Abstract
The assembly of human cytomegalovirus (HCMV) with recombinant systems has not been accomplished. An understanding of specific interactions between individual capsid proteins could point to unique characteristics of the assembly process of HCMV capsids. Similar to its herpes simplex virus counterpart, VP26 (UL35), the HCMV smallest capsid protein (SCP; UL48/49) decorates hexons in the mature capsid. In contrast to VP26, the HCMV SCP is essential for virus assembly. In this study we have shown that the major capsid protein (MCP) and the SCP interact in the cytoplasm of transfected cells and can be coprecipitated from insect cells expressing the MCP and the SCP. Using a two-hybrid reporter assay, we demonstrated that two linear sequences within the SCP are sufficient for SCP and MCP interactions.
The capsids of herpesviruses display a high degree of structural homology and likely share closely related assembly pathways. Extensive studies of herpes simplex virus type 1 (HSV-1) have indicated that the mature virion capsid of this virus is composed primarily of four viral proteins (5, 10, 12, 13, 16, 19, 20). The most abundant of these proteins, VP5 (UL19), is a protein with an approximate mass of 149 kDa, and the 150 hexons and 12 pentons of the mature capsid are made up of this viral protein (1, 23, 26). The smallest capsid protein, VP26 (UL35), is present in nearly equimolar amounts and has been shown to decorate hexons in both recombinant derived capsids and capsids from virus-infected cells (23, 25). Interestingly, this abundant capsid protein was shown to be dispensable for capsid formation both in infected cells and in a cell-free capsid assembly system; however, a VP26 deletion mutant exhibited an altered phenotype when mice were infected with this viral mutant (3, 6, 21). Similarly, four abundant virion proteins have been detected in the virion capsid of human cytomegalovirus (HCMV) (7-9, 11). These include the major capsid protein (MCP; pUL86), the minor capsid proteins (MnCPs; pUL85 and pUL46), and the smallest capsid protein (SCP; pUL48/49) (9). Cryoelectron microscopy of HCMV capsids has revealed structural features of the HCMV capsid that are shared with the HSV-1 capsid, including the fact that the SCP and VP26 decorate only the hexons of the mature capsids (4). In contrast to the role of VP26 in HSV-1 assembly, the HCMV SCP was found to be essential for the assembly of infectious virus in HCMV-infected cells (2). A cell-free capsid assembly system for HCMV has not been reported, nor have HCMV capsids been assembled in cells expressing only individual capsid proteins.
Previous studies have demonstrated interactions between individual capsid proteins of herpesviruses that are presumably important for mature capsid assembly. Early studies demonstrated interactions between HSV-1 VP5 and preVP22a, and more recent studies have demonstrated that VP5 also interacts with VP26 (14, 16). Perhaps most interesting for understanding capsid assembly has been the finding by at least two groups that HSV-1 VP5 and VP26 interact but that within capsids the binding of VP26 with VP5 is restricted to the hexons (23, 25). This finding is consistent with the possibility that VP5 exists in different conformations in the pentons and hexons of the capsid, an explanation supported by the reactivities of conformation-specific anti-VP5 monoclonal antibodies (MAbs) (22). We have investigated the binding between the HCMV MCP and the SCP by using several different experimental systems. In this study, we demonstrated that the SCP and the MCP interacted in the cytoplasm of cells transiently expressing these proteins, a finding similar to previously reported findings for cells transfected with plasmids encoding HSV-1 VP5 and VP26. In addition, we have defined two linear sequences in the SCP that together appear to be sufficient for binding to the MCP.
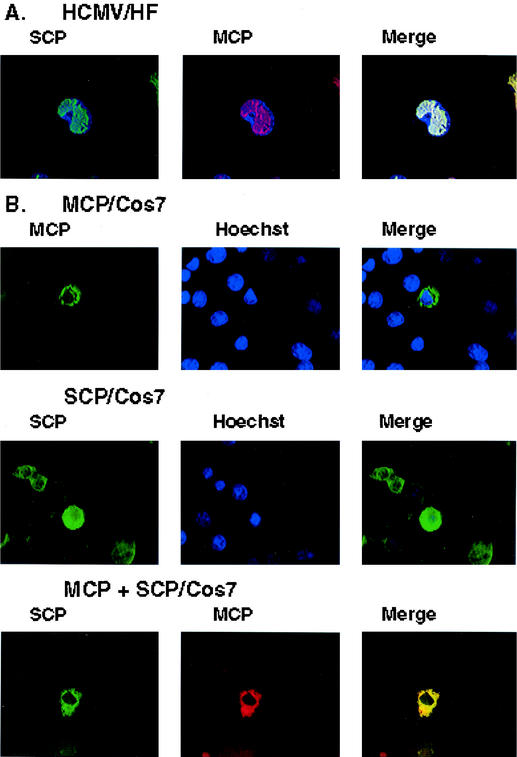
In early studies utilizing murine MAbs specific for the HCMV MCP and SCP, we noted the nuclear localization of both proteins in HCMV-infected fibroblasts. We utilized these MAbs to confirm these earlier findings and to determine if the proteins were spatially associated in the nucleus of infected cells. The expression of both the SCP and the MCP was restricted to the nucleus, and the signals from both proteins completely colocalized in human fibroblasts infected 5 days earlier with HCMV strain AD169 (Fig. 1A). When we studied the intracellular localization of several different HCMV capsid proteins expressed in cells infected with recombinant vaccinia viruses that expressed individual capsid proteins, we observed that the MCP and the SCP colocalized in the cytoplasm of infected cells (data not shown). We further investigated this observation by studying the coexpression of the SCP and the MCP in Cos 7 cells. Expression plasmids were generated by cloning the open reading frames (ORFs) for the SCP (UL48/49) and the MCP (UL86) into the plasmid pcDNA 3.1 (Invitrogen, Carlsbad, Calif.), and expression was verified by both Western blotting and image analysis of 293T cells transfected with these expression plasmids (data not shown). Following transfection of the SCP plasmid into Cos 7 cells, the protein was detected in both cytoplasmic and nuclear compartments (Fig. 1B). In contrast, the MCP was localized only in the cytoplasm in transfected cells (Fig. 1B). When plasmids encoding the SCP and the MCP were cotransfected into Cos 7 cells, we observed nearly complete colocalization of the signals from the SCP and the MCP in the cytoplasm (Fig. 1B). Furthermore, in cotransfected cells, the SCP was no longer detected in the nucleus of transfected cells, indicating that its interaction with the MCP restricted its intracellular distribution (Fig. 1B). These findings provide evidence that the MCP and the SCP may interact in the absence of other viral functions, including other capsid proteins.
FIG. 1.
Image analysis of cellular localization of the SCP and the MCP. (A) Human primary fibroblast (HF) cells infected with HCMV AD169 were fixed 5 days postinfection and processed for image analysis (18). Cells were stained with an anti-SCP MAb (11.2.23) or an anti-MCP MAb (28.4) and developed with a fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) or Texas Red-conjugated anti-mouse IgG (18). Nuclei of infected cells were visualized as blue with Hoechst dye. Colocalization of the signals from the MAbs can be seen in the merge panel as a white signal. (B) Transient expression of the SCP or the MCP in Cos 7 cells. The SCP or the MCP was transiently expressed in Cos 7 cells. In cells cotransfected with the SCP and the MCP, colocalization of the signals from the antibodies can be seen as a yellow signal.
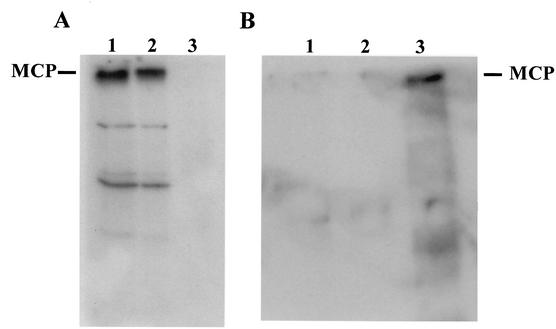
To obtain biochemical evidence of the interaction between the SCP and the MCP, we used an immobilized SCP to precipitate the MCP from a lysate of SF9 cells infected with recombinant baculoviruses encoding the MCP. The UL48/49 ORF (for the SCP) was expressed as an amino-terminal fusion with glutathione S-transferase (GST) and collected on glutathione beads. Following extensive washing, the beads were used to probe a lysate of insect cells infected with a recombinant baculovirus that expressed the MCP. Bound proteins were eluted from the SCP/GST beads and then analyzed by immunoblotting by using an anti-MCP MAb (18). A 150-kDa protein was detected with the MCP-specific MAb when a lysate of insect cells infected with the recombinant baculovirus encoding the MCP was loaded directly on the gel and after the lysate was transferred to membranes (Fig. 2A, lane 1). When the SCP/GST fusion protein immobilized on glutathione beads was used to precipitate proteins from a lysate of infected SF9 cells, a protein of 150 kDa was detected by the MCP-specific MAb, along with two minor species (Fig. 2A, lane 2). A control precipitation with beads containing only immobilized glutathione failed to precipitate the MCP (Fig. 2A, lane 3). To ensure that these interactions did not result from a nonspecific interaction between the MCP and GST, we also used two GST fusion proteins constructed by the fusion of GST with the cytoplasmic tail of gpTRL10 or gpUL100 (glycoprotein M [gM]) as controls. Beads containing either immobilized GST/gpTRL10 or GST/gpUL100 did not precipitate the MCP (Fig. 2B, lanes 1 and 2). In contrast, the SCP/GST precipitated the 150-kDa protein from a lysate of cells infected with recombinant baculovirus encoding the MCP (Fig. 2B, lane 3). These results provide biochemical evidence of an interaction between the SCP and the MCP that is consistent with the imaging findings described above.
FIG. 2.
Precipitation of the MCP by an immobilized SCP. (A) SCP/GST glutathione beads were used to precipitate the MCP from a lysate of SF9 cells infected with a recombinant baculovirus encoding the MCP. The precipitated protein was analyzed by immunoblotting by using a MCP-specific MAb (28.4) (lane 2) (18). Controls included a lysate containing the MCP that was loaded directly on the gel (lane 1) and glutathione beads incubated with a lysate containing the MCP (lane 3). Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the gel was transferred to nitrocellulose and developed with the MCP-specific MAb 28.4. MAb binding reactivity was detected with a rabbit anti-mouse IgG followed by 125I-protein A (17). The migration of the MCP is indicated in the left margin. (B) SCP/GST and GST fusion proteins with the cytoplasmic tail of gpTRL10 (gpULTRL10/GST) or the cytoplasmic tail of gM (gpUL100/GST) bound to glutathione beads were used to precipitate the MCP from a lysate of SF9 cells previously infected with a baculovirus encoding the MCP and analyzed as described above. The gpULTRL10/GST and the gpUL100(gM)/GST(lanes 1 and 2) failed to precipitate the MCP, whereas the SCP/GST beads (lane 3) precipitated the MCP.
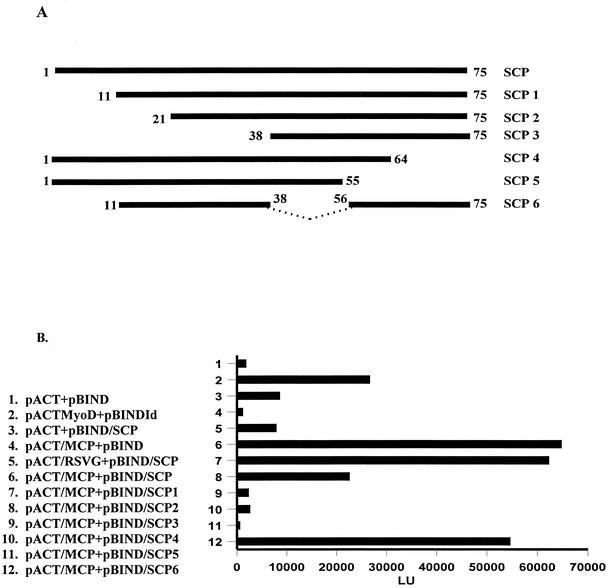
To further define the specificity of the interaction between the SCP and the MCP and to identify sequences within the SCP that may be critical for this interaction, we utilized a mammalian two-hybrid system that permitted us to assay the interactions between linear sequences of the SCP and the MCP following their expression in 293T cells. Initially, we confirmed the interaction of the SCP with the MCP by expressing the MCP as a VP16 fusion protein and the SCP as a Gal4 fusion protein by using a mammalian two-hybrid system (CheckMate; Promega, Madison, Wis). Coexpression of the SCP and the MCP resulted in an approximate sixfold increase of luciferase activity over that in negative controls and a more than twofold increase of luciferase activity over that in a positive control (Fig. 3B, lane 6). We then used this reporter system to analyze regions of the SCP that interacted with the MCP by generating NH2- and COOH-terminal truncations of the SCP followed by coexpression with the full-length MCP. All mutants were constructed by PCR, and nucleotide sequencing confirmed the presence of the ORF after it was inserted into the Gal4 coding sequence. All transfections were normalized by transfecting equivalent amounts of plasmid DNA. The deletion of the first 11 amino acids (aa) of the SCP had no effect on its interaction with the MCP, whereas the deletion of aa 1 to 21 decreased the signal following the interaction between the SCP and the MCP by approximately threefold (Fig. 3). The deletion of an additional 17 aa (aa 1 to 38) completely inhibited the interaction between these two proteins as measured by this assay (Fig. 3). Truncation of the molecule at aa 55 or 65 also eliminated interactions between the SCP and the MCP (Fig. 3). These data suggest that aa 21 to 75 of the SCP are required for the interaction between the SCP and the MCP. We further defined the interacting sequence by generating a polypeptide in which aa 11 to 38 were fused in frame with aa 56 to 75, resulting in a polypeptide of 47 aa. In the two-hybrid assay, the fusion polypeptide bound the MCP nearly as well as the wild-type SCP did, suggesting that aa 11 to 38 and 56 to 75 either contained a single assembled domain that interacted with the MCP or that two linear sequences of the SCP provided cooperative binding of the SCP to the MCP.
FIG. 3.
Mapping of the SCP sequences that bind the MCP with a mammalian two-hybrid system. (A) Constructs used in the two-hybrid assay. The full-length SCP (aa 1 to 75) and NH2 and COOH deletions were generated by PCR and cloned into a pBind vector (CheckMate mammalian two-hybrid system; Promega). (B) Two-hybrid analysis of binding regions of the SCP. The two-hybrid assay with the luciferase reporter was carried out according to the manufacturer's instructions by using equivalent amounts of pACT and pBIND plasmids transfected into 293T cells. In some cases, transfection efficiencies were normalized to control plasmids by using the dual luciferase reporter system. Negative controls included a plasmid with the activation domain of VP16 (pACT) transfected with a plasmid containing the Gal4 binding domain (pBIND; lane 1), pACT plus the coding sequence of the SCP fused with the Gal4 domain (lane 3), and pACT fused with the respiratory syncytial virus G protein coding sequence (RSVG; lane 5). Positive controls include the pACTMyoD and the pBINDId supplied by the manufacturer (lane 2). The mutants of the SCP were fused with pBIND and cotransfected with a plasmid containing the coding sequence of the MCP fused with the activation domain of VP16 (pACT/MCP). Results are reported as light units (LU).
Interactions between the smallest capsid proteins and the major capsid proteins of herpesviruses were initially described by Rixon and colleagues (16). In these studies, coexpression of the major capsid protein VP5 and the smallest capsid protein VP26 resulted in restricted expression of VP26 in the nucleus of transfected cells only when VP5 and VP26 were coexpressed with either preVP22a or VP19c (16). Although biochemical evidence of the binding of VP26 to VP5 was not provided in this report, a subsequent study utilizing recombinant VP26 demonstrated by using cryoelectron microscopy that VP26 bound to purified HSV capsids in a cell-free system (23). Furthermore, in that study and a previous study, VP26 was shown to occupy sites on the hexons but not the pentons of capsids (23, 25). Together with the results of the earlier study by Rixon et al. (16), these findings indicate that the interaction of VP26 with VP5 is likely dependent on the accessibility of the VP26 binding sites on VP5 (23). These sites are presumably not exposed on VP5 in pentons either because the conformation of VP5 in pentons differs from that of VP5 found in hexons or because these sites are occupied by other capsid proteins. We favor the former hypothesis because previous studies utilizing VP5-specific antibodies and biochemical analysis have indicated that the VP5 conformations found in pentons and hexons differ (22). Furthermore, the observation that recombinant VP26 binds only to hexons but not to pentons provides additional evidence for the difference in the conformations of VP5 in pentons and hexons (23). Similarly, in a cryoelectron microscopic analysis of HCMV capsids, the SCP (UL48/49) was shown to decorate only the hexons and not the pentons formed by the MCP (UL86) (4).
Our results were consistent with previous findings of Rixon et al. and indicated that the coexpression of the HCMV SCP with the MCP resulted in restricted expression of the SCP in the cytoplasm of transfected cells (16). In addition, our study also provided biochemical evidence of this interaction by using coprecipitation assays. Together, these findings indicate that the SCP interacts with the MCP in the absence of other capsid proteins and raises the possibility that only the conformation of the MCP that is found in the hexons is sufficient for this interaction. We demonstrated that the interaction occurred in the cytoplasm and that nuclear localization and/or association with UL80 was not required for the interactions between the SCP and the MCP. In addition, we noted only cytoplasmic localization of the SCP when the MCP was coexpressed by using three different recombinant expression systems and several different cell lines (data not shown). These results suggest that the interaction of the SCP with the MCP was not saturable under these conditions and that overexpression of the SCP did not lead to a large pool of protein that nonspecifically interacted with several partners. Furthermore, the interaction between the SCP and the MCP is also suggested by the finding that imaging signals from virus-infected cells indicated that both proteins colocalized and were present only in the nucleus of virus-infected cells. Finally, the use of the mammalian two-hybrid system provided additional evidence of the specificity of the interaction between these two proteins and suggested that a domain or specific linear sequence of the SCP was sufficient for the observed interaction.
Although the capsids of herpesviruses are assembled within the nucleus of infected cells, findings presented in this report indicate that neither the MCP nor the SCP has functional nuclear localization signals. Previously, it has also been shown that the MCP lacks a nuclear localization signal (24). Similarly, neither VP26 nor VP5 has been reported to contain nuclear localization signals, although in contrast to the HCMV MCP, the HSV VP5 has been reported to be distributed throughout the cell in cells transfected with a plasmid encoding VP5 (16). The HSV VP5 and VP26 proteins have been proposed to localize to the nucleus in transfected cells after their interaction with either VP19c or preVP22a (16). Although the possibility was not directly tested, our finding indicates that the interaction between the SCP and the MCP is similar to that described by the latter proposal and, because previous studies have demonstrated that the coexpression of UL80 and the MCP allows translocation of the MCP to the nucleus, suggests that the MCP and the SCP enter the nucleus together (15, 24). Consistent with this proposed nuclear translocation of the SCP together with the MCP is the finding that the SCP can be detected only in the nucleus of virus-infected cells and, even during the late phases of infection, cannot be detected in the cytoplasm. A similar pathway for nuclear importation of VP26 and VP5 of HSV has been proposed (16, 25). An unresolved question is whether the VP26 associated with VP5 is retained in VP5-containing hexons and therefore lost during the conformational changes that occur during penton formation or, alternatively, whether VP26 binds to hexons only after it dissociates from the VP5 molecules that shuttle it into the nucleus (23). Although we have no direct evidence that either model accounts for our findings, we favor the former explanation and suggest that the SCP (UL48/49) interacts with the MCP (UL86) in the cytoplasm and enters the nucleus as a protein complex. The formation of the MCP-containing pentons results in a conformational change and, therefore, the loss of binding by the SCP. If valid, this model suggests that mature HCMV capsid formation could potentially be inhibited at several points along this pathway, such as (i) the interaction between the SCP and the MCP, (ii) the interaction between the SCP/MCP complex and UL80, (iii) the transport of the complex into the nucleus, and (iv) the locking of the MCP into a hexon-only conformation by engineered forms of the SCP, which thus limits penton formation. The recent demonstration by Borst et al. that the SCP is an essential capsid protein and that expression of a green fluorescent protein/SCP fusion from a different loci in the viral genome acted as a dominant negative for virus production raises the possibility that inhibitors could be designed to interrupt or impede capsid assembly and thus function as antiviral agents (2). In this report we have described two amino acid sequences within the SCP that when expressed together appear to be sufficient for its interaction with the MCP. These domains could serve as the basis for the design of small molecule inhibitors of SCP-MCP interactions.
Acknowledgments
We thank Robert LaFemina for the gift of the MCP (UL86) construct and Peter Prevelige for helpful discussions.
This work was supported by PHS grant AI-35602 from the NIAID.
REFERENCES
- 1.Booy, F. P., B. L. Trus, W. W. Newcomb, J. C. Brown, J. F. Conway, and A. C. Steven. 1994. Finding a needle in a haystack: detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus). Proc. Natl. Acad. Sci. USA 91:5652-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borst, E. M., S. Mathys, M. Wagner, W. Muranyi, and M. Messerle. 2001. Genetic evidence of an essential role for cytomegalovirus small capsid protein in viral growth. J. Virol. 75:1450-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, D. H., J. Jakana, D. McNab, J. Mitchell, Z. H. Zhou, M. Dougherty, W. Chiu, and F. J. Rixon. 2001. The pattern of tegument-capsid interaction in the herpes simplex virus type 1 virion is not influenced by the small hexon-associated protein VP26. J. Virol. 75:11863-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, D. H., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 260:10-16. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, G. H., M. Ponce de Leon, H. Diggelmann, W. C. Lawrence, S. K. Vernon, and R. J. Eisenberg. 1980. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J. Virol. 34:521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai, P., N. A. DeLuca, and S. Person. 1998. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology 247:115-124. [DOI] [PubMed] [Google Scholar]
- 7.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, W., M. K. Baxter, and K. S. Clopper. 1996. Cytomegalovirus “missing” capsid protein identified as heat-aggregable product of human cytomegalovirus UL46. J. Virol. 70:7454-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, W., K. S. Clopper, W. J. Britt, and M. K. Baxter. 1996. Human cytomegalovirus (HCMV) smallest capsid protein identified as product of short open reading frame located between HCMV UL48 and UL49. J. Virol. 70:5680-5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson, W., and B. Roizman. 1972. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J. Virol. 10:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irmiere, A., and W. Gibson. 1985. Isolation of human cytomegalovirus intranuclear capsids, characterization of their protein constituents, and demonstration that the B-capsid assembly protein is also abundant in noninfectious enveloped particles. J. Virol. 56:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newcomb, W. W., F. L. Homa, D. R. Thomsen, Z. Ye, and J. C. Brown. 1994. Cell-free assembly of the herpes simplex virus capsid. J. Virol. 68:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newcomb, W. W., B. L. Trus, F. P. Booy, A. C. Steven, J. S. Wall, and J. C. Brown. 1993. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J. Mol. Biol. 232:499-511. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson, P., C. Addison, A. M. Cross, J. Kennard, V. G. Preston, and F. J. Rixon. 1994. Localization of the herpes simplex virus type 1 major capsid protein VP5 to the cell nucleus requires the abundant scaffolding protein VP22a. J. Gen. Virol. 75:1091-1099. [DOI] [PubMed] [Google Scholar]
- 15.Plafker, S. M., and W. Gibson. 1998. Cytomegalovirus assembly protein precursor and proteinase precursor contain two nuclear localization signals that mediate their own nuclear translocation and that of the major capsid protein. J. Virol. 72:7722-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rixon, F. J., C. Addison, A. McGregor, S. J. Macnab, P. Nicholson, V. G. Preston, and J. D. Tatman. 1996. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J. Gen. Virol. 77:2251-2260. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez, V., P. C. Angeletti, J. A. Engler, and W. J. Britt. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication. Characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatman, J. D., V. G. Preston, P. Nicholson, R. M. Elliott, and F. J. Rixon. 1994. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J. Gen. Virol. 75:1101-1113. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen, D. R., L. L. Roof, and F. L. Homa. 1994. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J. Virol. 68:2442-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trus, B. L., F. L. Homa, F. P. Booy, W. W. Newcomb, D. R. Thomsen, N. Cheng, J. C. Brown, and A. C. Steven. 1995. Herpes simplex virus capsids assembled in insect cells infected with recombinant baculoviruses: structural authenticity and localization of VP26. J. Virol. 69:7362-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trus, B. L., W. W. Newcomb, F. P. Booy, J. C. Brown, and A. C. Steven. 1992. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc. Natl. Acad. Sci. USA 89:11508-11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingfield, P. T., S. J. Stahl, D. R. Thomsen, F. L. Homa, F. P. Booy, B. L. Trus, and A. C. Steven. 1997. Hexon-only binding of VP26 reflects differences between the hexon and penton conformations of VP5, the major capsid protein of herpes simplex virus. J. Virol. 71:8955-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood, L. J., M. K. Baxter, S. M. Plafker, and W. Gibson. 1997. Human cytomegalovirus capsid assembly protein precursor (pUL80.5) interacts with itself and with the major capsid protein (pUL86) through two different domains. J. Virol. 71:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou, Z. H., J. He, J. Jakana, J. D. Tatman, F. J. Rixon, and W. Chiu. 1995. Assembly of VP26 in herpes simplex virus-1 inferred from structures of wild-type and recombinant capsids. Nat. Struct. Biol. 2:1026-1030. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, Z. H., B. V. Prasad, J. Jakana, F. J. Rixon, and W. Chiu. 1994. Protein subunit structures in the herpes simplex virus A-capsid determined from 400 kV spot-scan electron cryomicroscopy. J. Mol. Biol. 242:456-469. [DOI] [PubMed] [Google Scholar]