporter gene assay (Galacto-Light Plus; Tropix, Bedford, Mass.) according to the manufacturer's protocol. The protein content of the cell lysates was monitored in parallel with the DC protein assay (Bio-Rad).
Assay of inhibition of adenovirus vector infection.
Approximately 105cells in monolayers were incubated in medium with or without recombinant Ad5 fiber knob and Ad2 penton base protein as well as various amounts of soluble heparin (Sigma). The cells were incubated with recombinant adenovirus vector and washed with medium to remove the unbound virus, followed by addition of culture medium. After incubation at 37°C for 18 h, β-galactosidase activity was determined as described above.
Animal experiments.
The adenovirus vector (2 × 1010 particles) or PBS alone was administered intravenously to C57BL/6 mice. At 2 h postinjection, the animals were killed, and total DNA was extracted from different organs with the Dneasy tissue kit (Qiagen) and assessed by quantitative TaqMan PCR (see below). To detect transgene expression, the animals were killed 4 days postinfection, and LacZ activity in the tumor and various tissues was measured with the Galacto-Light Plus kit as described previously (34).
TaqMan PCR analysis.
The amount of adenovirus genomic DNA in the different organs was quantified with the TaqMan fluorogenic detection system (ABI Prism 7700 sequence detector; Perkin-Elmer Applied Biosystems) as described previously (10). Briefly, samples were prepared with isolated DNA templates from each organ (50 ng) and amplified in 50-μl reactions for 40 cycles with the QuantiTect Probe PCR (Qiagen) kit. Data were collected by continuous fluorescence monitoring and processed with sequence detection system software according to the manufacturer's instructions (Perkin-Elmer Applied Biosystems).
Cyto-electron microscopy.
All purified viral samples were negatively stained with 1% uranyl acetate as described previously (43) and viewed with a JEOL JEM-1010 transmission electron microscope at 100 kV according to the manufacturer's instructions.
RESULTS
Construction and production of chimeric fiber mutant adenovirus vectors.
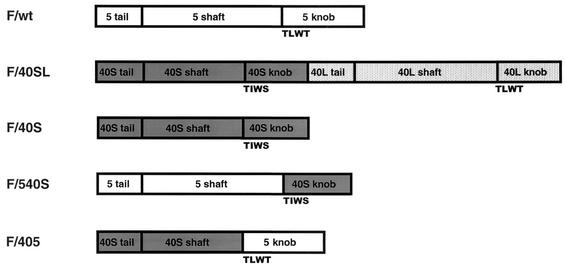
We generated Ad5-based mutants with chimeric fibers. As shown in Fig. 1, each chimeric fiber protein was incorporated into the Ad5 capsid. F/40SL carries the two distinct short and long fibers of Ad40 instead of the Ad5 fiber, while F/40S contains only the Ad40 short fiber. F/540S is composed of the tail and shaft domains from the Ad5 fiber and the knob domain from the Ad40 short fiber, while F/405 is composed of the tail and shaft domains from the Ad40 short fiber and the knob domain from the Ad5 fiber. Recombinant viruses expressing the lacZ gene under the control of a CAG promoter were successfully propagated within 293 cells. There was no evidence of contamination of the viral preparations with E1, as determined by PCR amplification. The correct open reading frame of each of the chimeric fibers was confirmed by DNA sequence analysis (data not shown).
FIG. 1.
Schematic representation of chimeric fiber proteins incorporated into Ad5 capsid. The adenovirus fiber can be divided into three domains, tail, shaft, and knob. The long shafts from the Ad5 and Ad40 long fiber contain 22 and 21 β sheets, respectively; the short shaft from the Ad40 short (40S) fiber contains 12 β sheets. The N-terminal knob domains from the Ad5 and Ad40 long (40L) fiber begin with a TLWT hinge sequence and bind to CAR, whereas the N-terminal knob domain from the Ad40 short fiber begins with a TIWS hinge sequence and is unable to bind to CAR.
All viral stocks were prepared at the same time, and experiments were carried out in a parallel fashion. Particle numbers and infectious titers of adenovirus vector preparations are shown in Table 1. Each chimeric fiber mutant adenovirus vector was readily propagated in 293 cells, with approximately the same number of viral particles produced as by the control, F/wild-type (F/wt). In contrast, the infectivity of F/40S and F/540 was two or three orders of magnitude lower than that of F/wt, F/40SL, and F/405, as shown by an increased particle/PFU ratio (see Discussion).
TABLE 1.
Particle numbers and infectious titers of chimeric adenovirus vectors
| Vector | Titer
|
Particle/PFU ratio | |
|---|---|---|---|
| Particles/mla | PFU/mlb | ||
| F/wt | 1.91 × 1012 | 1.98 × 1011 | 9.65 |
| F/40SL | 9.77 × 1011 | 1.67 × 1010 | 58.5 |
| F/40S | 7.52 × 1011 | 5.40 × 108 | 1,393 |
| F/540S | 8.36 × 1011 | 5.02 × 108 | 1,665 |
| F/405 | 2.20 × 1012 | 2.05 × 1011 | 10.7 |
| F/40S-K7 | 6.23 × 1011 | 5.02 × 109 | 124 |
Calculated from total virion protein and OD260 (1.1 × 1012 virions per OD260).
Assayed by plaquing on 293 cells.
In vitro transduction properties of chimeric fiber mutant adenovirus vectors: use of primary human hepatocytes.
The initial recognition and binding of adenovirus vectors to the host cell surface is mediated by interaction between the adenovirus fiber knob protein and its receptor, CAR. This interaction with CAR needs to be ablated in order to achieve targetable adenovirus vector-based gene transfer. To this end, we noted that Ad40 contains two distinct long and short fibers; the short fiber is unable to recognize CAR, while the long fiber binds CAR. It has been hypothesized that replacement of the Ad5 fiber with that of Ad40, via genetic manipulation, leads to ablation of the natural CAR-binding tropism of Ad5. Therefore, we generated four kinds of chimeric fiber mutant adenovirus vector with Ad40 fiber and investigated the transduction properties of these adenovirus vector mutants.
Since intravenous administration of adenovirus vector is well known to result mostly in hepatocyte transduction (2, 10, 16, 20), we examined the efficiency of gene transfer to primary human hepatocytes of the generated adenovirus vector mutants. First, we evaluated the relative expression levels of CAR as well as αvβ3- and αvβ5-type integrins in primary hepatocytes by flow cytometry. The hepatocytes displayed a high expression level of CAR and αvβ5-type integrins and slight expression of αvβ3-type integrins (Fig. 2A). To test whether replacement of the endogenous fiber would affect the infectivity of the chimeric adenovirus vector mutants, the efficiency of gene transfer to primary hepatocytes was examined at a multiplicity of infection of either 100 or 1,000 particles/cell.
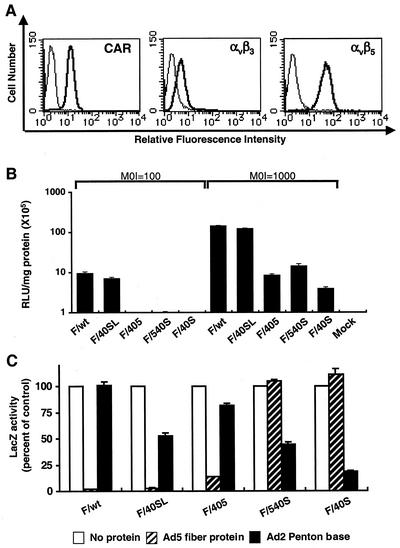
FIG. 2.
In vitro gene transfer to primary hepatocytes with adenovirus vectors containing chimeric fiber and inhibition of gene transfer by recombinant fiber knob or penton base protein. (A) CAR, αvβ3-type, and αvβ5-type integrin expression (heavy lines) in human primary hepatocytes was determined by flow cytometric analysis. The thin lines show the results for the corresponding negative controls. (B) The primary hepatocytes were incubated with the adenovirus vectors at a multiplicity of infection (MOI) of either 100 or 1,000 particles/cell for 1 h and then assayed for β-galactosidase activity at 30 h postinfection. RLU, relative light units. (C) The specificity of gene delivery to the primary hepatocytes of adenovirus vectors containing chimeric fiber (1,000 particles/cell) was examined by competition with recombinant Ad5 fiber (25 μg/ml) or Ad2 penton base (50 μg/ml) protein. After competition for 30 min at 4°C, the cells were washed with Dulbecco's modified Eagle's medium and infected with the adenovirus vectors for 15 min at room temperature. LacZ activity at 18 h was expressed as the percentage of ithe activity determined in the absence of the competing protein. The results represent means ± standard deviation of triplicate determinations.
LacZ-expressing adenovirus vectors F/wt (containing wild-type Ad5 fiber) and F/40SL efficiently infected the CAR- and αv-integrin-positive hepatocytes. Interestingly, the degree of gene transduction exhibited by F/405 was >20-fold lower than that of F/wt, even though F/405 is composed of the Ad40 short fiber shaft and CAR-binding Ad5 fiber knob. Regarding adenovirus vector mutants containing the CAR-nonbinding Ad40 short fiber, F/40S and F/540 were >40-fold and >10-fold lower, respectively, than F/wt in transduction efficiency. These results suggest that adenovirus-mediated infection of primary human hepatocytes is influenced not only by fiber-CAR interaction but also by fiber shaft length (Fig. 2B).
To determine the specificity of gene transduction with the chimeric fiber adenovirus vector mutants, we used recombinant fiber knob or penton base proteins as competitors in the hepatocyte transduction studies. As shown in Fig. 2C, the Ad5 fiber protein blocked >90% of gene transduction observed with F/wt, F/40SL, and F/405 (each carrying the CAR-binding fiber knob), whereas Ad5 fiber protein did not block transduction of CAR-nonbinding F/40S or F/540. These results showed that the CAR-mediated entry pathway is responsible for the infectivity of CAR-binding F/wt, F/40SL, and F/405. In contrast, the Ad2 penton base protein blocked gene transduction by 82% in the case of F/40S and partially inhibited gene transduction of F/40SL and F/540 by 47% and 55%, respectively (Fig. 2C). These data demonstrated that the integrin-mediated entry pathway is mainly responsible for the infectivity of CAR-ablated F/40S and may be at least partially involved in the infectivity of F/540. These results also showed that for F/40SL, the CAR-mediated and integrin-mediated entry pathways depend on the Ad40 long and short fibers, respectively.
Biodistribution of chimeric fiber mutant adenovirus vectors.
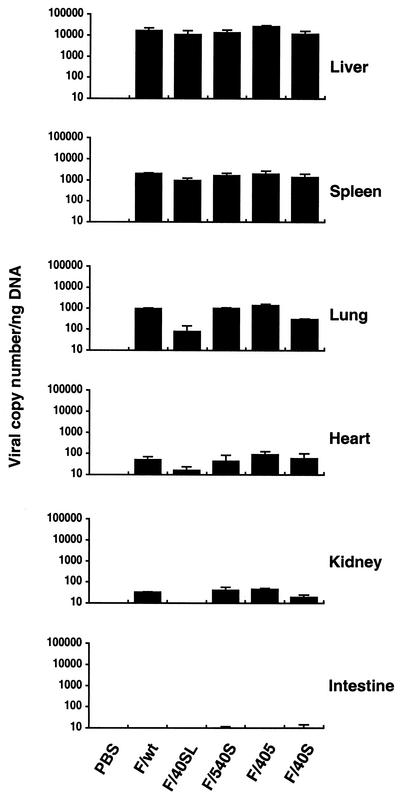
Tropism to the liver prevents adenovirus vectors from being used to target specific cells by intravenous injection. To address this question, we evaluated the in vivo biodistribution of CAR-binding and CAR-ablated chimeric fiber adenovirus vector mutants. The adenovirus vector (2 × 1010 particles) or PBS was administered intravenously to C57BL/6 mice. At an early stage (2 h) post-adenovirus injection, more than 80% or 10% of the total viral DNA was detected in the liver and spleen, respectively, with no significant difference in the amount of viral DNA being found between CAR-binding and CAR-ablated adenovirus vector mutants (Fig. 3). Less than 10% or 1% of the viral DNA was detectable in the lung or heart and kidney, respectively. The viral DNA in the lung, heart, and kidney was reduced with F/40SL or F/40S, both of which carry the Ad40 short fiber. In addition, viral DNA was nearly undetectable in the intestine.
FIG. 3.
Biodistribution of viral DNA after intravenous injection with adenovirus vectors containing chimeric fiber. The adenovirus vector (2 × 1010 particles) or PBS was administrated intravenously to C57BL/6 mice (four mice per group). After 120 min, DNA extracted from various tissues was used in a quantitative TaqMan PCR assay. The results represent means ± standard deviation.
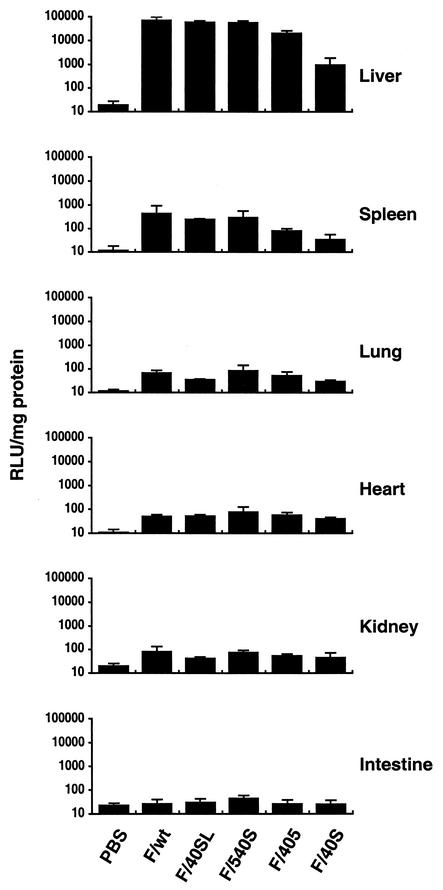
On the other hand, at a late stage (4 days) after intravenous administration, the majority of LacZ expression was detected in the liver, with expression levels being two or more orders of magnitude higher than that found in other tissues (Fig. 4). Most importantly, no differences in transduction efficiency in the liver were found among CAR-binding F/wt and F/40SL and CAR-ablated F/540. In contrast, the transduction efficiency observed with CAR-binding F/405 and CAR-ablated F/40 was about 4- and 64-fold lower, respectively, than with F/wt. The spleen displayed the second highest degree of LacZ expression after the liver.
FIG. 4.
Biodistribution of transgene expression after intravenous injection with adenovirus vectors containing chimeric fiber. The adenovirus vector (2 × 1010 particles) or PBS was administrated intravenously to C57BL/6 mice (four mice per group). After 4 days, LacZ activity was measured in various tissues. The results represent means ± standard deviation. RLU, relative light units.
The expression pattern of adenovirus vector mutants in the spleen was similar to that observed in the liver. While no significant difference in terms of transduction efficiency was found between F/wt, F/40SL, and 540S, the level of gene transduction observed with CAR-binding F/405 and CAR-ablated F/40S was about 5- and 13-fold lower, respectively, than with F/wt. In other tissues, such as the lung, heart, kidney, and intestine, where the level of transgene expression was less than 0.1% compared with F/wt, no significant difference in transduction efficiency was observed among the adenovirus vector mutants. These results demonstrate that the natural tropism of adenoviruses to the liver and spleen is influenced not only by fiber-CAR interaction but also by fiber shaft length. In addition, we generated cyto-electron micrographs of F/wt and F/40S, which displayed a reduced level of transduction to the liver. As shown in Fig. 5, F/wt has the long fiber of wild-type Ad5. The short fiber of F/40S, as in the case of the long fiber, could also be incorporated into the Ad5 capsid.
Incorporation of alternative ligand into chimeric fiber mutant F/40S.
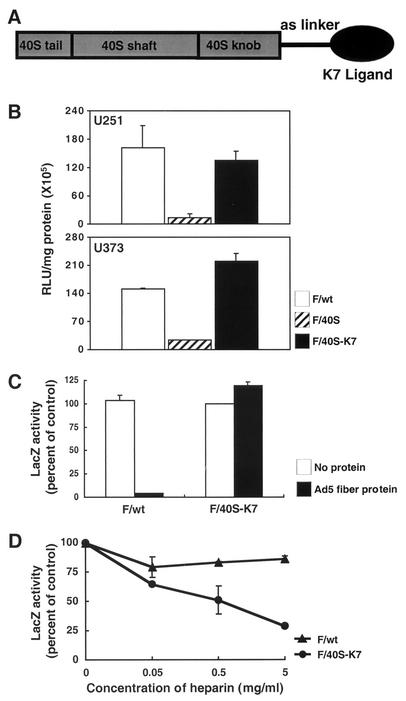
The second requirement for targeted adenovirus vectors is the ability to recognize specific receptors expressed on the surface of target cells. We hypothesized that retargeting of adenovirus vectors could be achieved by genetically incorporating a high-affinity ligand that does not recognize CAR into the C-terminal end of the Ad40 short fiber. To this end, we chose a positively charged seven-lysine-residue motif which binds to the heparan-containing receptor (20, 46, 47). As shown in Table 1, adenovirus vector with the K7 motif added at the C-terminal end of the Ad40 short fiber (F/40S-K7) achieved enhanced infectivity on heparan sulfate-containing 293 cells (particle/PFU ratio = 124), compared with F/40S (particle/PFU ratio = 1,393).
Furthermore, we investigated whether F/40S-K7 could be retargeted to recognize glioblastoma cells. In the glioblastoma cell lines U251 and U373, F/40S showed a low level of gene transduction. In contrast, the retargeted F/40S-K7 dramatically improved the extent of gene transduction, which was comparable to the transduction efficiency seen with F/wt (Fig. 6B). As shown in Fig. 6C, the Ad5 fiber protein blocked >90% of gene transduction observed with F/wt, whereas Ad5 fiber protein did not block transduction of F/40S-K7. In contrast, when a competition experiment was performed under conditions in which the U251 cells were preincubated with competitor (heparin) and infected in the presence of heparin, the transduction of F/40S-K7 was blocked in a dose-dependent manner, while that of F/wt was not (Fig. 6D). These results demonstrated that incorporation of the K7 motif into the C-terminal end of the Ad40 short fiber efficiently achieved adenoviral infection towards target cells via the heparan-containing receptor but not CAR.
FIG. 6.
Retargeted adenoviral infection. (A) Incorporation of a seven-lysine-residue peptide (K7) motif into the C-terminal end of the Ad40 short fiber, together with a linker, permitted binding to a novel target receptor on the cell surface. (B) The glioma cell lines U251 and U373 were incubated with the adenovirus vectors at a multiplicity of infection of 1,000 particles/cell and then assayed for β-galactosidase activity. The specificity of gene transfer to U251 cells of retargeted adenovirus vector (1,000 particles/cell) was assessed through competition with recombinant Ad5 fiber (25 μg/ml) (C) or various doses of heparin (D). After competition for 30 min at 4°C, the cells were infected with the adenovirus vector for 30 min at room temperature. LacZ activity at 18 h was expressed as a percentage of its activity in the absence of the competing protein. The results represent means ± standard deviation of triplicate determinations.
DISCUSSION
Both ablation of fiber-CAR interaction and use of replaceable short fiber were found to reduce natural adenovirus tropism to the liver, as determined with four different kinds of chimeric fiber mutant adenovirus vector. After titration of total adenovirus vector concentration (calculated from optical density measurements at 260 nm), the same physical particle titers (approximately 1012 particles/ml) were shown for each of the different viruses. In contrast, the titers, which show infectivity and replication of adenovirus vectors in 293 cells, correlated with the presence of the CAR-nonbinding Ad40 short fiber protein. The ratio of biologically infectious adenovirus to the total virion number (particle/PFU ratios) in the case of F/40S and F/540S containing the CAR-nonbinding fiber knob protein was two orders of magnitude lower than that of F/wt, F/40SL, and F/405 carrying the CAR-binding fiber knob. These results confirmed the critical role of the fiber-CAR interaction in adenovirus vector-mediated infection of 293 cells.
We also found that, when propagated in 293 cells, F/40S and F/540S formed plaques at a slower rate than F/wt, F/40SL, and F/405. Despite this slow formation of plaques, the plaque morphology exhibited by use of F/40S and F/540S was similar to that seen with F/wt, F/40SL, and F/405. These data suggest that F/40S and F/540S were able to infect, albeit inefficiently, via an alternative route not requiring CAR interaction and were capable of propagating in 293 cells in a standard fashion.
It has been reported that the natural tropism of Ad5 is ablated by altering specific amino acid residues that are involved in CAR binding in the fiber knob protein (2, 4, 18, 19, 20, 33). These studies demonstrated that the residues within the CAR-binding region that were directly involved in interaction were located in the AB loop, the B β-sheet, and the DE loop, while the residues indirectly involved were located in the FG loop. On the other hand, the infectivity of Ad5 has reported to be influenced not only by fiber-CAR interaction but also by other factors, such as the length of the fiber shaft domain (35). To test this concept, we used the short fiber of Ad40, which is hypothesized not to bind to any receptor, including CAR (32). As expected, the transduction efficiency in primary human hepatocytes of F/40S containing short CAR-nonbinding fiber was 40-fold lower than that observed with F/wt. On the other hand, the transduction efficiency of adenovirus vector mutants containing either CAR-binding short fiber or CAR-nonbinding long fiber was 20- and 10-fold lower, respectively, than that displayed by F/wt.
Furthermore, we determined the specificity of transduction with chimeric fiber adenovirus vector mutants in primary hepatocytes. The CAR-mediated entry pathway was critically responsible for the infectivity of CAR-binding F/wt and F/405. In contrast, the integrin-mediated entry pathway was mainly responsible for the infectivity of CAR-ablated F/40S and may be at least partially involved in determining the infectivity of F/540. The infectivity of F/40SL with both CAR-binding and CAR-nonbinding fiber depended on the two pathways, suggesting that only one fiber is incorporated into the Ad5 penton base, as in the case of Ad40 (17). Taken together, these results demonstrate that the differences in the transduction efficiencies observed with F/40S, F/540S, and F/405 were associated with fiber shaft length as well as CAR-binding ability and suggest that the short fiber plays an important role in reducing adenovirus vector native tropism.
Recently, Shayakhmetov et al. demonstrated that to achieve efficient infection via both fiber-CAR and RGD-integrin interactions, CAR-binding fiber requires a long shaft, such as that present in Ad5, which maintains the appropriate distance between the penton-localized RGD motif and CAR-binding knob (35). This concept was consistent with our results in that even if F/405, which has a CAR-binding short fiber, can infect via the fiber-CAR interaction, the level of gene transduction was reduced compared with its long-shafted counterpart, F/wt. Notably, the transduction efficiency of F/40S was reduced a further fourfold compared with that displayed by F/540S even though both mutants contain the CAR-ablated Ad40-derived short fiber knob. In addition, transduction mediated by F/40S was more susceptible to inhibition by penton base protein than that of F/540S (82% versus 55%).
Another difference between the two adenovirus vector mutants, apart from fiber shaft length, lies within the N-terminal tail region of the fiber, which takes part in incorporating the fiber in the penton base (7, 8). Since the tail of F/540 is derived from Ad5 fiber, it should be stably incorporated into Ad5 capsid. In the case of F/40S, the tail from the Ad40 short fiber may impact the interaction with the Ad5 penton base. In contrast to the fiber shaft and knob regions, the tail region is highly conserved among human adenovirus serotypes. In this context, it has been reported that the Ad5 fiber can be incorporated into the penton base of Ad3 particles (44). In addition, considering that the amino acid sequences of the Ad40 short and long fibers are more analogous to corresponding Ad5 fibers than fibers from other adenovirus serotypes (7), the Ad40 short fiber would be expected to incorporated into the Ad5 penton base in a similar manner to the Ad5 fiber.
Electron microscopy results confirmed that the Ad40 short fiber could be incorporated into the Ad5 capsid. On the other hand, a specific receptor for the Ad40 short fiber may contribute to infectivity. To test for this possibility, we demonstrated that neither F/40S nor F/540, which contain Ad40 short fiber, showed enhanced transduction efficiency in several human tumor cell lines, including A549, Colo320, HeLa, and HLF cells (data not shown), and in various tissues after systemic administration. In particular, although Ad40 is known to be associated with infantile gastroenteritis (39), intravenously administered adenovirus vector mutants do not exhibit intestinal transduction. This suggests that the Ad40 short fiber does not contribute to the natural tropism of Ad40. In the case of the A549 lung tumor cell line, which is positive for both CAR and αv integrin, the reduced transduction efficiency displayed by F/40S and F/540 was blocked by penton base protein but not by the recombinant Ad5 fiber protein or Ad40 short fiber (data not shown). These results support the concept that the cellular receptor recognized by the Ad40 short fiber of adenovirus vector mutants does not exist in vitro and in vivo. Taken together, these data indicate that the difference in transduction efficiency exhibited by the adenovirus vector mutants was related to fiber shaft length, although the elucidation of the exact mechanism(s) requires additional experiments.
It is very significant that while no differences in transduction efficiency were found in the liver among F/wt, F/40SL, and F/540S, the transduction efficiency observed with CAR-binding F/405 and CAR-ablated F/40S was about 4- and 64-fold lower, respectively, than with F/wt. In the spleen, which displayed the second highest degree of LacZ expression after the liver, the transduction efficiency observed with CAR-binding F/405 and CAR-ablated F/40 was about 5- and 13-fold lower, respectively, than that of F/wt, while no significant differences in transduction efficiency were found among F/wt, F/40SL, and F/540S. In other tissues with low levels of transgene expression, such as the lung, heart, kidney, and intestine, no significant differences in transduction efficiency were observed among adenovirus vector mutants.
To clarify the observed differences, we compared the biodistribution in vivo of each recombinant adenovirus by measuring the amount of viral DNA present at an early stage after injection. More than 80% of the viral DNA was detected in the liver, while 10% was detectable in the spleen. Notably, in both tissues, no significant differences in the amount of viral DNA were found between CAR-binding and CAR-ablated adenovirus vector mutants. The viral DNA copy number and expression pattern of adenovirus vector mutants observed in the spleen were similar to those seen in the liver. It is known that most of the intravenously injected adenovirus localizes to the macrophages of the liver and spleen (21, 49). In addition, Alemany et al. demonstrated that more than 99% of the adenovirus in blood was cleared by the reticuloendothelial system, within mainly Kupffer cells, in the first hour after injection (1). Based on these observations, our results indicate that the early-phase biodistribution of circulating adenovirus vector in the liver and spleen does not depend on CAR-dependent entry but is influenced by the size and charge of the adenovirus (22).
In this context, it has been reported that CAR- and αv integrin-independent interactions are closely related to the in vivo tropism of Ad5 after systemic administration (11, 50). Thus, the observed changes in the biodistribution of adenovirus vector mutants containing CAR-binding or CAR-ablated short fiber could be seen at a later stage following intravenous administration. In contrast to our strategy of using the Ad40 short fiber, the liver distribution of CAR-nonbinding virus having a genetic mutation of the Ad5 fiber was not altered compared with that of F/wt (2, 20). We also observed a similar phenomenon in that no differences in transduction of the liver and spleen were found between F/wt and F/540 carrying a CAR-nonbinding knob instead of the Ad5 fiber.
The difference in terms of shaft length may be one of the keys to understanding the mechanism of adenovirus vector native tropism to the liver and spleen. In the case of the Ad5 vector with a CAR-binding short-shafted fiber, such as that from Ad8 and Ad9, the exposed loops of hexon from Ad5 are highly negatively charged compared to each original adenovirus serotype (35). This suggested that the charge-dependent repulsion between the hexon of the Ad5 capsid and acidic cell surface proteins could prevent efficient transduction via CAR- or αv integrin-mediated binding (35). It should be noted that the entire capsid of Ad40 is considerably less negatively charged than the Ad5 virion due to lack of the highly acidic region present in Ad5 (41). From these observations, we postulated that when the Ad40 short fiber is incorporated into the Ad5 capsid, the adenovirus vector mutant should become strongly negatively charged.
Since many tissues, including the liver and spleen, commonly contain proteins with acidic residues at the cell membrane or in the extracellular matrix, electronic repulsion may be responsible for reducing the natural tropism of adenovirus vector mutants. This hypothesis was supported by our findings in both liver and spleen tissue. The level of gene transduction displayed by F/405, which has a CAR-binding short fiber, was reduced compared to that of F/wt, which has a CAR-binding long fiber. The CAR-nonbinding short-shafted F/40S further decreased the efficiency of transduction compared to F/405, although the CAR-nonbinding, long-shafted F/540S demonstrated the same degree of transduction as exhibited by F/wt. Taken together, these results demonstrated that the natural tropism of adenoviruses to the liver and spleen is influenced by not only fiber-CAR interaction but also fiber shaft length.
When conventional adenovirus vectors are applied systemically, the great majority of the vector viruses are distributed to the liver (2, 10, 16, 20). This liver tropism prevents adenovirus vectors from being used to target specific cells by intravenous administration. To address this question, we generated chimeric fiber mutants with CAR-nonbinding short fiber and demonstrated that the efficiency of transduction to the liver (and spleen) was reduced up to 64-fold in the chimeric adenovirus vector compared with control Ad5 vectors. Recently, Einfeld et al. showed that a combined CAR- and αv integrin-nonbinding adenovirus vector resulted in a >700-fold decrease of gene transduction to the liver, while the CAR-nonbinding adenovirus vector alone displayed only a 10-fold decrease in transduction efficiency (10).
Therefore, the construct F/40S, with the possibility of integrin interaction having been removed, might be more useful in terms of ablating infection to the liver. However, the penton base-αv integrin interaction has been reported to play important roles in viral internalization on the cell surface via receptor-mediated endocytosis as well as in cell signaling and in release of viral cores from endosomes (27). In this regard, when integrin interaction-ablated adenovirus vectors were used as in vivo targeting vectors, either the incorporated ligands and/or the targeting receptor may also be required to facilitate internalization via endocytosis. Alternatively, it has been noted that transcriptional targeting, including the use of a cell type-specific promoter, was applied to adenovirus vectors with a bispecific molecule which blocks the CAR interaction and recognizes a targeting receptor (9, 31). This adenovirus vector with only the biospecific molecule for targeting to angiotensin-converting enzyme resulted in a 67% reduction in liver transduction following systemic administration (31). Considering that gene transduction of the liver by F/40S carrying CAR-ablated short fiber could be reduced to 98% of that of the control F/wt, the residual liver transduction may be overcome by transcriptional targeting.
The purpose of this study was to develop targeted adenovirus vectors via genetic manipulation in the hope of restricting gene delivery to specific cells both efficiently and safely. Several strategies have been reported to ablate CAR binding and introduce novel tropisms in cultured cells by incorporating specific ligands into the Ad5 fiber with a CAR-nonbinding mutation (20, 33), although further modifications would be necessary for in vivo targeting applications. In this study, we demonstrated that the reduced infectivity of the CAR-nonbinding F/40S was restored in vitro by the incorporation of a K7 motif into the C-terminal end of the Ad40 short fiber. In addition, competition experiments clearly showed that F/40S with the K7 peptide achieved ligand-mediated infection via the heparan sulfate receptor.
The very low infectivity of F40/S appears to be due to the lack of the first step of adenovirus infection, i.e., attachment of the fiber to CAR, but not due to critical impairment of the subsequent steps, such as internalization (45), intracellular trafficking (24), and endosome escape (25). This was also supported by our results showing that αv integrin-mediated transduction of F/wt in CAR-negative primary human dermal fibroblasts was comparable to that of F/40S (data not shown).
Finally, our studies focused on tumor-specific gene transduction by systemic delivery. The phage display system is a powerful tool to identify multiple peptide ligands that could be used to specifically target adenovirus vectors to particular organs, such as the brain, lungs, skin, kidneys, and pancreas (29, 30), as well as various kinds of tumors in vivo (5, 13). Modification of the capsid protein of adenovirus by the Ad40 short fiber ablated CAR binding and introduced a novel tropism. If a variety of other peptide ligands work just as well as the K7 peptide in the 40S fiber, our strategy would be very useful for the development of targeted adenovirus vectors. Although improvements remain to be made, further modifications, including the use of transcriptional targeting and ablation of the αv integrin-interaction, should lead to more specific and efficient infection.
Acknowledgments
We thank M. Kitabatake, Y. Ito, and N. Kawano for technical assistance with adenovirus development, histological analysis, and animal experiments. We are grateful to J. M. Bergelson for providing the anti-CAR monoclonal antibody RmcB. We also thank Stephen J. Russell (Mayo Clinic, Rochester) for helpful discussions.
This work was supported in part by grants from the Ministry of Education, Culture and Science of Japan and from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Alemany, R., K. Suzuki, and D. T. Curiel. 2000. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 81:2605-2609. [DOI] [PubMed] [Google Scholar]
- 2.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 28:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 5.Burg, M. A., R. Pasqualini, W. Arap, E. Ruoslahti, and W. B. Stallcup. 1999. NG2 proteoglycan-binding peptides target tumor neovasculature. Cancer Res. 59:2869-2874. [PubMed] [Google Scholar]
- 6.Chillon, M., A. Bosch, J. Zabner, L. Law, D. Armentano, M. J. Welsh, and B. L. Davidson. 1999. Group D adenoviruses infect primary central nervous system cells more efficiently than those from group C. J. Virol. 73:2537-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chroboczek, J., R. W. H. Ruigrok, and S. Cusack. 1995. Adenovirus fiber, p. 164-200. In W. Doerfler and P. Bohm (ed.), The molecular repertoire of adenovirus. Springer-Verlag, Berlin, Germany.
- 8.Devaux, C., M. L. Caillet-Boudin, B. Jacrot, and P. Boulanger. 1987. Crystallization, enzymatic cleavage, and the polarity of the adenovirus type 2 fiber. Virology 161:121-128. [DOI] [PubMed] [Google Scholar]
- 9.Douglas, J. T., B. E. Rogers, M. E. Rosenfeld, S. I. Michael, M. Feng, and D. T. Curiel. 1996. Targeted gene delivery by tropism-modified adenoviral vectors. Nat. Biotechnol. 14:1574-1578. [DOI] [PubMed] [Google Scholar]
- 10.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. R. King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75:11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fechner, H., A. Haack, H. Wang, X. Wang, K. Eizema, M. Pauschinger, R. Schoemaker, R. Veghel, A. Houtsmuller, H. P. Schultheiss, J. Lamers, and W. Poller. 1999. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 6:1520-1535. [DOI] [PubMed] [Google Scholar]
- 12.Gall, J., A. Kass-Eisler, L. Leinwand, and E. Falck-Pedersen. 1996. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong, F. D., and G. L. Clayman. 2000. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res. 60:6551-6556. [PubMed] [Google Scholar]
- 14.Jolly, D. 1994. Viral vector systems for gene therapy. Cancer Gene Ther. 1:51-64. [PubMed] [Google Scholar]
- 15.Kanegae, Y., Y. Kanegae, G. Lee, Y. Sato, M. Tanaka, M. Nakai, T. Sakaki, S. Sugano, and I. Saito. 1995. Efficient gene activation in mammalian cells by with recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res. 23:3816-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kass-Eisler, A., E. Falck-Pedersen, D. H. Elfenbein, M. Alvira, P. M. Buttrick, and L. A. Leinwand. 1994. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1:395-402. [PubMed] [Google Scholar]
- 17.Kidd, A. H., J. Chroboczek, S. Cusack, and R. W. Ruigrok. 1993. Adenovirus type 40 virions contain two distinct fibers. Virology 192:73-84. [DOI] [PubMed] [Google Scholar]
- 18.Kirby, I., E. Davison, A. J. Beavil, C. P. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 1999. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR. J. Virol. 73:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby, I., E. Davison, A. J. Beavil, C. P. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 2000. Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J. Virol. 74:2804-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leissner, P., V. Legrand, Y. Schlesinger, D. A. Hadji, M. van Raaij, S. Cusack, A. Pavirani, and M. Mehtali. 2001. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 8:49-57. [DOI] [PubMed] [Google Scholar]
- 21.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei, Y. F., and G. Wadell. 1995. Molecular determinants of adenovirus tropism. Curr. Top. Microbiol. Immunol. 199:213-228. [DOI] [PubMed] [Google Scholar]
- 23.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, T., K. Sato, and H. Hamada. 2002. Effective gene transfer to human melanomas via Integrin targeted adenoviral vectors. Hum. Gene Ther. 13:613-626. [DOI] [PubMed] [Google Scholar]
- 27.Nemerow, G. R., and P. L. Stewart. 1999. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 29.Pasqualini, R., and E. Ruoslahti. 1996. Organ targeting in vivo with phage display peptide libraries. Nature 380:364-366. [DOI] [PubMed] [Google Scholar]
- 30.Rajotte, D., W. Arap, M. Hagedorn, E. Koivunen, R. Pasqualini, and E. Ruoslahti. 1998. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Investig. 102:430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds, P. N., S. A. Nicklin, L. Kaliberova, B. G. Boatman, W. E. Grizzle, I. V. Balyasnikova, A. H. Baker, S. M. Danilov, and D. T. Curiel. 2001. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 19:838-842. [DOI] [PubMed] [Google Scholar]
- 32.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing Adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 34.Shaper, N. L., A. Harduin-Lepers, and J. H. Shaper. 1994. Male germ cell expression of murine beta 4-galactosyltransferase. A 796-base-pair genomic region, containing two cAMP-responsive element (CRE)-like elements, mediates male germ cell-specific expression in transgenic mice. J. Biol. Chem. 269:25165-25171. [PubMed] [Google Scholar]
- 35.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stecher, H., D. M. Shayakhmetov, G. Stamatoyannopoulos, and A. Lieber. 2001. A capsid-modified adenovirus vector devoid of all viral genes: assessment of transduction and toxicity in human hematopoietic cells. Mol. Ther. 4:36-44. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson, S. C., M. Rollence, J. Marshall-Neff, and A. McClelland. 1997. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J. Virol. 71:4782-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiemessen, C. T., and A. H. Kidd. 1995. The subgroup F adenoviruses. J. Gen. Virol. 76:481-497. [DOI] [PubMed] [Google Scholar]
- 40.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toogood, C. I., R. Murali, R. M. Burnett, and R. T. Hay. 1989. The adenovirus type 40 hexon: sequence, predicted structure and relationship to other adenovirus hexons. J. Gen. Virol. 70:3203-3214. [DOI] [PubMed] [Google Scholar]
- 42.Trapnell, B. C., and M. Gorziglia. 1994. Gene therapy with adenoviral vectors. Curr. Opin. Biotechnol. 5:617-625. [DOI] [PubMed] [Google Scholar]
- 43.Valentine, R. C., and H. G. Pereira. 1965. Antigens and structure of the adenovirus. J. Mol. Biol. 13:13-20. [DOI] [PubMed] [Google Scholar]
- 44.Von Seggern, D. J., J. Kehler, R. I. Endo, and G. R. Nemerow. 1998. Complementation of a fibre mutant adenovirus by packaging cell lines stably expressing the adenovirus type 5 fibre protein. J. Gen. Virol. 79:1461-1468. [DOI] [PubMed] [Google Scholar]
- 45.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 46.Wickham, T. J., P. W. Roelvink, D. E. Brough, and I. Kovesdi. 1996. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 14:1570-1573. [DOI] [PubMed] [Google Scholar]
- 47.Wickham, T. J., E. Tzeng, L. L. Shears 2nd, P. W. Roelvink, Y. Li, G. M. Lee, D. E. Brough, A. Lizonova, and I. Kovesdi. 1997. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 71:8221-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wickham, T. J. 2000. Targeting adenovirus. Gene Ther. 7:110-114. [DOI] [PubMed] [Google Scholar]
- 49.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]
- 50.Ye, X., M. Jerebtsova, and P. E. Ray. 2000. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine, and kidney. Hum. Gene Ther. 11:621-627. [DOI] [PubMed] [Google Scholar]