Abstract
Three members of the αV integrin family of cellular receptors, αVβ1, αVβ3, and αVβ6, have been identified as receptors for foot-and-mouth disease virus (FMDV) in vitro. The virus interacts with these receptors via a highly conserved arginine-glycine-aspartic acid (RGD) amino acid sequence motif located within the βG-βH (G-H) loop of VP1. Other αV integrins, as well as several other integrins, recognize and bind to RGD motifs on their natural ligands and also may be candidate receptors for FMDV. To analyze the roles of the αV integrins from a susceptible species as viral receptors, we molecularly cloned the bovine β1, β5, and β6 integrin subunits. Using these subunits, along with previously cloned bovine αV and β3 subunits, in a transient expression assay system, we compared the efficiencies of infection mediated by αVβ1, αVβ3, αVβ5, and αVβ6 among three strains of FMDV serotype A and two strains of serotype O. While all the viruses could infect cells expressing these integrins, they exhibited different efficiencies of integrin utilization. All the type A viruses used αVβ3 and αVβ6 with relatively high efficiency, while only one virus utilized αVβ1 with moderate efficiency. In contrast, both type O viruses utilized αVβ6 and αVβ1 with higher efficiency than αVβ3. Only low levels of viral replication were detected in αVβ5-expressing cells infected with either serotype. Experiments in which the ligand-binding domains among the β subunits were exchanged indicated that this region of the integrin subunit appears to contribute to the differences in integrin utilizations among strains. In contrast, the G-H loops of the different viruses do not appear to be involved in this phenomenon. Thus, the ability of the virus to utilize multiple integrins in vitro may be a reflection of the use of multiple receptors during the course of infection within the susceptible host.
Foot-and-mouth disease virus (FMDV) is one of the most feared viral pathogens of livestock. Outbreaks can result in high morbidity and loss of production in infected animals, but the most devastating economic consequence to affected countries results from mass livestock culling and international trade restrictions imposed on animals and animal products. FMDV is the type species of the Aphthovirus genus of the Picornaviridae family and exists as many subtypes and variants within seven different serotypes.
Limited trypsin digestion of FMDV results in the generation of noninfectious virions that are unable to adsorb to susceptible cells (5) due to the cleavage of VP1 at the Arg (R) residue of a highly conserved Arg-Gly-Asp (RGD) motif (62) located within a flexible external loop between the βG and βH strands (G-H loop) (1, 36, 41). In addition, peptides containing the RGD sequence inhibited the adsorption of the virus to tissue culture cells (6, 21), and genetically engineered virions containing either mutations or deletions of the RGD sequence were unable to bind to cells or cause disease in susceptible animals (37, 45, 46). These observations have led to the conclusion that the virus utilizes cell surface integrin molecules as receptors via this RGD site. Subsequently, it has been demonstrated that at least three integrins, αVβ1, αVβ3, and αVβ6, can serve as receptors for FMDV in vitro (10, 32, 33, 52, 53).
Integrins are heterodimeric type I membrane glycoproteins composed of two subunits (α and β) that interact noncovalently at the cell surface (30). They mediate cell-cell interactions and the binding of cells to the extracellular matrix, and in doing so, they play a crucial role in cell division, differentiation, migration, and survival (23). The eighteen α and eight β mammalian integrin subunits can assemble into 24 different heterodimers (29). While the consensus binding motif of some integrins is unknown, at least eight integrins recognize and bind to ligands via an RGD sequence (63). Interestingly, despite recognizing this tripeptide sequence, these integrins bind different extracellular matrix ligands (40, 69). While the reasons for this specificity are obscure, there is evidence that ligand sequences either flanking the RGD motif or located in other regions of the ligand are responsible for binding specificity (12, 54). One subgroup within the integrin family is the αV integrins, comprising five heterodimers (αVβ1, αVβ3, αVβ5, αVβ6, and αVβ8), all of which recognize the RGD motif on their natural ligands (30, 63).
While the aforementioned observations strongly suggest that RGD-dependent integrins probably direct FMDV to target tissues during a natural infection in susceptible hosts, the fact that the virus can utilize multiple αV integrins in vitro leaves open the question of how these different receptors may function in determining viral pathogenesis. To begin to answer this question, we molecularly cloned the bovine β1, β5, and β6 integrin subunits and, using previously cloned bovine αV and β3 subunits (52), compared αV integrin receptor utilizations among several different representatives of FMDV serotypes A and O. Surprisingly, we found that viruses of these two serotypes utilized these integrins with different efficiencies. We also examined the roles of the β subunit ligand-binding domains (LBDs) and the G-H loops of the different viruses in αV integrin utilization.
MATERIALS AND METHODS
Sequencing of bovine integrin β1, β5, and β6 subunits.
Sequencing of the bovine integrin subunits was performed prior to molecular cloning in order to ascertain the exact sequences of the 5′ and 3′ ends. The primers used for cloning and sequencing of the integrin subunits are listed in Table 1. Total RNA was extracted from primary bovine tongue keratinocytes (55) (for the β1 and β5 subunits) and from secondary calf thyroid cells (for the β6 subunit) by using Trizol (Invitrogen) as described by the manufacturer. cDNA was amplified from total RNA, which was primed with oligo(dT), by using SuperScriptII (Invitrogen). All PCR amplifications were performed with Pfu polymerase (Stratagene). For the amplification of the β1 subunit, the forward primers 34 and 35 (Table 1) were selected from alignments of the GenBank human and feline β1 integrin sequences X07979 and U27351, respectively, and the reverse primers 36 and 37 (Table 1) were selected from the GenBank partial bovine sequence U10815. A 2.2-kbp β1 fragment was amplified by PCR with primers 34 and 36 followed by a nested PCR with primers 35 and 37. For the amplification of the β5 subunit, forward primer 9 and reverse primer 11 (Table 1) were selected from consensus sequences in the alignment of human and murine sequences (GenBank accession numbers J05633 and AF022110, respectively) and used to amplify a 2.1-kbp specific β5 fragment by PCR. For the amplification of the β6 subunit, forward primer 38 and reverse primer 40 (Table 1), selected from alignments of the human and murine β6 sequences (GenBank accession numbers NM000888 and AF115376, respectively), were used to amplify a 2.2-kbp fragment by PCR. The PCR products were inserted into the Zero Blunt TOPO vector (Invitrogen), as described by the manufacturer, and sequenced on an ABI 3700 DNA analyzer (Applied Biosystems) by using an ABI Prism Big Dye terminator cycle-sequencing ready reaction kit (Perkin-Elmer). The central sequences of these bovine integrin genes were used to design primers for the amplification and sequencing of the 5′ and 3′ ends by using a 5′/3′ RACE kit (Roche Molecular Biochemicals) as described by the manufacturer. Sequence analysis was done using the Lasergene analysis software package (DNASTAR Inc.).
TABLE 1.
Primers used in cloning and sequencing of bovine integrin β subunits
| Primer no. | Sequence (5′ → 3′) | Subunit(s) | Orientation | Source(s)a | Useb |
|---|---|---|---|---|---|
| 34 | CTGGATTGGACTGATCAGTTC | β1 | Forward | Human, cat | Sequencing |
| 35 | GCAAATGCCAAATCATGTGGAG | β1 | Forward | Human | Sequencing |
| 36 | CTCATACTTCGGATTAAC | β1 | Reverse | Bovine | Sequencing |
| 37 | GTGTCCCATTTGGCATTC | β1 | Reverse | Bovine | Sequencing |
| 9 | GGTCTCAACATATGCACTAGTG | β5 | Forward | Human | Sequencing |
| 11 | CCGGTCGTGGATGGTGAC | β5 | Reverse | Human, bovine | Sequencing |
| 38 | GTACAAGGTGGCTGTGCC | β6 | Forward | Human | Sequencing |
| 80 | GGTACCTCTAGATTATCAACTGGGGGCGGTCCCACAC | β5 | Reverse | Cloning | |
| 40 | TCCCGTTTGCCACTTGGC | β6 | Reverse | Human, murine | Sequencing |
| 84 | GGTCTAGACTCGAGCCGCGGGAGAAGATG | β1 | Forward | Cloning | |
| 71 | TCCACAGACACACTCTCC | β1 | Reverse | Cloning | |
| 70 | TTGATCCCTAAGTCAGCG | β1 | Forward | Cloning | |
| 97 | GGAATTCTTAAGGTACCTTATCATTTTCCCTCATACTTCGGATTAAC | β1 | Reverse | Cloning | |
| 91 | GGTCTAGACTCGAGGGCGCCCCACCATGCCG | β5 | Forward | Cloning | |
| 31 | GCTGCAAATGTGGCCATC | β5 | Reverse | Cloning | |
| 30 | CCCTGGTCAGAGGAAGTG | β5 | Forward | Cloning | |
| 92 | GGAATTCTTAAGCTTATCAGTCCACTGTGCCATTG | β5 | Reverse | Cloning | |
| 161 | TCTAGACTCGAGCTGAAACGGATGGGGATT | β6 | Forward | Cloning | |
| 162 | CTTAAGGGTACCTTACTATCCATCCGTGGAAAG | β6 | Reverse | Cloning | |
| 124 | CAGATTACCCAAGTCAGTCCACAGGAGGTCACGGTG | β3, β5 | Forward | LBD exchange | |
| 125 | ATCCAGCGCTATATGGGTCACGTCGTCCGTGGTGAA | β3, β5 | Reverse | LBD exchange | |
| 126 | GATGTCATCCAGCTGACACCCCAGAGGATTGCCCTC | β5, β3 | Forward | LBD exchange | |
| 127 | GTCCAGTGCGATGTGGGGCTTGGCATCAGTGGTAAA | β5, β3 | Reverse | LBD exchange | |
| 128 | CAGATTACCCAAGTCAGTCCACAGCAGTTGGTTCTG | β3, β1 | Forward | LBD exchange | |
| 129 | ATCCAGCGCTATATGGGTCACAGCATCTGTGGAAAA | β3, β1 | Reverse | LBD exchange | |
| 130 | GATATCACTCAAATCCAGCCCCAGAGGATTGCCCTC | β1, β3 | Forward | LBD exchange | |
| 131 | ATCTCCAGCGAAGTGAAACTTGGCATCAGTGGTAAA | β1, β3 | Reverse | LBD exchange | |
| 173 | CAGATTACCCAAGTCAGTCCTCAAAGCTTGGCTCTT | β3, β6 | Forward | LBD exchange | |
| 176 | ATCCAGCGCTATATGGGTGTCCGCGTCACTCACAAA | β3, β6 | Reverse | LBD exchange | |
| 174 | GACATCGTTCAGATTTCACCCCAGAGGATTGCCCTC | β6, β3 | Forward | LBD exchange | |
| 175 | GTCCATTCCAAAATGAGACTTGGCATCAGTGGTAAA | β6, β3 | Reverse | LBD exchange |
The GenBank accession numbers of the sequences used to derive the primers are given in the text. Where there is no source listed, the primer was generated using sequence determined during the cloning of the subunit.
See Materials and Methods.
Assembly of complete cDNA clones of bovine integrin β1, β5, and β6 subunits.
Two PCR amplicons, together comprising the complete integrin β1 subunit and containing some overlapping sequences, were amplified from cDNA prepared from bovine tongue keratinocyte RNA (see above) by using the primer pairs 84-71 and 70-97 (Table 1). Both amplicons were cloned independently into pCR-Blunt II-TOPO (Invitrogen) and assembled into a complete cDNA by using the restriction sites ClaI and XmaI. The assembled cDNA was transferred into the mammalian expression vector pcDNA3.1/zeo(−) (Invitrogen) to create pBovβ1 by using the restriction sites XbaI and HindIII included in the amplification primers. The β5 subunit was amplified from cDNA prepared from bovine tongue keratinocytes (see above) by using the primer pairs 91-31 and 30-92 (Table 1), assembled into pCR-Blunt II TOPO by using the BglII and XmaI sites, and transferred into pcDNA3.1/zeo(−) by using the restriction sites XbaI and HindIII to create pBovβ5. The bovine β6 subunit was amplified as a single amplicon by using cDNA prepared from secondary bovine thyroid cells (see above) and primers 161 and 162 (Table 1). This amplicon was ligated into pcDNA3.1/zeo(−) by using the KpnI and XhoI sites to create pBovβ6. The complete integrin subunit cDNAs were resequenced and analyzed by coupled in vitro transcription-translation using the rabbit reticulocyte TNT Quick Coupled system (Promega) as described previously (52). Plasmids pBovαVZEO and pBovβ3ZEO have been described previously (52).
Viruses and cells.
FMDV type A12 strain 119ab (A12) was derived from the infectious cDNA clone pRMC35 (61). The cDNA was assembled from a virus with an unknown high-passage history in both bovine kidney and BHK-21 cells and which, following recovery from transfected BHK-21 cells, has been passaged numerous times in this cell type. The virus exhibits mild virulence in cattle. An antigenic variant of type A12 (A12-SSP), harboring the VP1 sequence present in a bovine tongue tissue-derived virus, was assembled into a cDNA (vRM-SSP) as described previously (59). Following recovery from transfected BHK-21 cells, the virus was passaged twice in CHO cells expressing an engineered receptor consisting of a single-chain anti-FMDV antibody fused to intercellular adhesion molecule 1 (scAb/ICAM1) (60). The virulence of this virus in cattle has not been tested. FMDV type A24Cruziero (A24Cru) was recovered from a foot lesion of a steer experimentally inoculated with virus intradermally into the tongue. The virus was used directly from the vesicular fluid and not passaged in tissue culture. The cattle-virulent variant of type O1Campos (O1Camp) was derived from the infectious cDNA clone pCRM8, which contains capsid sequences isolated from a vaccine seed stock and has been described previously (64). FMDV O/Taw/2/99 was isolated from the esophagopharyngeal fluid of a bovine with a subclinical FMDV infection (27). The virus was passaged three times in BHK-21 cells in Taiwan and then sent to the Institute for Animal Health, Pirbright, United Kingdom, where it was passaged once in primary bovine thyroid cells. It was then sent to the Plum Island Animal Disease Center, where it was passaged twice in BHK-21 cells. This virus did not cause clinical disease when it was experimentally inoculated into the species of cattle from which it was originally isolated (27, 28), nor did it cause clinical disease when inoculated into cattle at the Plum Island Animal Disease Center, but all animals seroconverted (P. W. Mason, personal communication). The genetically engineered type A12 virus chimera, where the G-H loop has been replaced with the homologous loop sequences from type O1BFS (A/O) virus, has been described previously (58). A summary of the properties of these viruses is presented in Table 2.
TABLE 2.
Viruses used in this studya
| Virus | Derivation | Passage in tissue culture | Virulence in cattle |
|---|---|---|---|
| A12 | Infectious clone pRMC35 (61) | Multiple passages in BHK-21 cells | Mild |
| A12-SSP | Infectious clone pRM-SSP containing VP1 sequence from bovine tongue tissue virus (59) | Two passages in CHO cells expressing scab/ICAM1 receptor (60) | Not tested |
| A24Cru | Foot lesion of experimentally infected bovine | None | Virulent |
| O1Camp | Infectious clone pCRM8 (64) | Less than five passages in BHK-21 cells | Virulent |
| O/Taw/2/99 | Esophagopharyngeal fluid from bovine (27) | Three passages in BHK-21 cells, one passage in bovine thyroid cells, and two passages in BHK-21 cells | Subclinical infection (27, 28) |
| A/O | Infectious clone pRM-A/O; type A12 virus with G-H loop from type O1BFS virus (58) | Multiple passages in BHK-21 cells | Not tested |
Numbers in parentheses indicate references.
BHK-21 cells were maintained in minimum essential medium containing 10% calf serum and 10% tryptose phosphate broth. COS-1 cells were maintained in Dulbecco's minimum essential medium (Life Technologies) containing 10% fetal calf serum, an additional 2 mM concentration of l-glutamine, and 1 mM sodium pyruvate.
Exchanging putative LBDs among β subunits.
The putative LBDs of the β1, β5, and β6 integrin subunits were substituted into the β3 subunit, and the β3 LBD was substituted into the β1, β5, and β6 subunits. The exchanges were accomplished by a three-step recombinant PCR method that resulted in clean swapping of the LBDs into the paralogous integrin backbones cloned in the pcDNA3.1/zeo(−) background. The LBD to be exchanged was amplified with the specific primers (listed in Table 1) containing 5′ extensions, comprising approximately one-half the length of the primer, corresponding to the integrin subunit contributing the backbone sequences. The primer 5′ extensions allowed the PCR-mediated insertion of the LBD amplicons to backbone integrin fragments upstream and downstream of the LBD. The recombinant fragment containing the heterologous LBD was inserted into plasmids expressing the backbone integrin by digestion with appropriate restriction enzymes. The chimeric full-length cDNAs were designated pβ3[β1], pβ3[β5], pβ5[β3], pβ1[β3], pβ3[β6], and pβ6[β3], where the first β designation corresponds to the donor of the integrin subunit backbone and the subunit in brackets corresponds to the LBD donor. All chimeric β subunit cDNAs were totally resequenced to confirm the presence of the exchanged regions and to rule out the introduction of other mutations. Plasmids were also analyzed by in vitro transcription-translation as described above.
Transient expression of integrin subunits in COS-1 cells and viral replication assays.
Expression of integrin subunits in COS-1 cells and analysis of viral replication were performed as described previously (52). Briefly, cells plated on six-well tissue culture plates were transfected with 2 μg each of pBovαVZEO and the appropriate β subunit cDNA plasmid by using the transfection reagent FuGENE6 (Roche Molecular Biochemicals). Transfected cells were incubated overnight, followed by trypsinization and replating onto 24-well plates. After a further overnight incubation, transfected cultures were infected with FMDV type A12, A24Cru, O1Camp, or O/Taw/99 at a multiplicity of infection of 10 PFU/cell or A12-SSP at a multiplicity of infection of 1 PFU/cell. Infected cells were labeled with [35S]methionine between 4 and 18 h postinfection, and viral replication was determined on infected cell lysates by radioimmunoprecipitation (RIP) of equal amounts of trichloroacetic acid-precipitable counts per minute of lysate by using monoclonal antibody (MAb) 6EE2 directed against FMDV type A12 (8) or 10GA4 directed against type O1 (67) as described previously (52). Immunoprecipitated proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12.5% polyacrylamide gel. Transfected cells in one of the wells were not infected but were analyzed for integrin expression by immunohistochemistry as described previously (52) by using MAbs LM609, 6S6, and CSβ6 directed to the human integrins αVβ3, β1, and β6, respectively, or a polyclonal rabbit serum directed against the bovine β5 subunit.
Nucleotide sequence accession numbers.
The nucleotide and amino acid sequences for the bovine β1, β5, and β6 integrin subunit cDNAs have been deposited in GenBank and assigned the accession numbers AF468058, AF468059, and AF468060, respectively.
RESULTS
Cloning and sequence analysis of bovine integrin β subunits.
At least three different αV integrins, αVβ1, αVβ3, and αVβ6, have been reported to function as FMDV receptors in vitro (10, 32, 33, 53), and Neff et al. have previously shown that the virus utilized bovine αVβ3 more efficiently than its human homolog (52). In preliminary experiments using K562 cells stably transfected with human αVβ5, we were unable to demonstrate receptor utilization of that integrin by FMDV type A12 (unpublished observation). In light of the aforementioned observations, we cloned and analyzed the cDNAs encoding bovine β1, β5, and β6 subunits. The β1 and β5 subunit-coding sequences were amplified from cDNA prepared from primary bovine tongue keratinocytes, while sequences coding for the β6 subunit were amplified from cDNA prepared from secondary bovine thyroid cell cultures (see Materials and Methods).
The complete coding sequence for the bovine β1 subunit comprised 2,397 nucleotides coding for a 798-amino-acid-residue protein, consisting of a 20-residue signal peptide, a 708-residue ectodomain, a 29-residue transmembrane domain, and a 41-residue cytoplasmic domain. The coding sequence for the β5 subunit comprised 2,403 nucleotides coding for an 800-amino-acid-residue protein, consisting of a 23-residue signal peptide, a 697-residue ectodomain, a 29-residue transmembrane domain, and a 51-residue cytoplasmic domain. A comparison with the human β5 sequence revealed that the bovine homolog contained one additional codon located within the ectodomain. The coding sequence for the β6 subunit comprised 2,367 nucleotides coding for a 788-amino-acid-residue protein consisting of a 26-residue putative signal peptide, a 681-residue ectodomain, a 29-residue transmembrane domain, and a 52-residue cytoplasmic domain.
The nucleotide and predicted amino acid sequence similarities within the different subunit functional regions among the human and bovine β subunits are shown in Table 3. Consistent with the previously analyzed bovine and human αV and β3 homologs (52), the greatest degree of amino acid sequence divergence of the mature β1 and β6 subunits occurred within the ectodomain. The β5 subunit exhibited the highest amino acid sequence divergence within the ectodomain of the three subunits and, surprisingly, had a higher degree of amino acid sequence divergence within the transmembrane domain (Table 3). The amino acid sequences of the cytoplasmic domain were totally conserved for the β1 and β5 subunits, but there was a single codon change in the β6 subunit. The cytoplasmic domain of the human β6 subunit has been shown to be required for the integrin to function as a receptor for FMDV (49), which is in contrast to the bovine β3 subunit, where deletion of almost the entire cytoplasmic domain does not affect the viral receptor function of the integrin (51). Characteristic of all integrin β subunits is the high content of cysteine residues and four tandem cysteine-rich epidermal growth factor (EGF)-like domains known as the cysteine-rich repeats (24). Alignments of the human and bovine predicted amino acid sequences of the β1, β5, and β6 subunits showed that all cysteines were conserved, with the exception of a C → Y change in the bovine β1 ectodomain at residue 671, just downstream of the last EGF-like domain. Neff et al. have previously found a C → R substitution in the bovine β3 subunit within the second EGF-like domain (52).
TABLE 3.
Nucleotide and amino acid similarities between bovine and human integrin β subunits
| Subunit | % Nucleotide similarity/ % amino acid similarity ina:
|
||||
|---|---|---|---|---|---|
| Mature subunitb | Signal peptide | Ecto- domain | Transmembrane domain | Cytoplasmic domain | |
| β1 | 89.2/94.1 | 87.3/90.0 | 88.8/93.5 | 94.3/100 | 92.9/100 |
| β5 | 88.1/92.0 | 85.3/79.2 | 88.0/91.5 | 84.9/89.7 | 89.9/100 |
| β6 | 88.4/93.4 | 91.4/62.1 | 88.3/93.0 | 87.4/96.6 | 89.3/98.0 |
The first number of each pair represents the sequence similarity of nucleotides aligned using the Martinez/Needleman-Wunsh alignment method and determined using a gap penalty of 1.10 and a gap length penalty of 0.33. The second number represents the sequence similarity of amino acids aligned using the Lipman-Pearson alignment method and determined using a gap penalty of 4 and a gap length penalty of 12. The putative boundaries between the domains of the bovine subunits were defined from alignments to the human homologs and the reported borders of the domains in the human sequences.
Sequence comparison of the complete ectodomain, transmembrane domain, and cytoplasmic domain.
Replication of type A12 and O1Camp viruses in COS-1 cells expressing bovine integrins.
COS-1 cells were cotransfected with pBovαVZEO and pBovβ1, pBovβ3ZEO, pBovβ5, or pBovβ6 as described in Materials and Methods. Cells were monitored for integrin expression by immunohistochemistry (52). More than 80% of the cells in the cultures transfected with αVβ3, αVβ5, or αVβ6 cDNA stained positively for the appropriate integrin (data not shown). COS-1 cells, however, express the β1 subunit naturally, possibly as the α5β1 heterodimer. Since the available anti-human αV MAbs did not cross-react with the bovine homolog, we were unable to effectively monitor αVβ1 expression in these cultures. The integrin expression in the transfected cells was always tested in parallel cell culture preparations in the subsequent FMDV infection experiments, and only the results of experiments where at least 80% of the cells expressed the proper integrins are reported.
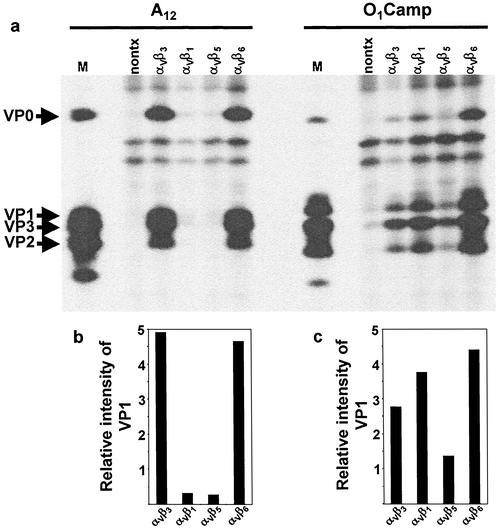
Transfected and nontransfected COS-1 cultures were infected with either FMDV type A12 or type O1Camp to evaluate integrin usage preference between these two serotypes. Both viruses have been demonstrated to utilize both bovine and human αVβ3 as receptors, although they utilized the bovine integrin more efficiently (52, 53). In addition, neither virus utilizes heparan sulfate as a receptor, as has been demonstrated for some tissue culture-adapted FMDV strains (31, 53, 64). Infected cells were labeled with [35S]methionine between 4 to 18 h after infection, and viral replication was analyzed by RIP as described in Materials and Methods. In this and subsequent experiments, only cells originating from the same transfected culture were infected with the different viruses. In addition, within each strain, equal numbers of trichloroacetic acid-precipitable counts per minute were immunoprecipitated, allowing direct comparison of viral replication between cultures expressing different integrins. The results of a typical transfection-infection assay are shown in Fig. 1a. Under these conditions, virus-specific proteins could not be detected in nontransfected cells infected with type A12 virus. In contrast, low levels of virus-specific proteins were present in nontransfected cells infected with type O1Camp virus. This finding, which has been observed previously (52), may be due to contaminating virus which can utilize heparan sulfate as a receptor. In cells transfected with cDNAs encoding the bovine integrins and infected with either type A12 or O1Camp virus, virus-specific proteins were detected in all the integrin-transfected cell cultures. However, the level of viral protein synthesis in type A12 virus-infected cells expressing either αVβ3 or αVβ6 was much greater than that in cells transfected with either αVβ1 or αVβ5. In contrast, type O1Camp virus appeared to utilize the αVβ6 integrin more efficiently than any of the other integrins and utilized αVβ1 more efficiently than either αVβ3 or αVβ5.
FIG. 1.
Viral replication in type A12 or O1Camp virus-infected COS-1 cells expressing bovine integrins. COS-1 cells were cotransfected with cDNA plasmids encoding the bovine integrin αV subunit and the β1, β3, β5, or β6 subunit. (a) Transfected cells were infected with FMDV type A12 or O1Camp and labeled with [35S]methionine, and viral protein synthesis was analyzed by RIP and SDS-PAGE as described in Materials and Methods. Lanes: nontx, immunoprecipitated proteins from nontransfected infected cell lysates; M, the locations of the viral structural proteins from lysates prepared from FMDV-infected BHK-21 cells are indicated. (b and c) The autoradiogram shown in panel a was digitized in a MultiImage Light Cabinet, and the intensities for VP1 bands in cells infected with type A12 (b) or type O1Camp (c) virus were quantitated with the spot density utility of AlphaEase software, version 4.0. The bars represent the intensities of the VP1 band in transfected cells relative to that of the same region in nontransfected cells.
To quantitate the level of viral replication in the transfected cells, we digitized the autoradiogram and quantitated the intensities of the VP1 bands relative to the intensity determined for nontransfected cells. αVβ3 and αVβ6 were equally efficient in mediating infection by type A12 virus, while αVβ1 and αVβ5 were poor receptors for this virus (Fig. 1b). In contrast, replication of type O1Camp virus was mediated by αVβ6 and αVβ1 to a greater degree than by either αVβ3 or αVβ5 (Fig. 1c).
To determine whether the transfected cells became susceptible to FMDV as a specific consequence of integrin expression, we pretreated transfected cells with function-blocking anti-integrin MAbs against human αVβ3, αVβ6, or αVβ1, followed by infection with either type A12 or O1Camp virus in the presence of the antibodies. Viral replication in either αVβ1- or αVβ3-transfected cells was inhibited in the presence of the appropriate antibody (data not shown). Treatment with the available anti-αVβ6 blocking MAb resulted in a low degree of inhibition of viral replication (data not shown). While this antibody has been demonstrated to inhibit the replication of a type O1 virus in cells transfected with human αVβ6 (33), we found by using flow cytometry that it reacts poorly with the bovine integrin (data not shown). The available function-blocking antibodies against human αVβ5 did not cross-react with the bovine integrin, so we were unable to perform this study with cells transfected with this integrin.
Role of the β subunit LBD in mediating virus infection.
The LBD of the intact integrin consists of discrete regions of both the α and β subunits (20, 74). To analyze what role, if any, the β subunit LBD plays in distinguishing different virus strains, we exchanged homologous regions corresponding to the putative LBDs of the individual subunits, which resulted in a series of β subunit chimeras, as described in Materials and Methods. These chimeras were cotransfected with the bovine αV subunit into COS-1 cells, followed by infection with either type A12 or O1Camp virus. Viral replication mediated by the chimeras was analyzed as described in Materials and Methods and compared to replication mediated by the wild-type integrins, and a representative experiment is shown in Fig. 2.
FIG. 2.
Viral replication in COS-1 cells expressing β subunit LBD chimeras. Cells were cotransfected with cDNA plasmids encoding the bovine αV subunit and either wild-type β subunits or β subunit LBD chimeras as indicated in the figure and infected with either type A12 or O1Camp virus. Viral replication was analyzed by RIP and SDS-PAGE as described in Materials and Methods. Lanes: nontx, immunoprecipitated proteins from nontransfected infected cell lysates; M, the locations of the viral structural proteins from lysates prepared from FMDV-infected BHK-21 cells are indicated.
In transfected cells infected with type A12 virus, replacement of the LBD of the β3 subunit with the LBD of either the β1 or β5 subunit reduced the efficiency of the αVβ3 integrin as a receptor. Conversely, replacing the β1 LBD with the β3 LBD did not increase the efficiency of the αVβ1 integrin as a receptor for this virus; however, replacing the β5 LBD with the β3 LBD resulted in a small increase in the efficiency of the αVβ5 integrin. Exchanging LBDs of the β1 and β3 subunits appeared to lower the efficiencies of the β1 and β3 subunits in mediating type O1Campos virus infection, similar to what was observed with type A12 virus. Likewise, exchanging the LBDs of the β3 and β5 subunits resulted in a decrease in β3 receptor efficiency and a small increase in β5 receptor efficiency. Interestingly, while exchange of the LBDs of the β3 and β6 integrin subunits lowered the efficiencies of both of these integrins as viral receptors for both types A12 and O1Camp, type A12 virus utilized the β6[β3] subunit and type O1Camp virus utilized the β3[β6] subunit slightly more efficiently than the subunits with the reciprocal exchanges.
Analysis of integrin utilization by other type A and O viruses.
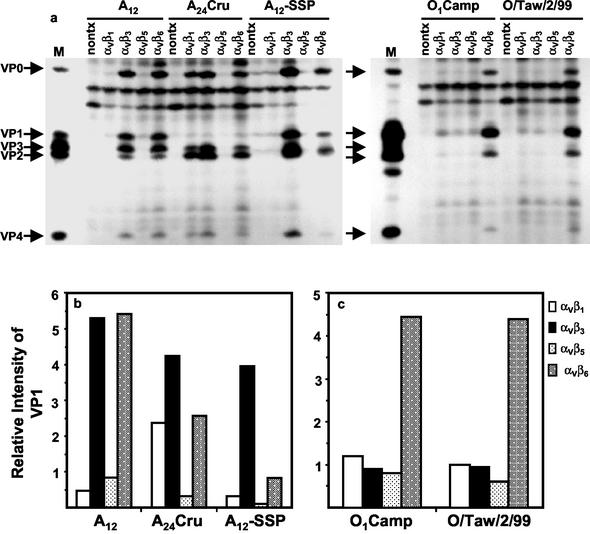
In order to ascertain whether the preference for different integrins was unique to types A12 and O1Camp or was common to the individual serotypes, we analyzed viral replication in cells transfected with each of the cloned integrin cDNAs of two additional type A viruses and one additional type O virus. These viruses and their passage histories are described in Materials and Methods and summarized in Table 2. The results of this experiment are shown in the autoradiogram in Fig. 3a, and the quantitation is shown in Fig. 3b and c. All of the type A viruses efficiently utilized the αVβ3 integrin as a receptor. Types A12 and A24Cru also utilized the αVβ6 integrin efficiently as a receptor, while type A12-SSP does so to a lesser extent. Surprisingly, A24Cru also utilized the αVβ1 integrin as a receptor with relatively high efficiency. In contrast, the two type O viruses utilized only the αVβ6 integrin with high efficiency and utilized αVβ1 slightly better than αVβ3.
FIG. 3.
Viral replication in FMDV-infected COS-1 cells expressing bovine integrins. COS-1 cells were cotransfected with cDNA plasmids encoding the bovine integrin αV subunit and the β1, β3, β5, or β6 subunit. (a) Transfected cells were infected with different FMDV types as noted in the figure and labeled with [35S]methionine, and viral protein synthesis was analyzed by RIP and SDS-PAGE as described in Materials and Methods. Lanes: nontx, immunoprecipitated proteins from nontransfected infected cell lysates; M, the locations of the viral structural proteins from lysates prepared from FMDV-infected BHK-21 cells are indicated. (b and c) The autoradiogram shown in panel a was digitized in a MultiImage Light Cabinet, and the intensities for VP1 bands in cells infected with type A (b) or type O (c) viruses were quantitated with the spot density utility of AlphaEase software, version 4.0. The bars represent the intensities of the VP1 band in transfected cells relative to that of the same region in nontransfected cells.
Influence of the G-H loop on αV integrin utilization.
The above-mentioned results indicate that the viral serotype, not the tissue culture history or the relative bovine virulence of the individual isolates, appeared to determine the efficiency of integrin utilization. While there could be many factors which determine the integrin specificity of the individual serotypes, we focused on the G-H loop. A comparison of the sequences of the loops for all of the viruses utilized in this study (Fig. 4) showed that the sequences are more conserved within the serotype than between the serotypes. All of the loops have a conserved Tyr near the N terminus of the loop and a conserved Leu-Ala at the RGD + 4 position. The two type O viruses have a Leu at the RGD + 1 position, and the DLXXL sequence has been suggested to be an αVβ6 recognition sequence (35). The most striking difference between the serotypes is the presence of a Cys at the base of the loop in the type O viruses which forms a disulfide bond with a Cys residue in VP2 (41, 56). The type A viruses do not have this disulfide bond, allowing the G-H loop to assume a conformation, relative to the rest of the capsid, that is different from what is seen in the type O viruses (16). Reduction of the disulfide bond in the type O1 G-H loop results in the rearrangement of the loop (41) into a conformation similar to that seen in the type A viruses (16). To examine the role of the loop in integrin differentiation, we transfected COS-1 cells with cDNA encoding either αVβ3 or αVβ6, followed by infection with type A12 or O1Camp virus that was treated with 10 mM dithiothreitol (DTT) or a type A12 virus chimera where the G-H loop was replaced with sequences representing the G-H loop of type O1BFS (58). Treatment of either type A12 or O1Camp virus with DTT did not reduce the infectivity of the virus, as determined by plaque assay in BHK-21 cells (data not shown), confirming previously reported results (41). Our results, shown in Fig. 5, indicate that neither treatment with DTT nor replacement of the type A12 G-H loop with a type O1 loop had any dramatic effects on the differentiation of the two receptors by either type A12 or O1Camp virus.
FIG. 4.
Sequences of the G-H loops of type A and O viruses.
FIG. 5.
Replication of DTT-reduced FMDV or an FMDV G-H loop chimera in COS-1 cells expressing αVβ3 or αVβ6. COS-1 cells were cotransfected with cDNA plasmids encoding the bovine integrin αV subunit and either the β3 or β6 subunit. FMDV type A12 or O1Camp was incubated with 10 mM DTT for 30 min at room temperature. Transfected cells were immediately infected with either DTT-treated (+) or untreated (−) FMDV or the type A/O G-H loop chimera as noted in the figure and labeled with [35S]methionine, and viral protein synthesis was analyzed by RIP and SDS-PAGE as described in Materials and Methods. Lanes M, the locations of the viral structural proteins from lysates prepared from FMDV-infected BHK-21 cells are indicated.
DISCUSSION
In addition to integrins, FMDV has been shown to utilize various cell surface molecules as receptors in vitro. These include Fc receptors (7, 43, 45), heparan sulfate (4, 31, 42, 53), and a single-chain antibody fused to intercellular adhesion molecule 1 (60). Since the original identification of the αVβ3 integrin as a receptor for FMDV (10, 52, 53), two additional integrins, αVβ6 (33) and αVβ1 (32), have been shown to function as FMDV receptors in cell culture. The present report expands on these earlier studies by using molecularly cloned bovine integrin subunits and demonstrates differences in αV integrin receptor utilizations by different FMDV strains.
We employed a transient integrin expression assay system in COS-1 cells (51, 52) to compare the efficiencies of each of the αV integrins in mediating infection by strains representing two different FMDV serotypes. In each experiment, the transfected cells used for infection originated from the same transfected culture and all transfections and infections were done at the same time. Thus, we minimized the degree of variation that may result from differences in the expressions of the different integrins in the transient expression assay system. Our results demonstrated that the efficiencies by which these four bovine integrins are able to mediate infection differed between the two virus serotypes. While the type O viruses utilized αVβ6 with the highest efficiency, followed in descending order by αVβ1, αVβ3, and αVβ5, all of the type A viruses utilized αVβ3 with high efficiency and utilized αVβ6 with equal or lesser efficiency. Only one type A virus (A24Cru) utilized αVβ1 with moderate efficiency, and all utilized αVβ5 very poorly. These results, obtained by using integrins from a species susceptible to FMDV, confirmed the finding of Jackson et al. (32, 33) that type O1 FMDV utilizes αVβ6 and αVβ1 as receptors in vitro.
While interactions between the α and β subunits contribute to the LBD of integrins, specific regions of both subunits have been shown to interact directly with ligands (20). Since the same α subunit was present in all of the integrins in this study, we concentrated on differences in the β subunits as possible explanations for differences in receptor specificities between the two viruses. To further define the reasons for the observed specificities among the β subunits, we exchanged regions of the subunits' putative LBDs. The regions we chose to exchange have been identified functionally as LBDs by various biochemical criteria, and exchanging these regions among β subunits resulted in changes in the ligand specificities of the integrins (11, 18, 40, 69, 70). The recently solved crystal structure of the αVβ3 integrin, which has led to the structural definition of the integrin's LBD, showed that the region exchanged in this study interacts with the α subunit within the ligand-binding cleft and contains the RGD binding site, but the entire LBD of the β subunit appears to encompass a larger region of the ectodomain than that exchanged in this study (74, 75). In all cases, however, removing even this portion of the LBD from its natural context and replacing it with an LBD of another β subunit lowered the efficiency of the integrin as a receptor for FMDV. The only exception appeared to be in the β5 subunit, where replacing the β5 LBD with the β3 LBD resulted in a slight increase in the efficiency of the αVβ5 receptor. When the LBDs for the β3 and β6 subunits were exchanged, we observed that the type A12 virus utilized the β6 subunit containing the β3 LBD with higher efficiency than the β3 subunit with the β6 LBD. Conversely, the O1Camp virus utilized the β3 subunit with the β6 LBD with slightly higher efficiency than the β6 subunit with the β3 LBD. These results suggest that the β subunit LBD plays a role in the recognition of the different viral serotypes; however, with some of the chimeric constructs, we were unable to directly measure integrin expression since reactivity with the anti-integrin MAbs appeared to be either reduced or abolished. Thus, some of the differences in viral replication could be related to differences in the expressions of the chimeric integrins on the cell surface. Neff et al. have previously shown that the increased efficiency of the bovine β3 subunit compared with that of the human homolog as a receptor for FMDV appeared to map to the EGF-like cysteine-rich repeat region downstream of the LBD (52), raising the possibility that differences in receptor specificities for FMDV among the integrins may be related to either the LBD or the LBD and other regions of the subunit ectodomain.
The five viruses we analyzed in this study have different degrees of virulence in the bovine (Table 2). The A24Cru and O1Camp viruses are virulent to bovines and cause a rapid and severe clinical disease upon exposure, while the A12 and O/Taw/2/99 viruses cause either a mild or subclinical disease. The A12-SSP virus has not been tested directly in animals. This suggests that differences in receptor recognition do not appear to be related to the virus' ability to cause disease but rather to the serotype of the virus. Eight integrins recognize RGD as a binding motif sequence on their natural ligands (63). Since each of these integrins displays unique ligand specificities, other regions of the ligands are likely to influence recognition by the receptor. It is interesting that the G-H loops of the type A viruses used in this study contain a conserved leucine at the RGD + 4 position but lack a leucine at the RGD + 1 position (Fig. 4). The DLXXL motif has been shown to be involved in recognition of the αVβ6 integrin (35). These viruses, however, utilize the αVβ6 integrin quite efficiently. Thus, the differences in receptor specificities displayed by the type A and O viruses in vitro may be the result of the amino acid sequence divergence within the G-H loop surrounding the RGD sequence (Fig. 4), differences in loop structure, or interactions with other capsid regions outside of the G-H loop. To examine the first possibility, we made use of an engineered chimeric type A virus in which the G-H loop of a type O virus (O1BFS) was substituted for the type A loop (58). The sequence of the substituted loop is identical to that of the G-H loop from type O1Camp virus, with the exception of a Val → Leu change at the RGD − 1 position. This chimeric virus appears to utilize αVβ3 and αVβ6 to the same extent as does the wild-type A12 virus (Fig. 5). This result indicates that the amino acid residues immediately surrounding the RGD sequence in the G-H loop do not appear to greatly influence the receptor utilization by the virus, although we have not generated and tested a type O virus with a type A loop.
To analyze the influence that the conformation of the G-H loop has on receptor utilization, we infected cells expressing either αVβ3 or αVβ6 with type O1Camp virus that was pretreated with DTT. In type O viruses, the base of the G-H loop is linked, via a disulfide bond, between a Cys at residue 134 and a Cys at residue 130 of VP2 (56). Reduction of this disulfide bond by DTT results in a rearrangement of the G-H loop within the viral particle, allowing for the determination of the loop structure (41). For the type A viruses, only the structure of the type A22 virus has been determined, and within the crystal, the G-H loop was disordered (16). Type A viruses, however, do not contain the Cys residue at the base of the G-H loop in VP1, and the structure of the residues at the base of the loop was very similar to that seen in the structure of DTT-treated, but not native, type O1 virus (16). In addition, DTT treatment of type O1 virus resulted in a shift of the G-H loop of VP3 (41) into a conformation almost identical to that seen in the type A22 particle (16). These results suggested that there are differences in the dispositions of the G-H loop between type A and O viruses, and these differences are eliminated by reduction of the disulfide bond in type O viruses. It has also been shown that the disulfide bond is reduced in newly released virus, suggesting that the reduced loop conformation may be biologically relevant (41). We therefore reasoned that by placing the G-H loop of type O1 virus into a more type A-like conformation, the virus would resemble the type A viruses in its receptor utilization. The results in Fig. 5 indicate, however, that treatment of the type O1 virus with DTT did not affect receptor usage. In fact, the reduced virus utilized αVβ3 to a lesser extent than did the native virus. Treatment of type A12 virus with DTT did not affect receptor utilization. In this experiment, we did not further modify the type O virus to prevent reformation of the disulfide bond; however, DTT was present during the entire adsorption period. Experiments examining the reoxidation of the disulfide bond in vitro have estimated that the half time for reformation of the bond at room temperature is 4 days (41). Thus, it appears that, since neither the primary sequence nor the structural conformation of the loop play a role in the relative utilization of the αVβ3 and αVβ6 integrins by types A and O, other regions of the viral capsid must be involved in this interaction.
Little information pertaining to the viral receptors important in the pathogenesis of FMDV in susceptible species is available. If the virus uses only one of the RGD-dependent integrins in vivo, then a correlation should exist between the sites where the virus replicates and causes the lesions and the tissue distribution of that particular integrin. The other possibility is that more than one integrin is used during different stages of the disease. In this scenario, the initial stages of infection of an aerosol-infected animal could involve the utilization of receptors in the upper respiratory tract, whereas later replication cycles and amplification in epithelial sites in the mouth and feet could involve the utilization of alternate receptors. While it appears that the disease process in susceptible animals is mediated by virus-integrin interactions (46, 53, 64), a type C virus containing an RGGD sequence has been isolated from a bovine which was not protected from virus challenge following immunization with an experimental peptide vaccine (68). In addition, a tissue culture-adapted type C virus with a genetically engineered RGG sequence, which was unable to bind to heparin, replicated in BHK-21 cells which express both heparan sulfate and FMDV integrin receptors and in heparan sulfate-negative CHO cell mutants which do not express FMDV integrin receptors (2, 3). The ability of these viruses to cause disease in susceptible animals, however, has not been demonstrated. More recently, a tissue culture-adapted derivative of a Cathay topotype O1 virus isolated in China has also been shown to replicate in tissue culture in both a heparan sulfate- and integrin-independent manner and cause mild disease in swine (76). However, it should be stressed that, with the exception of the virus isolated from a bovine (68), the other viruses were obtained through either tissue culture adaptation or genetic engineering. Thus, the possible role of nonintegrin receptors in FMDV pathogenicity needs to be more closely examined.
While there are many reports on the distribution of some of the αV integrins in human tissues, little information on the tissue expression of the integrins in FMDV-susceptible animals is available. In human tissues, the αVβ3 integrin is found on vascular endothelium and smooth muscle (15, 19, 39) but is not found on bronchiolar epithelium (47). This integrin has been shown to be expressed in an estrous cycle-dependent manner in bovine endometrial epithelium (34) and is expressed weakly in bovine and porcine airway epithelium (65). In contrast, the αVβ6 integrin is restricted to epithelial cells (14) but is rarely found in normal tissues in humans (13, 25, 72, 73). High levels of αVβ6 have also been found in the macula densa of the kidneys and the endometrial epithelium of secretory phase uterus (14). In general, the expression of αVβ6 is highly regulated and is found during development, healing processes, and neoplasia (13). There is very little information available on the expression or function of the αVβ1 integrin in specific tissues or organs, although this integrin is expressed on malignant cells (22, 48), in smooth muscle (17), and in the central nervous system (50). It has also been demonstrated to be a receptor for human parechovirus 1 (57, 71) and a coreceptor for human adenovirus (38). The β1 subunit forms dimers with at least 10 different α subunits, making the β1 integrins the largest subgroup within the integrin family (30). However, only three of the β1 integrins, αVβ1, α5β1, and α8β1, utilize the RGD sequence as a binding recognition motif (63).
Obtainment of information on integrin expression in susceptible animals would allow some correlation to be made between integrin use by the virus in cell culture and sites of virus replication in vivo. The tissues we utilized for integrin cloning gave us some indication of integrin expression in cattle. cDNAs for the β1 and β5 subunits were easily amplified from RNA extracted from primary bovine tongue keratinocytes. We have also been able to amplify both αV and β3 cDNAs from these keratinocytes by PCR (unpublished data). In contrast, we were unable to amplify sequences coding for the β6 subunit from cDNAs prepared from a number of bovine tissues collected at necropsy, including tongue epithelium, tongue keratinocytes, lung, and kidney. This was surprising given that some of these organs are targets for the virus during natural infection. We were able, however, to amplify cDNA encoding this integrin subunit from RNA prepared from secondary cultures of bovine thyroid. These cells are considered to be highly sensitive to FMDV infection and are often used for primary isolation of virus from field samples (26, 66). The role of the thyroid, if any, in FMDV pathogenesis in vivo is not known.
While this report has concentrated on the role of the viral receptor in pathogenesis, other viral and cellular factors may also affect both host range and virulence (44). In particular, it has recently been demonstrated that attenuation of virulence in bovines and reduced ability to replicate in bovine cells are associated with deletions in the nonstructural protein 3A (9, 55) At this time, it is difficult to reconcile why this virus would utilize at least three different receptors to cause disease. While infection with different FMDV strains may result in different degrees of disease severity, in most cases there are no apparent differences in clinical symptoms within a species. There are, however, differences in the clinical courses of disease among the different species which are susceptible to FMDV. Thus, it is possible that these may be related to differences in either expression patterns or degrees of expression of the known integrin receptors for FMDV among different species. Analysis of the distribution of the integrin receptors in susceptible species may be necessary to explain viral pathogenesis within different species.
Acknowledgments
We thank Vivian O'Donnell and Juan Pacheco for providing bovine tongue keratinocytes and Michael LaRocco for excellent technical assistance. We also express our appreciation to Terry Jackson for sharing his data with us prior to publication.
REFERENCES
- 1.Acharya, R., E. Fry, D. Stuart, G. Fox, D. Rowlands, and F. Brown. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature 337:709-716. [DOI] [PubMed] [Google Scholar]
- 2.Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. [DOI] [PubMed] [Google Scholar]
- 3.Baranowski, E., C. M. Ruiz-Jarabo, N. Sevilla, D. Andreu, E. Beck, and E. Domingo. 2000. Cell recognition by foot-and-mouth disease virus that lacks the RGD integrin-binding motif: flexibility in aphthovirus receptor usage. J. Virol. 74:1641-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranowski, E., N. Sevilla, N. Verdaguer, C. M. Ruiz-Jarabo, E. Beck, and E. Domingo. 1998. Multiple virulence determinants of foot-and-mouth disease virus in cell culture. J. Virol. 72:6362-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxt, B., and H. L. Bachrach. 1982. The adsorption and degradation of foot-and-mouth disease virus by isolated BHK-21 cell plasma membranes. Virology 116:391-405. [DOI] [PubMed] [Google Scholar]
- 6.Baxt, B., and Y. Becker. 1990. The effect of peptides containing the arginine-glycine-aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes 4:73-83. [DOI] [PubMed] [Google Scholar]
- 7.Baxt, B., and P. W. Mason. 1995. Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor-mediated adsorption. Virology 207:503-509. [DOI] [PubMed] [Google Scholar]
- 8.Baxt, B., D. O. Morgan, B. H. Robertson, and C. A. Timpone. 1984. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J. Virol. 51:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beard, C. W., and P. W. Mason. 2000. Genetic determinants of altered virulence of Taiwanese foot-and-mouth disease virus. J. Virol. 74:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitan, G., L. Scheibler, Z. Greenberg, M. Rosenblatt, and M. Chorev. 1999. Mapping the integrin αVβ3-ligand interface by photoaffinity cross-linking. Biochemistry 38:3414-3420. [DOI] [PubMed] [Google Scholar]
- 12.Bowditch, R. D., M. Hariharan, E. F. Tominna, J. W. Smith, K. M. Yamada, E. D. Getzoff, and M. H. Ginsberg. 1994. Identification of a novel integrin binding site in fibronectin. Differential utilization by β3 integrins. J. Biol. Chem. 269:10856-10863. [PubMed] [Google Scholar]
- 13.Breuss, J. M., J. Gallo, H. M. DeLisser, I. V. Klimanskaya, H. G. Folkesson, J. F. Pittet, S. L. Nishimura, K. Aldape, D. V. Landers, W. Carpenter, N. Gillett, D. Sheppard, M. A. Matthay, S. M. Albelda, R. H. Krammer, and R. Pytela. 1995. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108:2241-2251. [DOI] [PubMed] [Google Scholar]
- 14.Breuss, J. M., N. Gillett, L. Lu, D. Sheppard, and R. Pytela. 1993. Restricted distribution of integrin β6 mRNA in primate epithelial tissues. J. Histochem. Cytochem. 41:1521-1527. [DOI] [PubMed] [Google Scholar]
- 15.Brooks, P. C., R. A. Clark, and D. A. Cheresh. 1994. Requirement of vascular integrin αVβ3 for angiogenesis. Science 264:569-571. [DOI] [PubMed] [Google Scholar]
- 16.Curry, S., E. Fry, W. Blakemore, R. Abu-Ghazaleh, T. Jackson, A. King, S. Lea, J. Newman, D. Rowlands, and D. Stuart. 1996. Perturbations in the surface structure of A22 Iraq foot-and-mouth disease virus accompanying coupled changes in host cell specificity and antigenicity. Structure 4:135-145. [DOI] [PubMed] [Google Scholar]
- 17.Dahm, L. M., and C. W. Bowers. 1998. Vitronectin regulates smooth muscle contractility via αV and β1 integrin. J. Cell Sci. 111:1175-1183. [DOI] [PubMed] [Google Scholar]
- 18.D'Souza, S. E., T. A. Haas, R. S. Piotrowicz, V. Byers-Ward, D. E. McGrath, H. R. Soule, C. Cierniewski, E. F. Plow, and J. W. Smith. 1994. Ligand and cation binding are dual functions of a discrete segment of the integrin β3 subunit: cation displacement is involved in ligand binding. Cell 79:659-667. [DOI] [PubMed] [Google Scholar]
- 19.Felding-Habermann, B., and D. A. Cheresh. 1993. Vitronectin and its receptors. Curr. Opin. Cell Biol. 5:864-868. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez, C., K. Clark, L. Burrows, N. R. Schofield, and M. J. Humphries. 1998. Regulation of the extracellular ligand binding activity of integrins. Front. Biosci. 3:D684-D700. [DOI] [PubMed] [Google Scholar]
- 21.Fox, G., N. R. Parry, P. V. Barnett, B. McGinn, D. J. Rowlands, and F. Brown. 1989. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 70:625-637. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander, D. R., D. Zagzag, B. Shiff, H. Cohen, J. C. Allen, P. J. Kelly, and M. Grumet. 1996. Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves αV and β1 integrins. Cancer Res. 56:1939-1947. [PubMed] [Google Scholar]
- 23.Gonzalez-Amaro, R., and F. Sanchez-Madrid. 1999. Cell adhesion molecules: selectins and integrins. Crit. Rev. Immunol. 19:389-429. [PubMed] [Google Scholar]
- 24.Green, L. J., A. P. Mould, and M. J. Humphries. 1998. The integrin β subunit. Int. J. Biochem. Cell Biol. 30:179-184. [DOI] [PubMed] [Google Scholar]
- 25.Hakkinen, L., H. C. Hildebrand, A. Berndt, H. Kosmehl, and H. Larjava. 2000. Immunolocalization of tenascin-C, α9 integrin subunit, and αVβ6 integrin during wound healing in human oral mucosa. J. Histochem. Cytochem. 48:985-998. [DOI] [PubMed] [Google Scholar]
- 26.House, C., and J. A. House. 1989. Evaluation of techniques to demonstrate foot-and-mouth disease virus in bovine tongue epithelium: comparison of the sensitivity of cattle, mice, primary cell cultures, cryopreserved cell cultures and established cell lines. Vet. Microbiol. 20:99-109. [DOI] [PubMed] [Google Scholar]
- 27.Huang, C. C., M. H. Jong, and S. Y. Lin. 2000. Characteristics of foot and mouth disease virus in Taiwan. J. Vet. Med. Sci. 62:677-679. [DOI] [PubMed] [Google Scholar]
- 28.Huang, C. C., Y. L. Lin, T. S. Huang, W. J. Tu, S. H. Lee, M. H. Jong, and S. Y. Lin. 2001. Molecular characterization of foot-and-mouth disease virus isolated from ruminants in Taiwan in 1999-2000. Vet. Microbiol. 81:193-205. [DOI] [PubMed] [Google Scholar]
- 29.Hynes, R. O. 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9:M33-M37. [PubMed] [Google Scholar]
- 30.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 31.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin αVβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αVβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimmins, S., and L. A. MacLaren. 1999. Cyclic modulation of integrin expression in bovine endometrium. Biol. Reprod. 61:1267-1274. [DOI] [PubMed] [Google Scholar]
- 35.Kraft, S., B. Diefenbach, R. Mehta, A. Jonczyk, G. A. Luckenbach, and S. L. Goodman. 1999. Definition of an unexpected ligand recognition motif for αVβ6 integrin. J. Biol. Chem. 274:1979-1985. [DOI] [PubMed] [Google Scholar]
- 36.Lea, S., J. Hernandez, W. Blakemore, E. Brocchi, S. Curry, E. Domingo, E. Fry, R. Abu-Ghazaleh, A. King, J. Newman, D. Stuart, and M. G. Mateu. 1994. The structure and antigenicity of a type C foot-and-mouth disease virus. Structure 2:123-139. [DOI] [PubMed] [Google Scholar]
- 37.Leippert, M., E. Beck, F. Weiland, and E. Pfaff. 1997. Point mutations within the βG-βH loop of foot-and-mouth disease virus O1K affect virus attachment to target cells. J. Virol. 71:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αVβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liaw, L., M. P. Skinner, E. W. Raines, R. Ross, D. A. Cheresh, S. M. Schwartz, and C. M. Giachelli. 1995. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of αVβ3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Investig. 95:713-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, E. C., B. I. Ratnikov, P. M. Tsai, C. P. Carron, D. M. Myers, C. F. Barbas III, and J. W. Smith. 1997. Identification of a region in the integrin β3 subunit that confers ligand binding specificity. J. Biol. Chem. 272:23912-23920. [DOI] [PubMed] [Google Scholar]
- 41.Logan, D., R. Abu-Ghazaleh, W. Blakemore, S. Curry, T. Jackson, A. King, S. Lea, R. Lewis, J. Newman, N. Parry, D. Rowlands, D. Stuart, and E. Fry. 1993. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature 362:566-568. [DOI] [PubMed] [Google Scholar]
- 42.Martinez, M. A., N. Verdaguer, M. G. Mateu, and E. Domingo. 1997. Evolution subverting essentiality: dispensability of the cell attachment Arg-Gly-Asp motif in multiply passaged foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 94:6798-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason, P. W., B. Baxt, F. Brown, J. Harber, A. Murdin, and E. Wimmer. 1993. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology 192:568-577. [DOI] [PubMed] [Google Scholar]
- 44.Mason, P. W., M. J. Grubman, and B. Baxt. Molecular basis of pathogenesis of foot-and-mouth disease virus. Virus Res., in press. [DOI] [PubMed]
- 45.Mason, P. W., E. Rieder, and B. Baxt. 1994. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc. Natl. Acad. Sci. USA 91:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenna, T. S., J. Lubroth, E. Rieder, B. Baxt, and P. W. Mason. 1995. Receptor binding site-deleted foot-and-mouth disease (FMD) virus protects cattle from FMD. J. Virol. 69:5787-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mette, S. A., J. Pilewski, C. A. Buck, and S. M. Albelda. 1993. Distribution of integrin cell adhesion receptors on normal bronchial epithelial cells and lung cancer cells in vitro and in vivo. Am. J. Respir. Cell Mol. Biol. 8:562-572. [DOI] [PubMed] [Google Scholar]
- 48.Meyer, T., J. F. Marshall, and I. R. Hart. 1998. Expression of αV integrins and vitronectin receptor identity in breast cancer cells. Br. J. Cancer 77:530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller, L. C., W. Blakemore, D. Sheppard, A. Atakilit, A. M. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the β-subunit of integrin αVβ6 in infection by foot-and-mouth disease virus. J. Virol. 75:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milner, R., G. Edwards, C. Streuli, and C. ffrench-Constant. 1996. A role in migration for the αVβ1 integrin expressed on oligodendrocyte precursors. J. Neurosci. 16:7240-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neff, S., and B. Baxt. 2001. The ability of integrin αVβ3 to function as a receptor for foot-and-mouth disease virus is not dependent on the presence of complete subunit cytoplasmic domains. J. Virol. 75:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neff, S., P. W. Mason, and B. Baxt. 2000. High-efficiency utilization of the bovine integrin αVβ3 as a receptor for foot-and-mouth disease virus is dependent on the bovine β3 subunit. J. Virol. 74:7298-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neff, S., D. Sa-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αVβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obara, M., M. S. Kang, and K. M. Yamada. 1988. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell 53:649-657. [DOI] [PubMed] [Google Scholar]
- 55.O'Donnell, V. K., J. M. Pacheco, T. M. Henry, and P. W. Mason. 2001. Subcellular distribution of the foot-and-mouth disease virus 3A protein in cells infected with viruses encoding wild-type and bovine-attenuated forms of 3A. Virology 287:151-162. [DOI] [PubMed] [Google Scholar]
- 56.Parry, N., G. Fox, D. Rowlands, F. Brown, E. Fry, R. Acharya, D. Logan, and D. Stuart. 1990. Structural and serological evidence for a novel mechanism of antigenic variation in foot-and-mouth disease virus. Nature 347:569-572. [DOI] [PubMed] [Google Scholar]
- 57.Pulli, T., E. Koivunen, and T. Hyypia. 1997. Cell-surface interactions of echovirus 22. J. Biol. Chem. 272:21176-21180. [DOI] [PubMed] [Google Scholar]
- 58.Rieder, E., B. Baxt, J. Lubroth, and P. W. Mason. 1994. Vaccines prepared from chimeras of foot-and-mouth disease virus (FMDV) induce neutralizing antibodies and protective immunity to multiple serotypes of FMDV. J. Virol. 68:7092-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rieder, E., B. Baxt, and P. W. Mason. 1994. Animal-derived antigenic variants of foot-and-mouth disease virus type A12 have low affinity for cells in culture. J. Virol. 68:5296-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rieder, E., A. Berinstein, B. Baxt, A. Kang, and P. W. Mason. 1996. Propagation of an attenuated virus by design: engineering a novel receptor for a noninfectious foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 93:10428-10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rieder, E., T. Bunch, F. Brown, and P. W. Mason. 1993. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J. Virol. 67:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson, B. H., D. M. Moore, M. J. Grubman, and D. G. Kleid. 1983. Identification of an exposed region of the immunogenic capsid polypeptide VP1 on foot-and-mouth disease virus. J. Virol. 46:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697-715. [DOI] [PubMed] [Google Scholar]
- 64.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh, B., N. Rawlings, and A. Kaur. 2001. Expression of integrin αVβ3 in pig, dog and cattle. Histol. Histopathol. 16:1037-1046. [DOI] [PubMed] [Google Scholar]
- 66.Snowdon, W. A. 1966. Growth of foot-and-mouth disease virus in monolayer cultures of calf thyroid cells. Nature 210:1079-1080. [DOI] [PubMed] [Google Scholar]
- 67.Stave, J. W., J. L. Card, D. O. Morgan, and V. N. Vakharia. 1988. Neutralization sites of type O1 foot-and-mouth disease virus defined by monoclonal antibodies and neutralization-escape virus variants. Virology 162:21-29. [DOI] [PubMed] [Google Scholar]
- 68.Taboga, O., C. Tami, E. Carrillo, J. I. Nunez, A. Rodriguez, J. C. Saiz, E. Blanco, M. L. Valero, X. Roig, J. A. Camarero, D. Andreu, M. G. Mateu, E. Giralt, E. Domingo, F. Sobrino, and E. L. Palma. 1997. A large-scale evaluation of peptide vaccines against foot-and-mouth disease: lack of solid protection in cattle and isolation of escape mutants. J. Virol. 71:2606-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takagi, J., T. Kamata, J. Meredith, W. Puzon-McLaughlin, and Y. Takada. 1997. Changing ligand specificities of αVβ1 and αVβ3 integrins by swapping a short diverse sequence of the beta subunit. J. Biol. Chem. 272:19794-19800. [DOI] [PubMed] [Google Scholar]
- 70.Tozer, E. C., P. E. Hughes, and J. C. Loftus. 1996. Ligand binding and affinity modulation of integrins. Biochem. Cell Biol. 74:785-798. [DOI] [PubMed] [Google Scholar]
- 71.Triantafilou, K., M. Triantafilou, Y. Takada, and N. Fernandez. 2000. Human parechovirus 1 utilizes integrins αVβ3 and αVβ1 as receptors. J. Virol. 74:5856-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang, A., Y. Yokosaki, R. Ferrando, J. Balmes, and D. Sheppard. 1996. Differential regulation of airway epithelial integrins by growth factors. Am. J. Respir. Cell Mol. Biol. 15:664-672. [DOI] [PubMed] [Google Scholar]
- 73.Weinacker, A., R. Ferrando, M. Elliott, J. Hogg, J. Balmes, and D. Sheppard. 1995. Distribution of integrins αVβ6 and α9β1 and their known ligands, fibronectin and tenascin, in human airways. Am. J. Respir. Cell Mol. Biol. 12:547-556. [DOI] [PubMed] [Google Scholar]
- 74.Xiong, J. P., T. Stehle, B. Diefenbach, R. Zhang, R. Dunker, D. L. Scott, A. Joachimiak, S. L. Goodman, and M. A. Arnaout. 2001. Crystal structure of the extracellular segment of integrin αVβ3. Science 294:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong, J. P., T. Stehle, R. Zhang, A. Joachimiak, M. Frech, S. L. Goodman, and M. A. Arnaout. 2002. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296:151-155. [DOI] [PubMed] [Google Scholar]
- 76.Zhao, Q., J. M. Pacheco, and P. W. Mason. Evaluation of genetically engineered derivatives of a Chinese strain of foot-and-mouth disease virus reveals a novel cell-binding site that functions in cell culture and in animals. J. Virol., in press. [DOI] [PMC free article] [PubMed]