Abstract
We describe the development of novel lentivirus vectors based on simian immunodeficiency virus from African green monkey (SIVagm) pseudotyped with Sendai virus (SeV) envelope glycoproteins. SeV fusion (F) and hemagglutinin-neuraminidase (HN) proteins were successfully incorporated into the SIVagm-based vector by truncation of the cytoplasmic tail of the F protein and by addition of the cytoplasmic tail of SIVagm transmembrane envelope protein to the N terminus of the HN protein. As with the vesicular stomatitis virus G glycoprotein-pseudotyped vector, the mutant SeV F- and HN-pseudotyped SIVagm vector was able to transduce various types of animal and human cell lines. Furthermore, the vector was able to transduce an enhanced green fluorescent protein reporter gene into polarized epithelial cells of rat trachea from the apical and basolateral sides. Therefore, SeV F- and HN-pseudotyped SIVagm vectors have considerable potential for effective use in gene therapy for various therapies, including respiratory diseases.
Replication-defective lentivirus vectors are useful tools in the field of gene therapy due to their ability to transduce a foreign gene in a cell cycle-independent manner, as most of the cells in normal tissues in humans are differentiated and arrested. In this feature, matrix (MA) protein, Vpr, and integrase have been shown to play important roles (2, 10, 23, 24). The efficiency of transduction of lentivirus vectors is highly dependent on the initial interaction between the envelope and the receptors on the cell surface. Therefore, pseudotyping lentivirus vectors is a highly effective system for changing their cell tropism and expanding their use in gene therapy.
Currently, vesicular stomatitis virus G glycoprotein (VSV-G) is widely used as an envelope glycoprotein for pseudotyped lentivirus vectors because the vectors are easily concentrated by centrifugation and transduce a wide range of cell types in animals. However, VSV-G-pseudotyped lentivirus vectors are unable to transduce airway epithelium without causing injury (11), and VSV-G is known to be cytotoxic to some cell types (12, 27). Sendai virus (SeV) is enveloped with fusion (F) and hemagglutinin-neuraminidase (HN) proteins, and recombinant SeV vectors have been shown to transfer a foreign gene to a wide spectrum of cells and tissues, including airway epithelia, where it is then expressed (14, 28). Therefore, SeV glycoprotein-pseudotyped vectors are expected to show expanded cell tropism, making them ideal tools for gene therapy, including therapy for respiratory diseases.
Incorporation of heterologous envelope proteins into the virus particles is an important factor for production of functional pseudotyped lentivirus vectors. The key role of the cytoplasmic tail of the transmembrane protein (TMP) in the process of envelope incorporation into the virions has been shown in a number of studies with homologous TMP, but these results are conflicting. TMPs with truncated cytoplasmic tails have been reported to be efficiently incorporated into virus particles (9, 25, 26, 31). In contrast, the cytoplasmic tail of TMP interacts with the Gag protein, making it essential for efficient envelope incorporation into virions (3, 5, 6, 18, 22). It is not clear what modifications in the cytoplasmic tail of the TMP are preferable for the generation of infectious pseudotyped vectors.
In this report, we investigated the incorporation of SeV F and HN glycoproteins with various modifications and showed successful generation of novel lentivirus vectors pseudotyped with the SeV F and HN proteins. Analysis of functional vector titer and incorporation of these proteins into the virions indicates that F protein with a truncated cytoplasmic tail was more efficiently incorporated. In contrast, HN protein was incorporated only after the addition of the cytoplasmic tail of simian immunodeficiency virus of African green monkey (SIVagm) TMP to its N terminus. One of the SeV mutant F- and HN-pseudotyped SIVagm vectors showed tropism for a wide range of cells, similar to the VSV-G-pseudotyped vector. In addition, the vector efficiently transduced polarized epithelial cells of rat trachea. SeV F- and HN-pseudotyped SIVagm vectors will be useful for various therapies, including respiratory diseases.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney 293T, African green monkey fibroblast COS-7, and human rhabdomyosarcoma TE671 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). BEAS-2B cells, a normal human bronchial epithelial cell line, were maintained in DMEM mixed with RPMI 1640 medium in a 1:1 ratio, supplemented with 10% FBS, 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate/ml (Gibco-BRL). Human cervical carcinoma HeLa cells and rhesus monkey kidney LLC-MK2 cells were maintained in minimal essential medium (MEM) supplemented with 10% FBS. Human hepatocellular carcinoma HepG2 cells were maintained in MEM supplemented with nonessential amino acids, sodium pyruvate, and 10% FBS.
Isolation and culture of RTE cells.
Rat tracheal epithelial (RTE) cells were isolated and cultured based on the methods of Matsui et al. (16). Male Fisher 344/N Slc rats (Japan SLC, Inc.) were euthanized by ethylether inhalation. The tracheas were cannulated, filled with a 1% solution of protease type 14 (Sigma) in Ham's F12 medium (Gibco-BRL), excised, and incubated for 24 h at 4°C. Epithelial cells were removed from the trachea by flushing with medium, counted, and plated onto the apical surface of Transwell-Col tissue culture inserts (Corning). The RTE cells were maintained in DMEM/Ham's F12 medium mixture in a 1:1 ratio, supplemented with insulin (10 μg/ml), hydrocortisone (0.1 μg/ml), transferrin (5 μg/ml), epidermal growth factor (25 ng/ml), bovine pituitary extract (1%), retinoic acid (5 × 10−8 M), gentamicin (50 μg/ml), and bovine serum albumin (3 mg/ml). All the animal studies were performed in accordance with recommendations for the proper care and use of laboratory animals.
Plasmid constructs. (i) Chimeric HN expression plasmids.
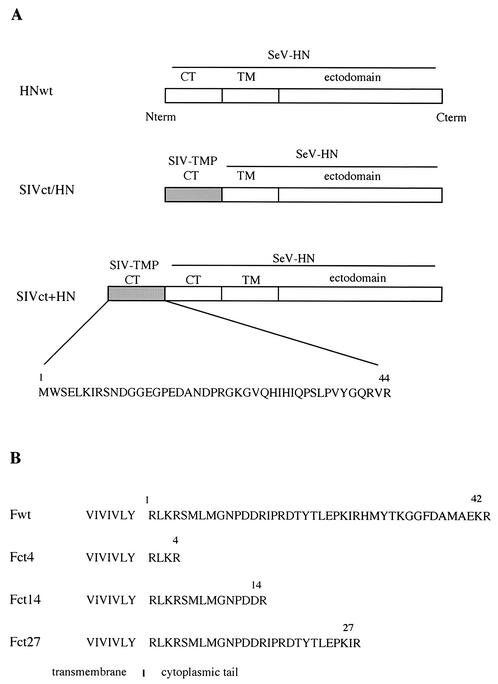
Plasmid SIVct/HN contains the gene encoding the cytoplasmic tail of SIVagm TMP fused to the ectodomain and transmembrane regions of SeV HN protein. Three oligonucleotide pairs were synthesized: pair 1, 5′-TCGAGATGTGGTCTGAGTTAAAAATCAGGAGCAACGACGGAGGTGAAGGACCAGACGCCAACGACCC-3′ and 5′-CCGGGGGTCGTTGGCGTCTGGTCCTTCACCTCCGTCGTTGCTCCTGATTTTTAACTCAGACCACATC-3′; pair 2, 5′-CCGGGGAAAGGGGGTGCAACACATCCATATCCAGCCATCTCTACCTGTTTATGGACAGA-3′ and 5′-ACCCTCTGTCCATAAACAGGTAGAGATGGCTGGATATGGATGTGTTGCACCCCTTTCC-3′; and pair 3, 5′-GGGTTAGGTGGTTGCTGATTCTCTCATTCACCCAGTGGG-3′ and 5′-GATCCCCACTGGGTGAATGAGAGAATCAGCAACCACCTA-3′. These oligonucleotide pairs were annealed and cloned into the XhoI and BamHI sites of pBluescript KS+ (Stratagene) to yield pKS+SIVct. pCAGGS-SIVct/HN was constructed by cloning the 160-bp XhoI-DraIII fragment from pKS+SIVct and a 1.5-kbp DraIII-Bsu36I fragment from pCAGGS-HN, which carries the wild-type HN gene (HNwt), in the XhoI site of pCAGGS (20) vector, into the XhoI and Bsu36I sites of pCAGGS. This plasmid was constructed so that the cytoplasmic tail of the HN protein was replaced with the cytoplasmic tail of SIVagm TMP (Fig. 1A).
FIG. 1.
Organization of SeV wild-type (wt) and mutant F and HN proteins. (A) HN expression vectors. Schematic representation of HN protein and the amino acid sequence of the cytoplasmic tail (CT) of SIVagm TMP are shown. The numbers represent amino acids derived from SIVagm TMP. term, terminus. (B) F expression vectors. The amino acid sequences of part of the transmembrane domain and the cytoplasmic tail are shown. The plasmid names (Fct4, Fct14, and Fct27) indicate the number of residues in the cytoplasmic tail of the F protein.
For construction of pCAGGS-SIVct+HN, the genes encoding the cytoplasmic tail of SIVagm TMP and the N terminus of HN protein were first amplified by PCR from pCAGGS-SIVct/HN with the primer pair 5′-GAGACTCGAGATGTGGTCTGAGTTAAAAATCAGG-3′ and 5′-AGAGGTAGACCAGTACGAGTCACGTTTGCCCCTATCACCATCCCTAACCCTCTGTCATAAAC-3′.The resulting PCR fragment was cloned into the XhoI and AccI sites of pKS+SIVct to generate pKS+SIVct-H. Then a XhoI-DraIII fragment from pKS+SIVct-H and a DraIII-Bsu36I fragment from pCAGGS-HN were cloned into the XhoI and Bsu36I sites of pCAGGS to yield pCAGGS-SIVct+HN (Fig. 1A).
(ii) Truncated F expression plasmids.
Three cytoplasmic tail truncation mutants of the SeV F protein were generated by PCR by introducing a stop TGA codon into the cytoplasmic tail gene. F mutants truncated to 27, 14, and 4 amino acids of the cytoplasmic tail were amplified by PCR with the primer pairs 5′-CCGCTCGAGCATGACAGCATA TATCCAGAGA and 5′-ATAGTTTAGCGGCCGCTCATCTGATCTTCGGCTCTAATGT-3′ (Fct27) or 5′-ATAGTTTAGCGGCCGCTCAACGGTCATCTGGATTACCCAT-3′ (Fct14) or 5′-ATAGTTTAGCGGCCGCTCACCTTCTGAGTCTATAAAGCAC-3′ (Fct4). The resulting PCR fragments were cloned into the XhoI and NotI sites of pCAGGS to yield pCAGGS-Fct27, pCAGGS-Fct14, and pCAGGS-Fct4, respectively (Fig. 1B).
Protein expression. (i) Immunofluorescence analysis.
Various truncated F and chimeric HN expression plasmids were transfected into 293T cells with Lipofectamine Plus reagent (Gibco-BRL). For detection of the SeV glycoproteins on the cell surface, transfected 293T cells were collected and incubated with monoclonal anti-F (γ-236) (21) or anti-HN (HN-2) (17) antibody. After washing and incubation with fluorescein-labeled anti-mouse immunoglobulin G (Alexa 488; Nakarai Tesque), the labeled cells were suspended in phosphate-buffered saline (PBS)-2% FBS for flow cytometric analysis with an Epics Elite EPS (Coulter).
(ii) Erythrocyte binding assay.
To investigate the functional expression of chimeric HN genes, we performed the erythrocyte binding assay by the following method. A 1% mixture of chicken erythrocyte in PBS was added to 293T cells transfected with various HN expression plasmids. The plates were incubated at 4°C for 45 min and then washed five times with PBS.
(iii) Cell fusion assay.
LLC-MK2 cells were cotransfected with a series of F and HN expression plasmids. After incubation at 37°C for 24 h, 7.5 μg of trypsin per ml was added to the medium. Cell fusion was examined 48 h after transfection.
Preparation of pseudotyped lentivirus vectors.
For vector preparation, the four-plasmid system was used. 293T cells were grown on six-well tissue culture plates. The medium was replaced with 0.8 ml of DMEM supplemented with 1% bovine serum albumin (BSA), and cells were cotransfected with 1.2 μg of pGL3C/CMVL.U3G2/RREc/s/CMVF EGFP/3LTRΔU3, which is a self-inactivating gene transfer vector containing the enhanced green fluorescent protein (EGFP) gene under the control of a cytomegalovirus early promoter (19), 0.36 μg of packaging vector pCAGGS/SIVagm Gag-Tat/Rev with a deletion in the long terminal repeat, the nef gene, and a packaging signal (19), and 0.24 μg each of SeV HN and F expression plasmids with Lipofectamine Plus reagent (Gibco-BRL).
Production of the Moloney murine leukemia virus-based vector was done with pMSCV-GFP, in which the EGFP gene was cloned into the pMSCVneo vector (Clontech) as a gene transfer vector. 293T cells were transfected with 1 μg of gene transfer vector, 0.3 μg of packaging vector (pVPack-GP [Stratagene]), and 0.2 μg each of SeV HN and F expression plasmids. After transfection, 1 ml of DMEM containing 1% BSA and 15 μg of trypsin per ml was added, and the cells were incubated. Sixteen hours after transfection, the medium was replaced with 2 ml of DMEM supplemented with 1% BSA and 7.5 μg of trypsin per ml, and the cells were further incubated. Twenty-four hours after replacing the medium, the vector-containing medium was collected after filtration through 0.45-μm filter.
HAU and vector titer.
The hemagglutination assay was performed as described by Condit (4). Briefly, after serial twofold dilutions of 50 μl of the culture supernatants with PBS in 96-well plates, 50 μl of a 0.5% suspension of chicken erythrocytes in PBS was added to the diluted samples. The plate was incubated for 1 h at 4°C, and hemagglutination units (HAU) were determined by observing the highest dilution at which agglutination occurred. The vector titer was determined on 293T cells by the following method. Vector-containing medium was used to infect 293T cells in six-well plates. After 3 h of infection, 1 ml of DMEM supplemented with 20% FBS was added, and the cells were incubated for 3 days at 37°C. Vector titer was determined by counting the number of EGFP-expressing cells by fluorescence microscopy and calculation of EGFP transduction units (T.U.).
Western blot analysis of viral pellets.
The supernatants of transfected producer cells were centrifuged at 16,000 × g for 1 h to pellet the viruses. The pellets were suspended in sample buffer (0.25 M Tris-HCl [pH 6.8], 1% sodium dodecyl sulfate, 5% β-mercaptoethanol, 15% glycerol, 0.005% bromophenol blue) and boiled for 10 min. The protein samples were subjected to 10% to 20% gradient SDS-polyacrylamide gel electrophoresis. The separated virus proteins were transferred to a polyvinylidene difluoride membrane (Amersham-Pharmacia) with a semidry blotter. Monoclonal anti-HN (HN-2) or monospecific anti-F1 antibody was used for specific binding. The protein bands were detected with either anti-mouse IgG (Cappel) or anti-rabbit IgG (ICN) antibody labeled with horseradish peroxidase with the ECL Plus Western blotting detection system (Amersham-Pharmacia).
RESULTS
Expression of chimeric SeV HN and F proteins with truncated cytoplasmic tails.
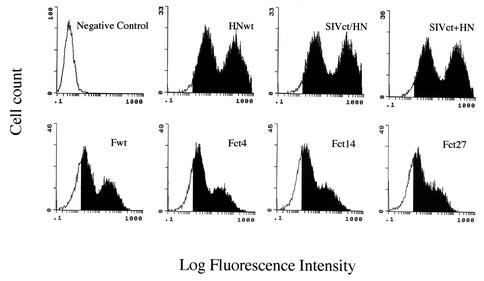
We constructed chimeric SeV HN proteins and F proteins with cytoplasmic tail truncations to incorporate into the vector particles. To investigate the expression of these envelope glycoproteins, 293T cells transfected with expression plasmids were stained with a monoclonal antibody specific for the F or HN protein, followed by flow cytometric analysis. As shown in Fig. 2, chimeric HN protein and F mutant protein with truncated cytoplasmic tails were expressed at the cell surface, and the amounts of these proteins were equivalent to those of the wild-type HN and F proteins.
FIG. 2.
Flow cytometric analysis of cell surface expression of SeV wild-type (wt) and mutant F or HN protein. 293T cells were transfected as described in Materials and Methods. At 24 h after transfection, cells were immunostained with a specific monoclonal antibody against F or HN protein and reacted with fluorescein-labeled anti-mouse immunoglobulin G. The solid area of each histogram indicates positive cells.
To determine whether the mutant proteins retained biological activity, an erythrocyte binding assay and cell fusion assay were performed after cotransfection of 293T or LLC-MK2 cells with the F and HN expression plasmids. Erythrocytes were tightly bound to the transfected 293T cells (data not shown), and cell fusion was observed in LLC-MK2 cells (Table 1). In cells transfected with either the F or HN expression plasmid alone, no cell fusion was observed. These results demonstrated that chimeric HN and truncated F proteins not only were efficiently expressed on the cell surface but also retained their biological activities.
TABLE 1.
Cell fusion activity of LLC-MK2 cells with SeV wild-type and mutant F and HN proteinsa
| Protein | Cell fusionb
|
|||
|---|---|---|---|---|
| No transfection | Wild-type HN | SIVct/HN | SIVct+HN | |
| None | ND | ND | ND | ND |
| F (wild type) | ND | + | 2+ | 3+ |
| Fct4 | ND | 3+ | + | 4+ |
| Fct14 | ND | 5+ | 7+ | 5+ |
| Fct27 | ND | + | + | 3+ |
LLC-MK2 cells were cotransfected with wild-type and mutant F and HN expression plasmids. At 24 h after transfection, trypsin (7.5 μg/ml) was added to the medium. Cell fusion activity was examined at 48 h.
Each plus sign represents 10 syncytia/field. ND, not detected.
Preparation of SeV F- and HN-pseudotyped SIVagm vector.
The four-plasmid system was employed for preparation of SeV F- and HN-pseudotyped SIVagm vectors. To generate vector particles, envelope plasmids encoding chimeric HN protein and the truncated F mutant were transfected into 293T cells with a gene transfer vector carrying the EGFP reporter gene and a packaging vector containing viral components. Transfection of F and HN expression plasmids did not induce visible syncytia, as it did in LLC-MK2 cells.
Forty-eight hours after the transfection, the culture medium was harvested and assayed to measure the degree of incorporation of HN protein into the vector particles. The HAU of culture supernatants transfected with SIVct+HN, which carries the cytoplasmic tail of SIVagm TMP in the N terminus of wild-type HN, in combination with any wild-type F or mutant F protein showed the highest levels, and the lowest were obtained in the case of wild-type HN (Table 2). These results suggested that mutant HN proteins including the cytoplasmic tail of SIVagm TMP (SIVct/HN and SIVct+HN) were incorporated into the vector particles more efficiently than wild-type HN, and incorporation was independent of F proteins.
TABLE 2.
Generation of SeV wild-type and mutant F- and HN-pseudotyped SIVagm vectora
| Virus envelope proteins | HAU | Virus titer on 293T cells (T.U./ml) | |
|---|---|---|---|
| F | HN | ||
| Wild type | Wild type | 1 | ND |
| SIVct/HN | 4 | 5.1 × 102 | |
| SIVct+HN | 16 | 1.4 × 103 | |
| Fct4 | Wild type | <1 | 8.5 × 102 |
| SIVct/HN | 2 | 9.2 × 103 | |
| SIVct+HN | 16 | 6.3 × 104 | |
| Fct14 | Wild type | <1 | ND |
| SIVct/HN | 2 | 3.4 × 102 | |
| SIVct+HN | 16 | 3.2 × 103 | |
| Fct27 | Wild type | <1 | ND |
| SIVct/HN | 4 | ND | |
| SIVct+HN | 16 | ND | |
293T cells were transfected with a gene transfer vector, a packaging vector, SeV wild-type or mutant F-expressing plasmids, and wild-type or mutant HN-expressing plasmids. The vector yields, HAU, and T.U. were determined as described in Materials and Methods. The HAV assay was performed 2 days after transfection. ND, not detected.
To investigate the generation of functional vectors, culture supernatants containing vector particles were used to transduce 293T cells, and the titer was measured by counting EGFP-expressing cells. As shown in Table 2, virus titers from SIVct+HN in combination with wild-type F, Fct4, and Fct14 proteins were higher than that from SIV/HN or wild-type HN, a tendency similar to the result for HAU. This suggests that the efficiency of incorporation of HN protein is what determines the functional titer. However, even the best combination, SIVct+HN and Fct4, the titer of the vector in 293T cells was 20- to 40-fold less than that of the VSV-G-pseudotyped vector.
Incorporation of chimeric HN and F proteins with truncated cytoplasmic tails into SIVagm vectors.
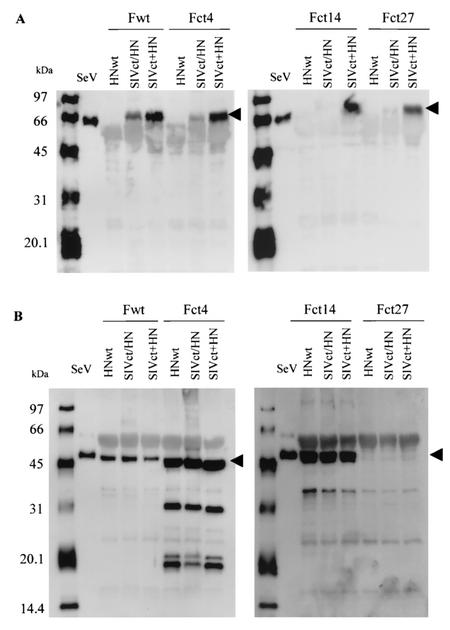
Generation of pseudotyped SIVagm vectors with different chimeric HN proteins and truncated F mutants from 293T cells showed different degrees of efficiency. To determine whether these proteins were indeed incorporated into SIVagm vectors, supernatants from 293T cells transiently transfected with a gene transfer vector, a packaging vector, and wild-type or mutant F and HN protein expression vectors were pelleted by ultracentrifugation. The pellets were then lysed and analyzed by Western blotting. As shown in Fig. 3, SIVct+HN protein was detected in all combinations by a panel of F proteins, and SIVct/HN protein was detected in small amounts in the presence of wild-type F protein or Fct4 protein. In contrast, wild-type HN protein was not found in the presence of wild-type F protein or any truncated F mutant proteins. In F proteins, wild-type F, Fct4 and Fct14 were detected in combination with any wild-type HN or mutant HN proteins. Fct27 was not detected in the pseudotyped virions. These results demonstrated that the incorporation of chimeric HN proteins and truncated F mutant proteins was not due to the level of expression of these proteins on the surface of the producer cells.
FIG. 3.
Western blot analysis of incorporation of SeV wild-type and mutant F and HN proteins into SIVagm vectors. Virion lysates of SeV wild-type (wt) and mutant F- and HN-pseudotyped vectors were prepared from pellets of supernatants of producer cells by ultracentrifugation. Samples were transferred to a polyvinylidene difluoride membrane and blotted with the anti-HN antibody HN-2 (A) or the anti-F1 antibody (B). SeV virion lysates were used as the positive control for each protein.
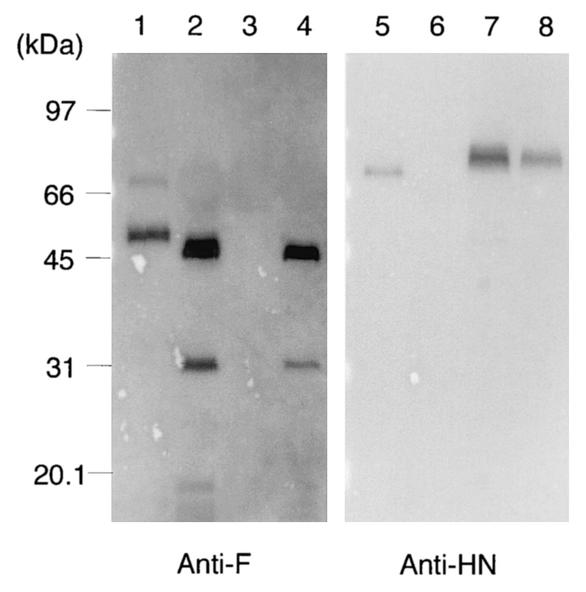
We next investigated whether SeV mutant F and HN proteins were individually incorporated into vector particles. The Fct4 or SIVct+HN expression plasmid was cotransfected with a gene transfer vector and a packaging vector into 293T cells, and virus pellets were subjected to Western blot analysis. Fct4 and SIVct+HN proteins were efficiently incorporated into vector particles, but 293T cells were not transduced by these vectors (Fig. 4).
FIG. 4.
Western blot analysis of incorporation of a SeV mutant F or HN protein into SIVagm vectors. 293T cells were cotransfected with the Fct4 or SIVct+HN expression plasmid in combination with gene transfer and packaging vectors. Preparation of pseudotyped virion lysates and Western blot analysis with the anti-F1 antibody (lanes 1 to 4) or the anti-HN antibody HN-2 (lanes 5 to 8) was performed as described for Fig. 3. SeV virion lysates were used as the positive control for each protein. Lanes: 1 and 5, SeV; 2 and 6, Fct4; 3 and 7, SIVct+HN; and 4 and 8, Fct4 and SIVct+HN.
Inhibition of gene transfer of SeV mutant F- and HN-pseudotyped SIVagm vector by neutral antibody to SeV.
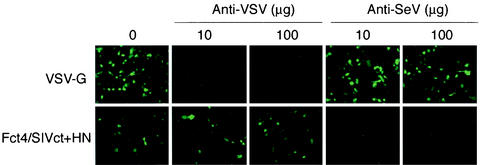
Pseudotyped vectors adopt the adsorption and entry characteristics of the incorporated envelope. It is likely that SeV HN protein binds to its receptor, sialic acid, and fusion of the viral envelope with the cell plasma membrane proceeds. To investigate this interaction further, transduction experiments were carried out in the presence of a neutralizing antibody specific to SeV. Fct4- and SIVct+HN- or VSV-G-pseudotyped vectors were incubated with anti-SeV-HN (HN-2), which inhibits cell-to-cell fusion. Anti-VSV-G (I-1 [13]), which neutralizes the infectivity of VSV, was used as a control. These mixtures were then used to transduce 293T cells. HN-2 completely inhibited transduction by the Fct4- and SIVct+HN-pseudotyped vector but had no effect on the titer of the VSV-G-pseudotyped vector. On the other hand, the titer of the Fct4- and SIVct+HN-pseudotyped vector was not changed in either the presence or absence of the anti-VSV-G antibody in the medium (Fig. 5). These results showed that the Fct4- and SIVct+HN-pseudotyped vector carries the Fct4 and SIVct+HN proteins on its surface and these proteins mediate cell adsorption and fusion.
FIG. 5.
Inhibition of transduction by SeV mutant F- and HN-pseudotyped SIVagm vector with a neutralizing antibody specific to SeV. The SeV Fct4- and SIVct+HN-pseudotyped vector at 0.8 × 104 T.U. or the VSV-G-pseudotyped vector at 1.2 × 106 T.U. was incubated with the immunoglobulin G fraction of a neutralizing antibody specific to SeV (anti-HN antibody HN-2) or VSV (anti-VSV-G antibody I-1) at 4°C for 30 min, followed by transduction of 293T cells. Seventy-two hours after the transduction, EGFP-expressing cells were detected by fluorescence microscopy.
Cell tropism of SeV mutant F- and HN-pseudotyped SIVagm vector.
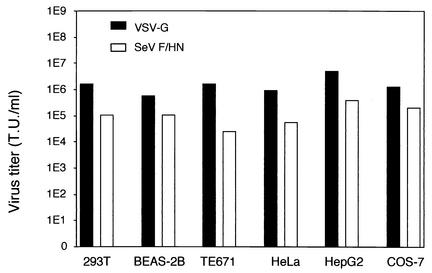
The virus adsorbs to the cell through the interaction of the envelope protein and cell receptor. The receptor of VSV is expressed on various cells, resulting in wide tropism of virus infection. To investigate the cell tropism of an SeV F- and HN-pseudotyped vector, several cell lines derived from animal and human organs were transduced with an Fct4- and SIVct+HN-pseudotyped SIVagm vector for comparison with a VSV-G-pseudotyped vector. Titers of Fct4/SIVct+HN- and VSV-G-pseudotyped SIVagm vectors in 293 T cells were 1 × 105 T.U./ml and 2 × 106 T.U./ml, respectively. The results demonstrated that the SeV mutant F- and HN-pseudotyped vector showed expanded cell tropism similar to that of VSV-G pseudotyped vectors, including fibroblasts and epithelial cells. However, the SeV Fct4- and SIVct+HN-pseudotyped vector displayed 5- to 70-fold less infectivity than the VSV-G-pseudotyped vector when the same volume of vector solution was applied (Fig. 6).
FIG. 6.
Cell tropism of SeV mutant F and HN vector. Cells were replated the day before the Fct4- and SIVct+HN- or VSV-G-pseudotyped SIVagm vector infection. About 106 cells in six-well plates were infected with 1 ml of the diluted vector solution. The number of EGFP-expressing cells was counted 72 h after infection. Titers of the vector solution measured in 293T cells were 1 × 105 T.U./ml in the Fct4- and SIVct+HN-pseudotyped vector and 2 × 106 T.U./ml in the VSV-G-pseudotyped vector.
SeV mutant F- and HN-pseudotyped SIVagm vector-mediated gene transfer to rat airway epithelial cells.
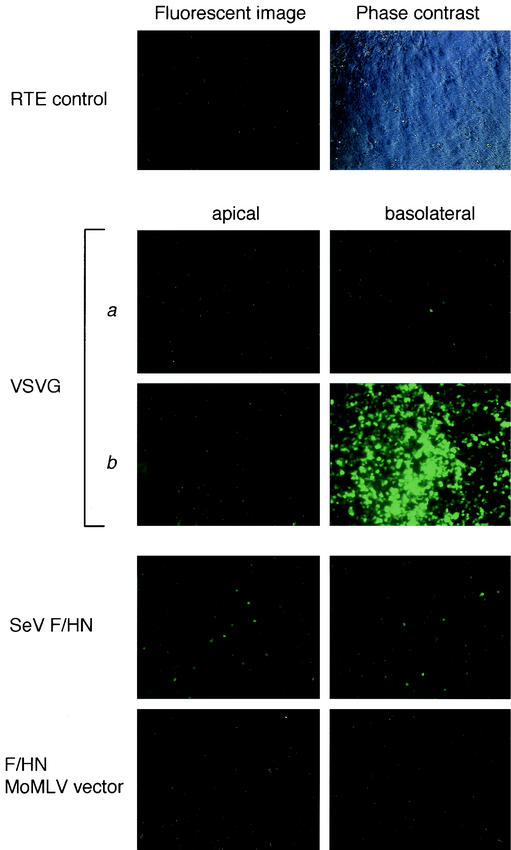
To investigate the transduction of different vectors into polarized airway epithelial cells, they were applied to RTE cells from the apical or basolateral surface. Transepithelial resistance of cultured RTE cells at 7 days after isolation was measured with a Millicell-ERS (Millipore) and confirmed to be more than 1,500 Ω/cm2. A VSV-G-pseudotyped SIVagm vector was able to effectively transduce the EGFP reporter gene from the basolateral surface but not the apical surface of RTE cells only when the vector was concentrated more than 200-fold (4.9 × 105 T.U to 1.2 × 108 T.U). In contrast, an SeV mutant Fct4- and SIVct+HN-pseudotyped SIVagm vector was able to transduce the EGFP reporter gene from both the basolateral and apical surfaces without any concentration (1.2 × 104 T.U.) (Fig. 7). In the case of a Moloney murine leukemia virus-based SeV Fct4- and SIVct+HN-pseudotyped vector, no transduction was observed after application from either the apical or basolateral surface when the vector was used at about the same amount as the SIVagm-based vector (1.7 × 104 T.U.). These data demonstrated that the SIVagm vector pseudotyped with SeV mutant F and HN proteins would be suitable for application to polarized epithelial cells.
FIG.7.
Transduction of RTE cells with pseudotyped SIVagm vectors. Fluorescent images of EGFP expression on day 3 and phase contrast microscopy of RTE cells. RTE cells were exposed to Fct4- and SIVct+HN-pseudotyped SIVagm vectors at the apical or basolateral side. The titers of the VSV-G-pseudotyped SIVagm vector were (a) 4.9 × 105 T.U./0.1 ml and (b) 1.2 × 108 T.U./0.1 ml; the titer of the SeV Fct4- and SIVct+HN-pseudotyped SIVagm vector was 1.2 × 104 T.U./0.1 ml; and the titer of the SeV Fct4- and SIVct+HN-pseudotyped Moloney murine leukemia virus vector was 1.7 × 104 T.U./0.1 ml.
DISCUSSION
We have developed novel SIVagm vectors pseudotyped with SeV F and HN envelope glycoproteins. SeV mutant F and HN proteins were incorporated efficiently into SIVagm vector particles and would be applicable to an extensive range of tissues in animals and humans, as sialic acid, which is a receptor for SeV, is ubiquitously present in several tissues.
In lentiviruses, the mechanism of incorporation of heterologous envelope proteins into virus particles is not well understood. Results from various genetic approaches indicate that the interactions that take place between MA and the C terminus of TMP (5, 7, 29), as well as mutations in Env that affect its incorporation into mature virions, inhibit envelope-matrix interaction (5). Another study showed that the cytoplasmic domain of gp41 is not required for the incorporation of Env into virions (9). Reports on Env of SIVmac indicated that truncation of the cytoplasmic domain enhances envelope incorporation into SIVmac or HIV virions (25, 31).
In the case of SIVagm, the cytoplasmic tail of TMP is shorter than that of SIVmac (8), and there was no report on the importance of the interaction between MA protein and Env. In this study, when SIVagm was pseudotyped with SeV wild-type and mutant F and HN proteins, the chimeric HN proteins SIVct/HN and SIVct+HN were efficiently incorporated into SIVagm particles. Although wild-type HN protein was efficiently expressed at the cell surface of transfected cells, the protein was not incorporated into pseudotyped virions. As the cytoplasmic tail of wild-type HN was 8 amino acids shorter in length than that of SIVct/HN (35 amino acids versus 43 amino acids), it appears that a specific domain in the cytoplasmic tail of TMP in SIVagm plays a major role in incorporation of HN proteins into virions.
SIVct+HN, with a longer cytoplasmic tail (78 amino acid) than SIVct/HN, was the most efficiently incorporated into virions and showed the highest titer. One explanation for this phenomenon is that the N terminus of SIVct+HN is located in close proximity to the viral matrix layer. In contrast to incorporation of HN proteins, chimeric F proteins carrying the cytoplasmic tail of SIVagm TMP were not expressed in producer cells (data not shown). There is as yet no logical explanation for why these chimeric F proteins are not expressed in the same way as chimeric HN proteins.
One report has demonstrated that a SIVmac Env glycoprotein with a short cytoplasmic tail is incorporated into particles in an MA-independent manner (7). In addition, there have been several reports of incorporation of distantly related retroviral glycoproteins bearing a short cytoplasmic tail into HIV-1 particles (15). In pseudotyping SIVagm with truncated F mutants, the Fct4, Fct14, and Fct27 proteins were expressed efficiently. However, the Fct27 protein was not incorporated into SIVagm particles. These results may support the suggestion that a short cytoplasmic tail in envelope glycoproteins avoids the steric constraints imposed by virus structure, leading to incorporation into viral particles. In the case of HN protein, however, cytoplasmic tail-truncated mutants were not incorporated into particles in spite of expression at the cell surface (data not shown).
Further analysis of the localization of these proteins and their interaction with lipid rafts will be required. Fct14 was more efficiently incorporated into virions than Fct4, but Fct14- and mutant HN (SIVct/HN and SIVct+HN)-pseudotyped vectors were more than 10-fold less infectious than Fct4- and mutant HN-pseudotyped vectors. Since these truncated F proteins had fusion activity, we conclude that interaction between F and HN, which is necessary for fusion promotion, is deficient, and the vector becomes less infectious in the case of the Fct14 and mutant HN proteins on vector particles.
SeV mutant F and HN proteins were sufficiently incorporated into SIVagm virions as examined by Western blot analysis. However, even under optimal conditions, the SIVagm vector pseudotyped with Fct4 and SIVct+HN was less infectious than the VSV-G-pseudotyped vector. Two explanations may account for this lower infectivity. First, because of the independent incorporation of each envelope glycoprotein, Fct4 and SIVct+HN, there are mixtures of various particles (Envless Fct4 or SIVct+HN vector particles; noninfectious Fct4 and SIVct+HN vector particles; and infectious Fct4 and SIVct+HN vector particles), resulting in a lower rate of functional vector. Second, the ratios of the Fct4 and SIVct+HN proteins on the virion surface are not optimum, and the interaction between the Fct4 and SIVct+HN proteins is inadequate.
To characterize this vector, we investigated the transduction of various cell types compared to transduction with the VSV-G-pseudotyped vector. An SeV mutant F- and HN-pseudotyped vector was able to transduce a panel of cell lines as observed with the VSV-G-pseudotyped vector. These results demonstrated that an SeV mutant F- and HN-pseudotyped vector had expanded cell tropism compared with SIVagm with the original envelope glycoprotein. Airway epithelial cells are targets for gene therapy of respiratory diseases such as cystic fibrosis. The VSV-G-pseudotyped vector, however, is unable to transduce these cells without causing them injury. In other reports, adeno-associated virus vector-mediated gene transfer to the airway was inhibited by a physical barrier at the apical surface (1) and required a high titer (30).
Localization of receptors is an important factor for virus infection. In polarized epithelia, expression of virus receptors on the apical or basolateral membrane was reported for several viruses. In the case of the VSV-G receptor, a distribution or uptake mechanism on differentiated airway cells has not been intensively studied. In contrast, a recombinant SeV has been reported to transduce a reporter gene to the airway epithelial cells efficiently from the apical surface (28). The envelope proteins derived from pneumotropic viruses are expected to break through this barrier and to be usable as potential pseudotypes. Indeed, our data demonstrated that an SeV mutant F- and HN-pseudotyped SIVagm vector transduced rat trachea polarized airway epithelial cells.
In conclusion, we have developed a novel pseudotyped SIV vector that has envelope glycoproteins derived from paramyxovirus and shows wide cell tropism. In addition, this vector was able to transduce polarized rat airway epithelial cells from the apical and basolateral surfaces. SeV glycoproteins-pseudotyped SIVagm vectors are likely to be a powerful tool for gene therapy of various diseases, including respiratory diseases.
Acknowledgments
We are grateful to J. Miyazaki for supplying pCAGGS, N. Miura for supplying anti-HN antibody, H. Taira for supplying anti-F antibody, N. Emi for supplying anti-VSV-G antibody, and M. Inoue for helpful suggestions. We thank to M. Ikegami and S. Komaba for technical assistance.
REFERENCES
- 1.Bals, R., W. Xiao, N. Sang, D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J. Virol. 73:6085-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubei, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celma, C. C. P., J. M. Manrique, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Domains in the simian immunodeficiency virus gp41 cytoplasmic tail required for envelope incorporation into particles. Virology 283:253-261. [DOI] [PubMed] [Google Scholar]
- 4.Condit, R. C. 2001. Principles of virology, p. 19-61. In D. M. Knipe and P. M. Howley (ed.), Fields virology, Lippincott-Williams & Wilkins, Philadelphia, Pa.
- 5.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 6.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freed, E. O., and M. A. Martin. 1995. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J. Biol. Chem. 270:23883-23886. [DOI] [PubMed] [Google Scholar]
- 8.Fukasawa, M., T. Miura, A. Hasegawa, S. Morikawa, H. Tsujimoto, K. Miki, T. Kitamura, and M. Hayami. 1988. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature 333:457-461. [DOI] [PubMed] [Google Scholar]
- 9.Gabuzda, D. H., A. Lever, E. Terwilliger, and J. Sodroski. 1992. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 66:3306-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzinger, N. K., M. I. Bukrinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M.-A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, L. G., J. C. Olsen, L. Naldini, and R. C. Boucher. 2000. Pseudotyped human lentivirus vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 7:568-574. [DOI] [PubMed] [Google Scholar]
- 12.Kafri, T., H. van Praag, L. Ouyang, F. R. Gage, and I. M. Verma. 1999. A packaging cell line for lentivirus vectors. J. Virol 73:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefrancois, L., and D. S. Lyles. 1982. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. Virology 121:157-167. [PubMed] [Google Scholar]
- 14.Li, H.-O., Y.-F. Zhu, M. Asakawa, H. Kuma, T. Hirata, Y. Ueda, Y.-S. Lee, M. Fukumura, A. Iida, A. Kato, Y. Nagai, and M. Hasegawa. 2000. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74:6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Gottlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsui, H., L. G. Johnson, S. H. Randell, and C. Boucher. 1997. Loss of binding and entry of liposome-DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J. Biol. Chem. 272:1117-1126. [DOI] [PubMed] [Google Scholar]
- 17.Miura, N., T. Uchida, and Y. Okada. 1982. HVJ (Sendai virus)-induced envelope fusion and cell fusion are blocked by monoclonal anti-HN protein antibody that does not inhibit hemagglutination activity of HVJ. Exp. Cell Res. 141:409-420. [DOI] [PubMed] [Google Scholar]
- 18.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima, T., K. Nakamaru, E. Ido, K. Terao, M. Hayami, and M. Hasegawa. 2000. Development of novel simian immunodeficiency virus vectors carrying a dual gene expression system. Hum. Gene Ther. 11:1863-1874. [DOI] [PubMed] [Google Scholar]
- 20.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 21.Segawa, H., M. Kato, T. Yamashita, and H. Taira. 1998. The role of individual cysteine residues of Sendai virus fusion protein in intracellular transport. J. Biochem. 123:1064-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent, M. J., L. R. Mersen, A. S. Martin, and R. W. Compans. 1999. Intracellular interaction of simian immunodeficiency virus Gag and Env proteins. J. Virol. 73:8138-8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Schwedler, U., R. S. Kornbluth, and D. Trono. 1994. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc. Natl. Acad. Sci. USA 91:6992-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vzorov, A. N., and R. W. Compans. 1996. Assembly and release of SIV Env proteins with full-length or truncated cytoplasmic domains. Virology 221:22-33. [DOI] [PubMed] [Google Scholar]
- 26.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189:167-177. [DOI] [PubMed] [Google Scholar]
- 27.Yang, Y., E. F. Vanin, M. A. Whitt, M. Fornerod, R. Zwart, R. D. Schneiderman, G. Grosveld, and A. W. Nienhuis. 1995. Inducible, high-level production of infectious murine leukemia retroviral vector particles pseudotyped with vesicular stomatitis virus G envelope protein. Hum. Gene Ther. 6:1203-1213. [DOI] [PubMed] [Google Scholar]
- 28.Yonemitsu, Y., C. Kitson, S. Ferrari, R. Farley, U. Griesenbach, D. Judd, R. Steel, P. Scheid, J. Zhu, P. K. Jeffery, A. Kato, M. K. Hasan, Y. Nagai, I. Masaki, M. Fukumura, M. Hasegawa, D. M. Geddes, and E. W. Alton. 2000. Efficient gene transfer to airway epithelium with recombinant Sendai virus. Nat. Biotechnol. 18:970-973. [DOI] [PubMed] [Google Scholar]
- 29.Yu, X., X. Yuan, Z. Matsuda, T.-H. Lee, and M. Essex. 1992. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66:4966-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 73:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases Env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]