Abstract
Hyperthyroidism can increase the renal excretion of magnesium and thus cause hypomagnesemia in various species. Anaerobically collected blood samples from 15 hyperthyroid and 40 normal, healthy cats were analyzed with an ion-selective electrode analyzer and a serum biochemical analyzer. There was no significant difference in ionized or total serum magnesium concentration between the 2 groups, but there was a significant difference (P = 0.004) in the ratio of ionized to total serum magnesium concentrations between the healthy cats and the hyperthyroid cats with thyroxine (T4) concentrations at or above the median. There was a significant correlation (r = 0.894, P = 0.000) between the ionized and total magnesium concentrations in the hyperthyroid cats. The hyperthyroid cats had a significantly lower (P = 0.003) total serum protein concentration than the healthy cats. A significant negative correlation (r = −0.670, P = 0.006) was detected between the ionized magnesium and logarithmically transformed total T4 concentrations in the hyperthyroid cats, which suggests that the severity of hyperthyroidism may contribute to a decrease in the ionized magnesium concentration.
Résumé
L’hyperthyroïdisme peut faire augmenter l’excrétion rénale du magnésium et ainsi causer une hypomagnésémie chez différentes espèces. Des analyses ont été effectuées sur des échantillons de sang prélevés de manière anaérobie de 15 chats hyperthyroïdiens et 40 chats en santé à l’aide d’un analyseur muni d’une électrode sélective d’ions ainsi que d’un analyseur des paramètres biochimiques sériques. Aucune différence significative entre les 2 groupes ne fut notée dans les concentrations sériques de magnésium ionisé ou total. Il y avait toutefois une différence significative (P = 0,004) dans le ratio des concentrations de magnésium ionisé sur le magnésium total entre les chats en santé et les chats hyperthyroïdiens avec des concentrations de thyroxine (T4) égales ou supérieures à la médiane. Chez les chats hyperthyroïdiens, il y avait une corrélation significative (r = 0,894, P = 0,000) entre les concentrations de magnésium ionisé et de magnésium total. Les concentrations sériques de protéines totales étaient significativement inférieures (P = 0,003) chez les chats hyperthyroïdiens comparativement à celles des chats en santé. De plus, chez les chats hyperthyroïdiens, une corrélation négative significative (r = −0,670, P = 0,006) a été trouvée entre les concentrations de magnésium ionisé et les concentrations de T4 totales transformées en logarithme, ce qui suggère que la sévérité de l’hyperthyroïdisme peut contribuer à une diminution de la concentration de magnésium ionisé.
(Traduit par Docteur Serge Messier)
Introduction
Evaluation of the serum magnesium concentration has become more important in recent years in human (1–3) and veterinary critical care and emergency medicine (4–6). Magnesium is integral to many bodily functions, especially those involving the production and use of adenosine triphosphate (ATP) (7). Magnesium is necessary for any reaction using ATP, because the complex MgATP is the required intracellular substrate (8–10). Magnesium also acts as a coenzyme for sodium–potassium ATPase, calcium ATPase, and proton pumps and is crucial for the synthesis of proteins and nucleic acids (7).
Magnesium is the 2nd most prevalent intracellular cation in the body after potassium (8,9). Most of the total body magnesium is in bones and soft tissues, predominantly skeletal muscle and liver (9); only about 1% is in serum, and therefore the serum magnesium concentration may not represent the true tissue magnesium concentrations (8–10). There are 3 forms of serum magnesium: the protein-bound, nonultrafilterable form (accounting for about 25% to 30%) and the ionized and chelated forms (together accounting for the remaining 70% to 75%), both ultrafilterable (11–13). The chelated form represents a small percentage of the ultrafilterable form and exists in a complex with phosphate, citrate, and other compounds (7,13). The ionized form is believed to be the physiologically active component and can be measured with an ion-selective electrode (ISE) (7,10).
Alterations in the total serum magnesium concentration are commonly reported in veterinary medicine (4–6). It has been reported that almost 50% of cats have hypomagnesemia or hypermagnesemia at some time during hospitalization (5).
Hypomagnesemia is most often due to disturbances with the gastrointestinal absorption or renal excretion of magnesium and less often due to changes in distribution within the body (14). Hyperthyroidism can increase the renal excretion of magnesium, thus leading to hypomagnesemia (9,14). Thyroid hormone affects the glomerular filtration rate (15), blood flow, and tubular sodium transport and has a direct effect on tubular calcium and magnesium resorption (16). A complete explanation for alterations in magnesium (including increased urinary excretion) in hyperthyroid patients has not been offered (17). Significant decreases in both ionized and bound (total minus ionized) serum magnesium concentrations have been documented in hyperthyroid human patients (18). Also, it was reported that 42% of hypokalemic human patients had concurrent hypomagnesemia (19). Hypokalemia can be refractory to potassium replacement until normal magnesium levels are restored (20). Magnesium is necessary for normal functioning of the sodium–potassium pump, which is needed for regulation of the intracellular potassium concentration (21). Extracellular magnesium slows the efflux of potassium from the cell on a biophysical basis (22). Therefore, hypomagnesemia can lead to increased loss of potassium from the cells and thus from the body in the urine (22). Because hypokalemia may develop in some hyperthyroid cats (23), the magnesium status could have important therapeutic implications.
The purposes of our study were to measure the ionized and total serum magnesium concentrations and determine their ratio in hyperthyroid cats, to compare the results with those for normal, healthy cats, and to determine whether any relationship exists between the ionized and total serum magnesium concentrations and other biochemical parameters in hyperthyroid cats.
Materials and methods
The study was approved by the Animal Care Committee of the University of Prince Edward Island, Charlottetown, and was carried out in accordance with the guidelines of the Canadian Council on Animal Care.
Samples from hyperthyroid cats
Anaerobically obtained blood samples from hyperthyroid cats were submitted to the Atlantic Veterinary College Diagnostic Services Laboratory, Charlottetown, Prince Edward Island, from regional veterinary clinics. The cats were from among those who were suspected to be hyperthyroid or who had hyperthyroidism that was poorly regulated with methimazole therapy. The samples were submitted in 4-mL glass serum separator tubes (SSTs) with clot activator and gel for serum separation (Vacutainer SST Gel and Clot Activator; Becton Dickinson, Franklin Lakes, New Jersey, USA). All but 2 of the samples were centrifuged prior to shipping. Immediately after the tube was opened, an aliquot of serum was placed in a 250-μL microcentrifuge tube with minimal exposure of the sample to air; the tube was capped and stored at 4°C until the total serum thyroxine (T4) concentration was measured, with a Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, Indiana, USA), less than 2 h later. If the sample had an increased serum T4 concentration, the ionized magnesium concentration was determined in the aliquot.
In this manner, hyperthyroidism was diagnosed in 14 cats, which were included in the study. An additional sample from a hyperthyroid cat was obtained during an initial validation study (24) of the ISE analyzer (Nova CRT8; Nova Biomedical, Mississauga, Ontario) used to measure the ionized serum magnesium concentration. This sample was also obtained anaerobically but was placed in a 10-mL glass tube containing no additives (Vacutainer; Becton Dickinson); the ionized magnesium concentration was determined within 1 hour of collection.
Of the 15 hyperthyroid cats, 3 had never been treated with methimazole, 7 were being treated with this agent, and 1 had previously been treated with methimazole but was not receiving it at the time of sampling; therapeutic information was not available for the remaining 4 cats. A serum biochemical profile (sodium, potassium, chloride, total calcium, total magnesium, urea, creatinine, glucose, and total protein concentrations, as well as alkaline phosphatase and alanine aminotransferase activities) was obtained for all 15 cats by means of a Hitachi 917 analyzer with the use of an aliquot from the collected blood.
Samples from normal, healthy cats
Blood samples were collected anaerobically from 40 owner-volunteered clinically healthy cats that were deemed normal on the basis of the outcome of a physical examination, the same serum biochemical profile as obtained for the hyperthyroid cats, and a serum T4 concentration. These results had recently been used to determine a reference interval for the ionized magnesium concentration in cats in our laboratory (24).
Ionized serum magnesium concentration
To measure the ionized serum magnesium concentration, we used the ISE analyzer and assay that had recently been validated in our laboratory for feline serum (24). The Nova CRT8 also provided a pH measurement for each sample.
Statistical analysis
Data were evaluated for normality by means of descriptive statistics and a normal probability plot. We used 2-sample t-tests to test for significant differences between the hyperthyroid cats and the healthy cats in ionized and total serum magnesium concentrations as well as the concentrations of total protein, potassium, and total calcium, the ratio of ionized to total magnesium concentrations, and pH. We used 1-way analysis of variance to test for significant differences in the ionized magnesium concentrations and the ratio of ionized to total magnesium concentrations between the healthy cats, the hyperthyroid cats with T4 concentrations less than the median (101.3 nmol/L), and the hyperthyroid cats with T4 concentrations equal to or greater than the median. Significant differences were evaluated further with Tukey’s pairwise comparisons. In addition, a 2-sample t-test was used to evaluate any difference in the ionized magnesium concentration between male and female hyperthyroid cats. The Pearson correlation coefficient (r) was calculated to determine relationships between the ratio of ionized to total magnesium concentrations and the total protein concentration in hyperthyroid and healthy cats. Pearson correlation coefficients were also established for the relationships in the hyperthyroid cats between ionized magnesium and logarithmically transformed T4 concentrations, total magnesium and logarithmically transformed T4 concentrations, ionized and total magnesium concentrations, ionized magnesium and total protein concentrations, total magnesium and total protein concentrations, ionized magnesium and potassium concentrations, total magnesium and potassium concentrations, ionized magnesium and total calcium concentrations, and total magnesium and total calcium concentrations.
All the statistical analyses were performed with the Minitab statistical software program, Release 13.32 (Minitab, State College, Pennsylvania, USA). The statistical significance level was set at P < 0.05.
Results
The 15 hyperthyroid cats had a mean age of 13.1 (range 8 to 18) y; 7 were spayed females, 7 were castrated males, and 1 was an intact female. There was no significant difference in the ionized serum magnesium concentration between the male and female cats in either the hyperthyroid group (P = 0.193) or the healthy group (P = 0.257). The data were normally distributed with the exception of the T4 concentrations, which were logarithmically transformed to achieve a normal distribution.
In the hyperthyroid cats, the range of ionized serum magnesium concentrations was 0.42 to 0.65 mmol/L and the range of total magnesium serum concentrations 0.75 to 1.23 mmol/L. In the healthy cats, the corresponding ranges were 0.47 to 0.67 mmol/L and 0.78 to 1.13 mmol/L. Table I presents the mean values for these and the other biochemical parameters evaluated. There were no significant differences between the 2 groups of cats in the mean ionized and total magnesium concentrations, but the hyperthyroid cats had a significantly lower mean ratio of ionized to total magnesium concentration (P = 0.000) and total protein concentration (P = 0.003) than the healthy cats. There were no significant differences between the 2 groups of cats in total calcium concentration, potassium concentration, or pH.
Table I.
Results of 2-sample t-tests of values for biochemical parameters in the serum of healthy and hyperthyroid cats
| Mean value (and standard deviation)
|
|||
|---|---|---|---|
| Healthy cats | Hyperthyroid cats | P-value | |
| Ionized magnesium concentration (mmol/L) | 0.55 (0.040) | 0.54 (0.054) | 0.271 |
| Total magnesium concentration (mmol/L) | 0.93 (0.081) | 0.95 (0.11) | 0.436 |
| Potassium concentration (mmol/L) | 4.12 (0.34) | 4.21 (0.58) | 0.589 |
| Total calcium concentration (mmol/L) | 2.57 (0.11) | 2.60 (0.16) | 0.500 |
| Ionized:total magnesium concentrations | 0.60 (0.037) | 0.56 (0.027) | 0.000a |
| Total protein concentration (g/L) | 72.5 (5.13) | 67.4 (5.03) | 0.003a |
| pH | 7.46 (0.033) | 7.38 (0.16) | 0.073 |
Significant at P < 0.05
Table II presents the Pearson correlation coefficients for the biochemical parameters evaluated in the hyperthyroid cats. There was a significant correlation between the ionized and total magnesium concentrations (r = 0.894, P = 0.000). There was no significant correlation between the total magnesium and logarithmically transformed T4 concentrations. There was, however, a significant negative correlation (r = −0.670, P = 0.006) between the ionized magnesium and logarithmically transformed T4 concentrations (Figure 1). To determine if significant differences existed between the healthy cats and the cats with markedly high T4 concentrations, we split the hyperthyroid group into a group with T4 values below the median of 101.3 nmol/L (n = 7, 4 receiving methimazole) and a group with values equal to or above the median (n = 8, 3 receiving methimazole). There was no significant difference in the ionized magnesium concentration between the healthy cats and each of the 2 groups of hyperthyroid cats (P = 0.054). However, the significant difference in the ratio of ionized to total magnesium concentration between the healthy and hyperthyroid cats was isolated to a difference between the healthy cats and the hyperthyroid cats with higher T4 concentrations by means of Tukey’s pairwise testing (P = 0.004). There was no significant correlation between the ionized magnesium and total protein, potassium, or total calcium concentrations or between the total magnesium and total protein, potassium, or total calcium concentrations in the hyperthyroid cats.
Table II.
Results of Pearson correlations of the biochemical test values for the hyperthyroid cats
| Parameters, concentrations | r | P-value |
|---|---|---|
| Ionized magnesium and logarithmically transformed T4 | −0.670 | 0.006a |
| Total magnesium and logarithmically transformed T4 | −0.426 | 0.114 |
| Ionized and total magnesium | 0.894 | 0.000a |
| Ionized magnesium and total protein | 0.214 | 0.443 |
| Total magnesium and total protein | 0.436 | 0.105 |
| Ionized magnesium and potassium | 0.397 | 0.143 |
| Total magnesium and potassium | 0.498 | 0.059 |
| Ionized magnesium and total calcium | 0.115 | 0.682 |
| Total magnesium and total calcium | 0.160 | 0.569 |
T4 — thyroxine
Significant at P < 0.05
Figure 1.
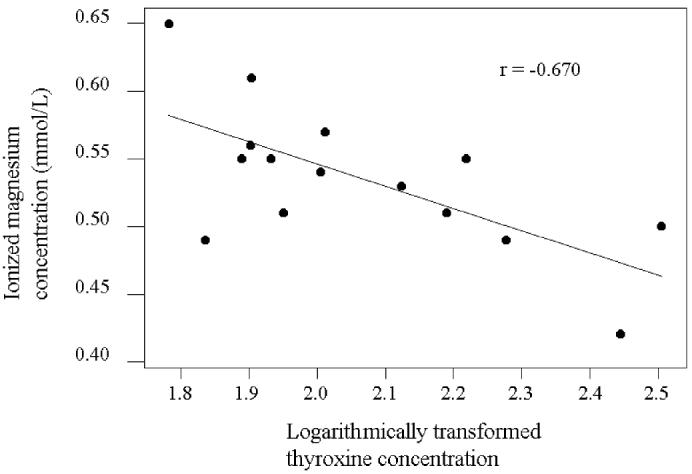
Correlation between ionized magnesium concentrations and logarithmically transformed thyroxine concentrations in hyperthyroid cats.
Discussion
This study found no significant difference in ionized and total serum magnesium concentrations between healthy and hyperthyroid cats. The results were, however, based on a small number of hyperthyroid cats. Although no previous work has evaluated hyperthyroid cats, these results do not agree with the majority of previous reports on magnesium concentrations in hyperthyroid patients of other species. Total plasma magnesium (25), total serum magnesium (26), and ionized and bound plasma magnesium (18) concentrations were decreased in human hyperthyroid patients. Similarly, total serum magnesium concentrations were decreased in untreated human hyperthyroid patients and increased slightly after treatment for hyperthyroidism with methimazole (27). As well, total serum magnesium concentrations increased significantly once euthyroidism was achieved in human hyperthyroid patients (17). A decrease in serum (28) and plasma (29) magnesium concentrations occurred in rats given oral thyroxine supplements. In contrast, 1 study did report that total plasma magnesium concentrations were increased in hyperthyroid rats, although their urinary magnesium excretion was similar to that of euthyroid rats (16).
The most interesting finding in our study was the significant negative correlation between the ionized magnesium and logarithmically transformed T4 concentrations in the hyperthyroid cats. The mean ionized magnesium concentration in the subgroup of hyperthyroid cats with higher T4 concentrations was not significantly lower than that of the healthy cats or the subgroup of hyperthyroid cats with lower T4 concentrations, but this may have reflected the low sample numbers, as the difference was approaching significance (P = 0.054). The negative correlation is not surprising. In humans, a significant negative correlation has been reported between ionized magnesium and free triiodothyronine concentrations (18). Another study in humans reported a significant negative correlation between total plasma magnesium and T4 concentrations, as well as between the erythrocyte magnesium concentration and the duration of the hyperthyroid illness (15). It is therefore possible that the degree of hypomagnesemia may be related to the degree or the duration of the hyperthyroid disease, or both, in feline patients as well, but further study is required.
Changes in the glomerular filtration rate that occur secondary to changes in thyroid hormone concentrations may affect the urinary excretion of magnesium (15). When human hyperthyroid patients underwent therapy for the disease, a significant correlation was found between the increase in the total serum magnesium concentration and the decrease in the renal fractional excretion of magnesium (26). Therefore, it appears that the more severe the hyperthyroid state, the more reduced is the capacity of the kidney to conserve magnesium (26). The other important factor for the regulation of magnesium is intestinal absorption (14). It is possible that the polyphagia present in some hyperthyroid patients may increase the oral intake of magnesium, which could delay the onset of hypomagnesemia (26). Hypomagnesemia may be more likely to develop in severely hyperthyroid patients who instead have decreased food intake (26). Clinical information, such as the presence of polyphagia, systemic hypertension, proteinuria, or diarrhea, which might have been useful in interpreting the magnesium concentration in our study patients, was not readily available. Polyphagia in a majority could assist in explaining the inability to a find a significant difference in magnesium concentrations between this group and the healthy cats. Although the alterations in various analytes, including magnesium, may be slight in hyperthyroidism in some instances and may not be an acute problem for the patient, it is possible that these disturbances may be important for a patient in the long term (17).
Another interesting finding of this study was the significantly lower ratio of ionized to total magnesium concentration in the hyperthyroid cats compared with the healthy cats. When the hyperthyroid group was split, those with higher T4 concentrations had a significantly lower mean value for this ratio than the healthy cats and those with lower T4 concentrations. Alterations in this ratio are usually due to changes in the binding of magnesium to proteins, most notably albumin (13). Because the mean total serum protein concentration for the hyperthyroid cats was lower than that of the healthy cats, a higher rather than a lower ratio of ionized to total magnesium concentration would be expected. This result was therefore surprising, but without knowing the albumin concentrations for the hyperthyroid cats and, ideally, having a larger sample, further conclusions cannot be made.
The reason for the low serum protein concentration in the hyperthyroid cats is not known. Serum proteins can be normal in hyperthyroid cats (23), but it is not uncommon for human hyperthyroid patients to be hypoalbuminemic (15). In the present study, further evaluation of the decreased protein concentrations in the hyperthyroid cats would have necessitated evaluation of the albumin and globulin concentrations, as well as further assessment for other diseases associated with hypoproteinemia.
It was important in the study to evaluate the sample pH, because ionized magnesium concentrations decrease as pH increases, owing to the presence of fewer hydrogen ions competing with magnesium for protein-binding sites (30). Since no difference was noted in pH between the healthy and the hyperthyroid cats, it is likely that any differences between these groups cannot be attributed to pH.
There was no significant difference in serum potassium concentration or in total serum calcium concentration between the healthy and the hyperthyroid cats. Potassium and calcium concentrations were evaluated because hypokalemia has been reported in hyperthyroid cats (23) and calcium disturbances have been reported in human hyperthyroid patients (17,29). As well, in humans, decreased concentrations of calcium or potassium are reported to be good predictors of magnesium deficiency (19). In this study, there was no significant correlation between potassium or total calcium and either ionized or total magnesium concentrations in the hyperthyroid cats. Therefore, the changes that were noted in the ionized magnesium concentrations were probably not due to the effects of potassium or total calcium.
This study found a significant and good correlation between ionized and total serum magnesium concentrations in hyperthyroid cats. This is not surprising: a weak but significant correlation has been reported between ionized and total magnesium concentrations in serum from healthy humans (31). However, even with a good correlation, it is important to note that the total magnesium concentration is not suitable as an estimator of the ionized magnesium concentration (13). This is especially evident in hypomagnesemia or mild hypermagnesemia, in which there is a poor correlation between the total and ionized magnesium concentrations (13). The relationship between total and ionized magnesium can be affected by many factors, such as the quantity of protein bound to or complexed with magnesium, the pH of the sample, and the serum water concentration (13). However, it is the protein concentration, and in particular the albumin concentration, that is the most important determinant of the relationship between ionized and total magnesium concentrations (13).
In conclusion, the hyperthyroid cats in this study had a significantly lower serum protein concentration and a significantly lower ratio of ionized to total magnesium than the healthy cats. We did not find a significant difference in ionized and total magnesium concentrations between the healthy and hyperthyroid cats. However, we did find a significant negative correlation between the ionized magnesium and logarithmically transformed T4 concentrations in the hyperthyroid cats. Therefore, it is possible that cats with more severe disease are more likely to have a decreased ionized magnesium concentration. Further research with larger numbers of hyperthyroid cats is warranted.
Acknowledgments
This study was funded by the Companion Animal Trust Fund, Atlantic Veterinary College.
References
- Salem M, Munoz R, Chernow B. Hypomagnesemia in critical illness: a common and clinically important problem. Crit Care Clin. 1991;7:225–252. [PubMed] [Google Scholar]
- Huijgen HJ, Soesan M, Sanders R, Mairuhu WM, Kesecioglu J, Sanders GT. Magnesium levels in critically ill patients. Am J Clin Pathol. 2000;114:688–695. doi: 10.1309/jr9y-pptx-ajtc-qdrd. [DOI] [PubMed] [Google Scholar]
- Dubé L, Granry J-C. The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: a review. Can J Anaesth. 2003;50:732–746. doi: 10.1007/BF03018719. [DOI] [PubMed] [Google Scholar]
- Martin LG, Matteson VL, Wingfield WE, Van Pelt DR, Hackett TB. Abnormalities of serum magnesium in critically ill dogs: incidence and implications. J Vet Emerg Crit Care. 1994;4:15–20. [Google Scholar]
- Toll J, Erb H, Birnbaum N, Schermerhorn T. Prevalence and incidence of serum magnesium abnormalities in hospitalized cats. J Vet Intern Med. 2002;16:217–221. doi: 10.1892/0891-6640(2002)016<0217:paiosm>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Johansson AM, Gardner SY, Jones SL, Fuquay LR, Reagan VH, Levine JF. Hypomagnesemia in hospitalized horses. J Vet Intern Med. 2003;17:860–867. doi: 10.1111/j.1939-1676.2003.tb02526.x. [DOI] [PubMed] [Google Scholar]
- Martin LG, Van Pelt DR, Wingfield WE. Magnesium and the critically ill patient. In: Kirk R, ed. Kirk’s Current Veterinary Therapy XII. 12th ed. Philadelphia, Pennsylvania: WB Saunders, 1995:128–131.
- Reinhart RA. Magnesium metabolism: a review with special reference to the relationship between intracellular content and serum levels. Arch Intern Med. 1988;148:2415–2420. doi: 10.1001/archinte.148.11.2415. [DOI] [PubMed] [Google Scholar]
- Rosol TJ, Capen CC. Calcium-regulating hormones and diseases of abnormal mineral (calcium, phosphorus, magnesium) metabolism. In: Kaneko JJ, Harvey JW, Bruss ML, eds. Clinical Biochemistry of Domestic Animals. 5th ed. San Diego, California: Academic Press, 1997:619–702.
- Saris N-EL, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- Altura BT, Shirey TL, Young CC, et al. Characterization of a new ion selective electrode for ionized magnesium in whole blood, plasma, serum and aqueous samples. Scand J Clin Lab Invest Suppl. 1994;217:21–36. doi: 10.3109/00365519409095208. [DOI] [PubMed] [Google Scholar]
- Kroll MH, Elin RJ. Relationships between magnesium and protein concentrations in serum. Clin Chem. 1985;31:244–246. [PubMed] [Google Scholar]
- Külpmann WR, Gerlach M. Relationship between ionized and total magnesium in serum. Scand J Clin Lab Invest Suppl. 1996;224:251–258. doi: 10.3109/00365519609088645. [DOI] [PubMed] [Google Scholar]
- Dhupa N, Proulx J. Hypocalcemia and hypomagnesemia. Vet Clin North Am Small Anim Pract. 1998;28:587–608. doi: 10.1016/s0195-5616(98)50057-5. [DOI] [PubMed] [Google Scholar]
- Shibutani Y, Yokota T, Iijima S, Fujioka A, Katsuno S, Sakamoto K. Plasma and erythrocyte magnesium concentrations in thyroid disease: relation to thyroid function and the duration of illness. Jpn J Med. 1989;28:496–502. doi: 10.2169/internalmedicine1962.28.496. [DOI] [PubMed] [Google Scholar]
- McCaffrey C, Quamme GA. Effects of thyroid status on renal calcium and magnesium handling. Can J Comp Med. 1984;48:51–57. [PMC free article] [PubMed] [Google Scholar]
- Ford HC, Crooke MJ, Murphy CE. Disturbances of calcium and magnesium metabolism occur in most hyperthyroid patients. Clin Biochem. 1989;22:373–376. doi: 10.1016/s0009-9120(89)80035-9. [DOI] [PubMed] [Google Scholar]
- Porta S, Epple A, Leitner G, et al. Impact of stress and triiodothyronine on plasma magnesium fractions. Life Sci. 1994;55:PL327–332. doi: 10.1016/0024-3205(94)00772-1. [DOI] [PubMed] [Google Scholar]
- Whang R, Oei TO, Watanabe AA, et al. Predictors of clinical hypomagnesemia. Arch Intern Med. 1984;144:1794–1796. [PubMed] [Google Scholar]
- Whang R, Flink EB, Dyckner T, Wester PO, Aikawa JK, Ryan MP. Magnesium depletion as a cause of refractory potassium repletion. Arch Intern Med. 1985;145:1686–1689. [PubMed] [Google Scholar]
- Martin LG, Wingfield WE, Van Pelt DR, Hackett TB. Magnesium in the 1990’s: implication for veterinary critical care. J Vet Emerg Crit Care. 1993;3:105–114. [Google Scholar]
- Berkelhammer C, Bear RA. A clinical approach to common electrolyte problems: 4. Hypomagnesemia. Can Med Assoc J. 1985;132:360–368. [PMC free article] [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. Feline hyperthyroidism (thyrotoxicosis). In: Canine and Feline Endocrinology and Reproduction. 3rd ed. Philadelphia: WB Saunders, 2004:152–218.
- Gilroy C, Burton S, Horney B, MacKenzie A. Validation of the Nova CRT8 for the measurement of ionized magnesium in feline serum. Vet Clin Pathol. 2005;34:124–131. doi: 10.1111/j.1939-165x.2005.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Frizel D, Malleson A, Marks V. Plasma levels of ionized calcium and magnesium in thyroid disease. Lancet. 1967;1:1360–1361. doi: 10.1016/s0140-6736(67)91766-7. [DOI] [PubMed] [Google Scholar]
- Disashi T, Iwaoka T, Inoue J, et al. Magnesium metabolism in hyperthyroidism. Endocr J. 1996;43:397–402. doi: 10.1507/endocrj.43.397. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Takeuchi K. Magnesium levels and thyroid disease. Lancet. 1967;2:1152–1153. doi: 10.1016/s0140-6736(67)90665-4. [DOI] [PubMed] [Google Scholar]
- Vitale JJ, Hegsted DM, Nakamura M, Connors P. The effect of thyroxine in magnesium requirement. J Biol Chem. 1957;226:597–601. [PubMed] [Google Scholar]
- Simsek G, Andican G, Ünal E, Hatemi H, Yigit G, Candan G. Calcium, magnesium and zinc status in experimental hyperthyroidism. Biol Trace Elem Res. 1997;57:131–137. doi: 10.1007/BF02778196. [DOI] [PubMed] [Google Scholar]
- Wang S, McDonnell EH, Sedor FA, Toffaletti JG. pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med. 2002;126:947–950. doi: 10.5858/2002-126-0947-PEOMOI. [DOI] [PubMed] [Google Scholar]
- Thode J, Juul-Jørgensen B, Seibæk M, Elming H, Borresen E, Jordal R. Evaluation of an ionized magnesium–pH analyzer — NOVA 8. Scand J Clin Lab Invest. 1998;58:127–133. doi: 10.1080/00365519850186706. [DOI] [PubMed] [Google Scholar]


