Abstract
Genetic strategies that reduce or block pathogen transmission by mosquitoes are being investigated as a means to augment current control measures. Strategies of vector suppression and replacement are based upon intracellular Wolbachia bacteria, which occur naturally in many insect populations. Maternally inherited Wolbachia have evolved diverse mechanisms to manipulate host insect reproduction and promote infection invasion. One mechanism is cytoplasmic incompatibility (CI) through which Wolbachia promotes infection spread by effectively sterilizing uninfected females. In a prior field test, releases of Wolbachia-infected males were used to suppress a field population of Culex pipiens. An additional strategy would employ Wolbachia as a vehicle to drive desired transgenes into vector populations (population replacement). Wolbachia-based population suppression and population replacement strategies require an ability to generate artificial Wolbachia associations in mosquitoes. Here, we demonstrate a technique for transferring Wolbachia (transfection) in a medically important mosquito species: Aedes albopictus (Asian tiger mosquito). Microinjection was used to transfer embryo cytoplasm from a double-infected Ae. albopictus line into an aposymbiotic line. The resulting mosquito line is single-infected with the wAlbB Wolbachia type. The artificially generated infection type is not known to occur naturally and displays a new CI crossing type and the first known example of bidirectional CI in Aedes mosquitoes. We discuss the results in relation to applied mosquito control strategies and the evolution of Wolbachia infections in Ae. albopictus.
Keywords: Wolbachia, Aedes albopictus, Microinjection
Abbreviation: CI, cytoplasmic incompatibility
1. Introduction
Wolbachia is a genus of obligate, intracellular, maternally inherited bacteria that occur in many insect species (O’Neill et al., 1997a). Cytoplasmic incompatibility (CI) is one of several reproductive manipulations caused by Wolbachia. CI occurs in matings between individuals that differ in their Wolbachia infection type and results in early embryonic death. Although the CI mechanism is unknown, a proposed modification/rescue model serves to explain much of the observed CI phenomena (Charlat et al., 2001; Poinsot et al., 2003; Dobson, 2004). In this model, Wolbachia in the male acts to ‘modify’ the sperm, such that karyogamy failure occurs following fertilization, resulting in embryo death. If the female (and resulting fertilized egg) have the same Wolbachia type as her mate, Wolbachia acts to ‘rescue’ the modification, resulting in normal embryo development. Thus, matings between uninfected females and infected males are incompatible, but the reciprocal cross is compatible (unidirectional CI). Unidirectional CI provides Wolbachia-infected females with a reproductive advantage relative to uninfected females, promoting the spread of maternally inherited Wolbachia into uninfected host populations (Hoffmann et al., 1990). The ability to spread into host populations has led to the proposed use of Wolbachia in population replacement strategies. Specifically, a desired transgene that is linked to Wolbachia could be ‘seeded’ into a mosquito disease vector population. The Wolbachia infection would then serve as a vehicle, driving the linked transgene into the targeted population.
Bidirectional CI can occur when two or more Wolbachia types infect the same host population. An example is provided by the parasitoid wasp Nasonia vitripennis (Perrot-Minnot et al., 1996). Crosses between N. vitripennis strains that are infected with divergent Wolbachia types (A type or B type) result in incompatibility in both cross directions. Theory predicts that bidirectionally incompatible Wolbachia types cannot persist within a panmictic host population (Rousset et al., 1991; Dobson et al., 2002). Bidirectional CI causes a ‘battle’ between the Wolbachia types, resulting in the elimination of infections until only one Wolbachia type predominates. The host population is a victim during this battle, as bidirectional incompatibility sterilizes many matings. The CI-induced suppression of the host population is transient however, lasting only until one Wolbachia infection type dominates the host population (Dobson et al., 2002). Therefore, known examples of bidirectional CI have been either artificially generated or isolated from allopatric populations.
Vector population suppression strategies are based upon artificially prolonging the bidirectional CI battle (Dobson et al., 2002). In a prior field test of the strategy, releases of bidirectionally incompatible males successfully eliminated a Culex mosquito vector population from a village in Burma (Myanmar) (Laven, 1967). However, the availability of naturally occurring bidirectionally incompatible strains that permitted the Culex strategy remains unique. Therefore, the use of the suppression strategy in additional mosquito vector populations requires the ability to artificially generate incompatible strains. Similarly, population replacement strategies also require an ability to generate novel infections. Although the artificial transfer of Wolbachia (transfection) has been successfully accomplished in other insect systems (Boyle et al., 1993; Sasaki et al., 2002; Hartmann et al., 2003; Kang et al., 2003), prior efforts to generate novel infections in mosquitoes have not proven successful (Sinkins and O’Neill, 2000).
Aedes albopictus (Asian tiger mosquito) is a medically important disease vector of multiple arboviruses and filaria (Francy et al., 1990; Moore and Mitchell, 1997; Cancrini et al., 2003). This mosquito is also an important invasive species, frequently spread by human transport (Reiter, 1998). Since its introduction to the United States, Ae. albopictus has spread to become a leading biting nuisance (Moore and Mitchell, 1997). Ae. albopictus individuals are naturally co-infected with two Wolbachia types (wAlbA and wAlbB) (Sinkins et al., 1995; Zhou et al., 1998). This type of co-infection is known as ‘superinfection’ and is commonly observed in insects, representing 34.6% of Wolbachia infections in one survey (Werren and Windsor, 2000). Superinfection results in additive unidirectional CI: superinfected females express both the A and B rescue and are compatible with all males in the population; super-infected males express both the A and B modification and are compatible only with superinfected females (Sinkins et al., 1995).
Although a majority of Ae. albopictus populations are superinfected (Armbruster et al., 2003), laboratory colonies of single-infected (wAlbA) strains have been established from the islands of Koh Samui and Mauritius (Sinkins et al., 1995). Crosses demonstrate that the superinfection is unidirectionally incompatible with the wAlbA infection (Sinkins et al., 1995). Two hypotheses have been proposed for the observation of the single-infected strains. The single-infected populations may represent an ancestral infection type, protected by geographic isolation from replacement with the superinfection (Sinkins et al., 1995; Dutton and Sinkins, 2004). An alternative hypothesis is that the single-infected lines are an experimental artifact and result from loss of the wAlbB infection during colony establishment (Kittayapong et al., 2002a, b).
The ability of wAlbB to induce CI has been speculated based upon crosses of superinfected and wAlbA-infected strains. Crosses of wAlbA-infected females and super-infected males are incompatible, resulting in high embryo mortality. Since the mates in the latter cross differ only by the wAlbB infection present in males, this suggests that the wAlbB infection is capable of inducing CI. However, the prior crosses cannot exclude an interaction between the wAlbA and wAlbB infections within superinfected males.
Here we demonstrate the use of embryonic microinjection to transfer Wolbachia from a naturally superinfected Ae. albopictus strain into an artificially generated aposymbiotic strain. The design was chosen due to concern that prior attempts to transfer Wolbachia in mosquitoes have failed due to an unsupportive host background or maladaptation of the Wolbachia infection to the recipient host (Sinkins and O’Neill, 2000). The results show that transfection efforts have generated an artificial Wolbachia infection type (wAlbB single infection) in Ae. albopictus. Crossing experiments with the artificial infection show a new CI crossing type, providing the first example of bidirectional incompatibility in Aedes. We discuss the results in relation to the evolution of Wolbachia infection in Ae. albopictus and to applied strategies for the control of mosquitoes and mosquito-borne disease.
2. Materials and methods
2.1. Mosquito strains
The Koh Samui strain of Ae. albopictus (Koh; Thailand, pre-1970) is infected with the wAlbA Wolbachia type (Sinkins et al., 1995). The Houston strain (Hou; Texas 1986) is superinfected with both wAlbA and wAlbB Wolbachia types (Sinkins et al., 1995). HT1 and UjuT are uninfected strains that were artificially generated by tetracycline treatment (Otsuka and Takaoka, 1997; Dobson and Rattanadechakul, 2001). Mosquitoes were maintained as previously described (Dobson et al., 2001).
2.2. Microinjection
Embryo injection was based upon techniques successfully used for mosquito transgenesis (Morris, 1997; Coates et al., 1998). Injection needles (Quartz with filament, O.D.: 1.0 mm, I.D.: 0.70 mm) were pulled with a P2000 micropipette puller (Sutter Instrument Co.; Novato, CA). Approximately ten blood-fed females (Hou or HT1) were held in Drosophila vials (Fisher Scientific) containing a wet filter paper funnel. HT1 embryos to be injected (recipient embryos) were collected after allowing females to oviposit for ≤90 min. Following a brief desiccation, gray embryos were aligned on double sided tape (Scotch 665; St. Paul, MN) and covered with halocarbon 700 oil (Sigma-Aldrich Co.). Donor Hou embryos were treated similarly but not desiccated. Cytoplasm was withdrawn from donor Hou embryos and injected into the posterior of recipient HT1 embryos using an IM300 microinjector (Narishige Scientific; Tokyo, Japan) as previously described (Morris, 1997). After injection, the embryos were incubated at 80% relative humidity and 27 °C for approximately 40 min. Embryos were then removed from oil and transferred to wet filter paper. Embryos were allowed to develop for 5 days on wet egg paper. Subsequently, the eggs were hatched (G0) and reared using standard maintenance conditions as above.
2.3. Crosses of transfected lines
To ensure a compatible mating, G0 females were isolated as virgins and mated with HT1 males. Following oviposition, G0 females were assayed for Wolbachia infection using PCR. G0 males were also PCR assayed for Wolbachia infection. G0 females testing negative for Wolbachia infection were discarded along with their progeny. Infected G1 females were sib mated, blood fed, isolated and allowed to oviposit. Following oviposition, G1 females were PCR assayed for Wolbachia infection. G1 females testing negative for Wolbachia infection were discarded along with their progeny. An introgressed line was generated by crossing wAlbB-infected females with UjuT males as previously described (Dobson et al., 2004). To determine CI levels, five virgin females were mated with five virgin males at G3. Mated females were blood fed weekly using mice. Oviposition sites were available constantly to females, and oviposition paper was changed weekly. Hatch rates were scored 3 days after eggs were immersed into water. A majority of Ae. albopictus eggs hatch within a few hours of being submerged in deoxygenated water, Thus, delaying observations beyond 3 days would not affect estimates of egg hatch.
2.4. PCR amplification
Ovaries or testis of adults were dissected and homogenized in 100 ul STE with 0.4 mg/ml proteinase K to extract DNA as previously described (O’Neill et al., 1992). General Wolbachia primers (81F-681R) and primers specific for the wAlbA (328F-691 R) and wAlbB (183F–691R) infections were used as previously described (Zhou et al., 1998).
2.5. Fluorescence in situ hybridization (FISH)
Dissected ovaries and oocytes were fixed for 15 min in freshly prepared 4% formaldehyde in PBS and then washed in PBS with 0.1% Tween 20. Hybridization was conducted following the manufacturers instruction (GeneDetect, Bradenton, FL) with buffer containing 200 ng probes at 37 °C overnight. Two FITC 5′-end labeled 16s rDNA Wolbachia probes (synthesized by Sigma-Genosys Ltd., Haverhill, UK) were used with the sequence as following: [5′-ACCAGATAGACGCCTTCGGCC-3′] (Heddi et al., 1999) and [5′-CTTCTGTGAGTACCGTCATTATC-3′]. Following hybridization, samples were washed at 45 °C and mounted on a glass slide with Vecta shield mounting media (Vector Laboratories; Burlingame, CA). Samples were viewed with Olympus IX70 fluorescence microscope and photographed using Magnafire software (Optronics; Goleta, CA).
3. Results
Cytoplasm from superinfected Ae. albopictus embryos (Hou) were microinjected into uninfected embryos (HT1). In one experiment, ten of 77 embryos (G0) survived microinjection (12% hatch rate). Two of the resulting adults were female. Since males are a dead end host for Wolbachia infection, males were not used to establish lines. Instead, the eight adult males were sacrificed for PCR Wolbachia detection assays (Fig. 1). Three males were PCR positive for both the wAlbA and wAlbB Wolbachia infection; two males were positive for only the wAlbB infection; Wolbachia was not detected in the remaining three males.
Fig. 1.
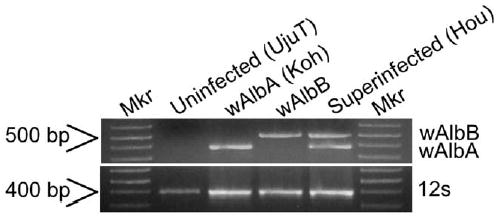
Strain-specific amplification of wAlbA and wAlbB Wolbachia type. Template quality is verified by amplification of mitochondria DNA with 12S primer. Mkr: 1 kb plus molecular weight marker (Invitrogen Life Technologies).
PCR tests of infected G0 females showed one G0 female to be positive for both the wAlbA and wAlbB infection. Wolbachia was not detected in the second G0 female. Twenty-one G1 isofemale lines were established from the infected G0 female. G1 PCR assays demonstrated 11 females to be positive for the wAlbB infection only. One G1 female was positive for the wAlbA infection only. Wolbachia was not detected in the remaining nine G1 females.
Three wAlbB-infected isofemale lines were established. Eggs from the wAlbA-infected G1 female failed to hatch, and thus this line was lost. To determine the stability of Wolbachia infection in the wAlbB-transfected lines, PCR was repeated in subsequent generations. Consistent PCR detection of the infection continued through the generation immediately prior to submission of this article (G8).
To characterize the distribution of Wolbachia in the transfected line, ovaries were dissected from G6 females and examined. Hou and wAlbB oocytes displayed a similar pattern of Wolbachia staining at both embryonic poles, which was absent from uninfected oocytes (Fig. 2). A reduced level of Wolbachia was observed in ovaries of wAlbB females compared to ovaries of superinfected Hou females.
Fig. 2.
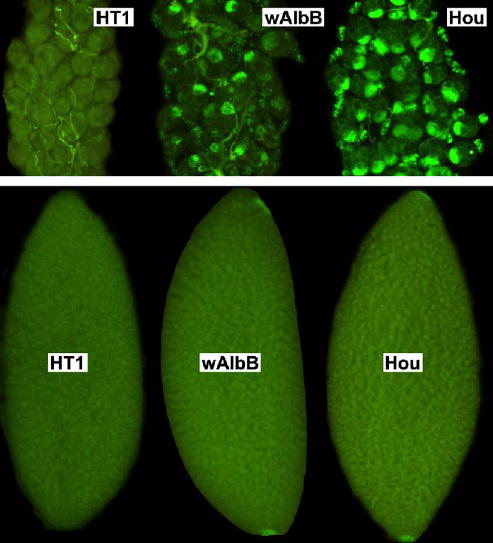
Distribution of Wolbachia in Ae. albopictus ovaries (top) and oocytes (bottom). HT1 is an aposymbiotic (uninfected) strain; wAlbB is the transfected strain; and Hou is the naturally superinfected strain.
Crosses to characterize the CI pattern of the transfected wAlbB strain resulted in a low egg hatch rate in crosses of wAlbB males with either uninfected or wAlbA-infected females (Table 1). Crosses of the wAlbB males with superinfected females are compatible. Crosses of the wAlbB females with uninfected or wAlbB-infected males were compatible, although relatively low egg hatch rate (38.0%) was observed in the latter crosses (Table 1). CI persists over the lifetime of wAlbB females. Egg hatch was observed to remain consistent in egg batches collected from the same females over a 4-week period (Fig. 3A, B). The CI level was re-examined at G7, resulting in similar results as G3. Greater than 86% hatch resulted in crosses of wAlbB males with superinfected Hou females. No egg hatch was observed in crosses of wAlbB males and uninfected HT1 females.
Table 1.
Crosses of the transfected wAlbB line (G3)
| Expected CI type | Crossa | Percent egg hatchb | Number of eggs/ovipositionb | Oviposition number |
|---|---|---|---|---|
| Bidirectional CI | wAlbB × Koh | 3.6±3.8 | 155±55 | 13 |
| Koh × wAlbB | 2.4±4.4 | 162±64 | 15 | |
| Unidirectional CI | HT1 × wAlbB | 0.0±0.0 | 170±45 | 14 |
| wAlbB × Hou | 3.0±2.1 | 150±52 | 6 | |
| Compatible | Hou × wAlbB | 73.8±12.1 | 192±60 | 15 |
| wAlbB × wAlbB | 38.0±21.3 | 139±48 | 6 | |
| HT1 × HT1 | 90.9±2.7 | 166±139 | 3 | |
| Koh × Koh | 88.0±2.8 | 177±85 | 4 | |
| Hou × Hou | 82.5±2.2 | 158±57 | 4 |
Female × male; HT1 is an aposymbiotic (uninfected) strain; wAlbB is the transfected strain; Koh is a wAlbA-infected strain; and Hou is the naturally superinfected strain.
Average ± standard deviation.
Fig. 3.
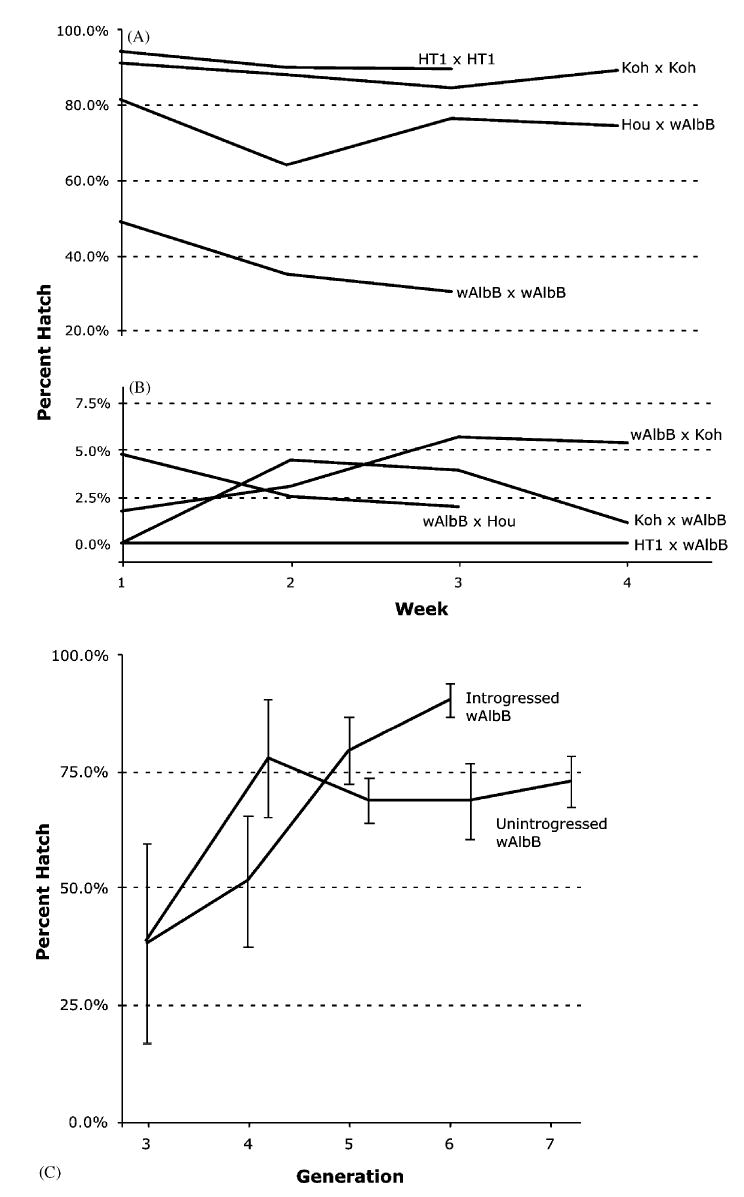
Egg hatch rate of (A) compatible G3 crosses, (B) incompatible G3 crosses, and (C) unintrogressed and introgressed wAlbB lines. Egg hatch was measured either weekly (A, B) or once per generation (C). Bars show standard deviation. Crosses are female × male. HT1 is an aposymbiotic (uninfected) strain; wAlbB is the transfected strain; Koh is a wAlbA infected strain; and Hou is the naturally superinfected strain.
To reduce potential inbreeding effects, one wAlbB line was introgressed with UjuT for three generations. As shown in Fig. 3C, the hatch rate in the introgressed strain increased to greater than 93%. The hatch rate observed in the unintrogressed wAlbB line also increased (72.8% in G7; Fig. 3C). Introgression did not affect CI. No egg hatch was observed in crosses of introgressed wAlbB males and uninfected HT1 females.
Maternal inheritance rate was examined in the wAlbB line by screening 20 G6 females using the PCR assay. Wolbachia infection was observed in all of the tested females. To examine for paternal transmission, rare progeny from incompatible crosses were reared to adult and then PCR assayed for infection type. Super-infection was not detected in the three progeny resulting from wAlbB × wAlbA and two progeny from wAl-bA × wAlbB (female × male). The infection type in each of the progeny was consistent with expectations for maternal inheritance only (i.e., progeny infection type was the same as the maternal type).
4. Discussion
Wolbachia in Ae. albopictus is known to represent a true superinfection (i.e., co-infection with two Wolbachia types) and not multiple copies of diagnostic genetic loci in a single Wolbachia type (Sinkins et al., 1995), based upon observations of the wAlbA single infection in mosquito lines and the wAlbB single infection in vitro (O’Neill et al., 1997b). However, the wAlbB single infection has not been observed naturally. Surveys show that >99.4% of natural Ae. albopictus populations are superinfected (Kittayapong et al., 2002a, b). Furthermore, prior efforts to segregate the wAlbA and wAlbB infections using antibiotics were unsuccessful (Dobson and Rattanadechakul, 2001).
Based upon the genetic divergence of the wAlbA and wAlbB infections and prior crossing experiments, bidirectional incompatibility has been predicted for crosses between individuals single-infected with wAlbA and wAlbB (Sinkins et al., 1995; Dobson et al., 2004). Here, crosses of the transfected wAlbB line were used to directly test predictions. Consistent with expectations for differing modification and rescue mechanisms, less than 4% egg hatch rate resulted in crosses between wAlbB males with either wAlbA or uninfected females (Table 1). Crossing results demonstrate that the wAlbB infection is capable of inducing and rescuing the CI modification independent of the wAlbA infection. Crosses of wAlbB females with either wAlbA or superinfected males demonstrate that the wAlbB infection is unable to rescue the wAlbA modification. Although prior characterization of Wolbachia infections in other insects shows that CI levels can be affected by host age (Singh et al., 1976; Reynolds et al., 2003), the wAlbB infection in females is able to rescue modified sperm until female death (Fig. 3).
Despite the observation that ovaries from the wAlbB line appeared to have lower infection levels relative to Hou ovaries (Fig. 2), the wAlbB infection was observed to be stably maintained in the transfected lines. PCR assays at G6 suggest maternal inheritance in excess of 95%, consistent with prior characterization of naturally infected lines (Kittayapong et al., 2002a, b). Wolbachia specific staining showed a similar infection level and Wolbachia distribution in wAlbB and Hou oocytes (Fig. 2).
Paternal transmission of Wolbachia infection provides a potential route for the evolution of superinfections. With paternal and maternal transmission, survivors of crosses between mates with different Wolbachia types would result in superinfected progeny. To examine for paternal transmission, the rare offspring from incompatible crosses between wAlbA and wAlbB strains were PCR tested. In each case, the Wolbachia infection was identical to the maternal infection type. Although this result is inconsistent with the hypothesized role of paternal transmission in superinfection evolution, we have examined few offspring and can only exclude high rates of paternal transmission. Future efforts should include repeating this experiment on a larger scale. In addition to the evolutionary significance, the results will also be important to proposed applied strategies. Paternal transmission resulting in superinfections would complicate strategies attempting to use Wolbachia to suppress insect populations, since super-infected field individuals would be compatible with released males.
Low hatch rate (38.0%) was observed in compatible crosses of wAlbB individuals. Hypotheses to explain this observation include inbreeding effects associated with the establishment of isofemale lines (i.e., increased homozygosity of deleterious loci) and high mortality associated with the artificially generated single wAlbB infection type. The observed increase in egg hatch with introgression (Fig. 3C) is consistent with predictions for an inbreeding effect. An increase in egg hatch was also observed in subsequent generations of a non-introgressed wAlbB line, reaching a plateau at approximately 70% (Fig. 3C). Thus, the wAlbB single infection does not appear to be associated with increased mortality.
Although superinfection was detected in G0 individuals surviving microinjection, only single infections were observed in G1. The presence of superinfection in G0 but not thereafter suggests that maternal transmission between G0 and G1 represents a bottleneck for transfected Wolbachia and may result from artificially low infection levels in microinjected embryos or somatic G0 infections that are not maternally inherited. Super-infection segregation following microinjection transfection has also been reported in Drosophila (Poinsot and Mercot, 2001; Riegler et al., 2004). It is useful to note that subsequent to G1, maternal transmission loss was not observed. This is similar to prior transfection experiments in Drosophila simulans (Xi and Dobson, 2005) and suggests that future transfection studies may be simplified by focusing PCR screening on G0 and G1 females.
Segregation of the superinfection in the transfection experiment was biased toward wAlbB infection. Only one wAlbA line was observed in the G1 lines, relative to 11 wAlbB G1 lines. This is similar to prior research generating a Wolbachia-infected cell line from super-infected Ae. albopictus, which resulted in an in vitro wAlbB single infection (O’Neill et al., 1997b). The observed wAlbB bias may reflect higher wAlbB infection levels relative to wAlbA in superinfected females (Dutton and Sinkins, 2004). Given the previously described wAlbB-bias in superinfected Ae. albopictus, it is somewhat surprising that a wAlbA-infected G1 line was observed. Unfortunately, the possibility that this line represented a PCR artifact or somatic infection could not be tested since the female failed to produce hatching G2 eggs and the line was lost. Egg hatch failure of the wAlbA line may have resulted from the experimental protocol. G1 progeny from the infected isofemale line were sib mated. Given that a majority of PCR tested G1 individuals were wAlbB infected, it is likely that the mate of the wAlbA female would be incompatible. This provides rationale for modifying the protocol presented here for future transfection experiments, such that virgin G1 females are mated with uninfected males.
Here, we have demonstrated a technique for Wolbachia transfection in Ae. albopictus. An ability to generate artificial Wolbachia infections and new CI crossing types represents an important advance toward implementation of proposed Wolbachia-based strategies for suppression and replacement of medically important mosquito vector populations. While the experiments described here demonstrate a successful transfection protocol, the artificial wAlbB infection will not be useful for the suppression or replacement of superinfected Ae. albopictus field populations. Releases of wAlbB males would not be incompatible with superinfected females of field populations and therefore would not result in CI or suppression. Similarly, wAlbB-infected females would not be useful for population replacement strategies. Released wAlbB females would be incompatible with superinfected field males, and thus the wAlbB single infection in females would be quickly eliminated. Therefore, future experiments should repeat the transfection protocol described here with the variation of using donor tissue infected with different Wolbachia types that do not naturally occur in Ae. albopictus. For suppression strategies, injection of aposymbiotic Ae. albopictus could be used to generate strains that are bidirectionally incompatible with the superinfected field population. Injection of superinfected Ae. albopictus could be used to generate a triple-infected Ae. albopictus strain that is unidirectionally incompatible with super-infected field population. The latter would be similar to prior transfection experiments with Drosophila (Rousset et al., 1999). To reduce complications associated with generation of artificial associations, closely related Wolbachia-infected Aedes mosquitoes may be initially selected as donors (Sherron and Rai, 1983; Meek and Macdonald, 1984; Dean and Dobson, 2004). However, prior transfers between divergent hosts have been successful, including the transfer of Wolbachia from Ae. albopictus to Drosophila simulans (Braig et al., 1994). Additional experiments could repeat the transfection protocol reported here, but using Ae. aegypti (Yellow fever mosquito) as the recipient. Ae. aegypti populations are naturally uninfected. If successful, the latter would generate a strain useful for population replacement strategies with Ae. aegypti populations, which are widely recognized to be important vectors of dengue, yellow fever, filaria and additional medically important pathogens.
Acknowledgments
We thank Craig Coates and his laboratory for their assistance with the embryo injection technique and Lok-Sze Ng for assistance with injection and screening. This work was supported by NIH grant (NIH-AI-51533) and a Dissertation Enhancement Award from the Graduate School of University of Kentucky. This is publication 04-08-174 of the University of Kentucky Agricultural Experiment Station.
References
- Armbruster P, Damsky WE, Jr, Giordano R, Birungi J, Munstermann LE, Conn JE. Infection of New- and Old-World Aedes albopictus (Diptera: Culicidae) by the intracellular parasite Wolbachia: implications for host mitochondrial DNA evolution. J Med Entomol. 2003;40:356–360. doi: 10.1603/0022-2585-40.3.356. [DOI] [PubMed] [Google Scholar]
- Boyle L, O’Neill SL, Robertson HM, Karr TL. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- Braig HR, Guzman H, Tesh RB, O’Neill SL. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature. 1994;367:453–455. doi: 10.1038/367453a0. [DOI] [PubMed] [Google Scholar]
- Cancrini G, Romi R, Gabrielli S, Toma L, Di-Paolo M, Scaramozzino P. First finding of Dirofilaria repens in a natural population of Aedes albopictus. Med Vet Entomol. 2003;17:448–451. doi: 10.1111/j.1365-2915.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Charlat S, Calmet C, Mercot H. On the mod resc model and the evolution of Wolbachia compatibility types. Genetics. 2001;159:1415–1422. doi: 10.1093/genetics/159.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JL, Dobson SL. Characterization of Wolbachia infections and interspecific crosses of Aedes (Stegomyia) polynesiensis and Ae. (Stegomyia) riversi (Diptera: Culicidae) J Med Entomol. 2004;41:894–900. doi: 10.1603/0022-2585-41.5.894. [DOI] [PubMed] [Google Scholar]
- Dobson SL. Evolution of Wolbachia cytoplasmic incompatibility types. Evolution. 2004;58:2156–2166. doi: 10.1111/j.0014-3820.2004.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Dobson SL, Rattanadechakul W. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2001;38:844–849. doi: 10.1603/0022-2585-38.6.844. [DOI] [PubMed] [Google Scholar]
- Dobson SL, Marsland EJ, Rattanadechakul W. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2001;38:382–387. doi: 10.1603/0022-2585-38.3.382. [DOI] [PubMed] [Google Scholar]
- Dobson SL, Fox CW, Jiggins FM. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc R Soc London B Biol Sci. 2002;269:437–445. doi: 10.1098/rspb.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL, Rattanadechakul W, Marsland EJ. Fitness advantage and cytoplasmic incompatibility in Wolbachia single-and superinfected Aedes albopictus. Heredity. 2004;93:135–142. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- Dutton TJ, Sinkins SP. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol. 2004;13:317–322. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Francy DB, Karabatsos N, Wesson DM, Moore CG, Jr, Lazuick JS, Niebylski ML, Tsai TF, Craig GB., Jr A new arbovirus from Aedes albopictus, an Asian mosquito established in the United States. Science. 1990;250:1738–1740. doi: 10.1126/science.2270489. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Stuckas H, Lucius R, Bleiss W, Theuring F, Kalinna BH. Trans-species transfer of Wolbachia: micro-injection of Wolbachia from Litomosoides sigmodontis into Acanthocheilonema viteae. Parasitology. 2003;126:503–511. [PubMed] [Google Scholar]
- Heddi A, Grenier AM, Khatchadourian C, Charles H, Nardon P. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci USA. 1999;96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Turelli M, Harshman LG. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics. 1990;126:933–948. doi: 10.1093/genetics/126.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Ma X, Cai L, Liao S, Sun L, Zhu H, Chen X, Shen D, Zhao S, Li C. Superinfection of Laodelphax striatellus with Wolbachia from Drosophila simulans. Heredity. 2003;90:71–76. doi: 10.1038/sj.hdy.6800180. [DOI] [PubMed] [Google Scholar]
- Kittayapong P, Baimai V, O’Neill SL. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am J Trop Med Hyg. 2002a;66:108–111. doi: 10.4269/ajtmh.2002.66.108. [DOI] [PubMed] [Google Scholar]
- Kittayapong P, Baisley KJ, Sharpe RG, Baimai V, O’Neill SL. Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. Am J Trop Med Hyg. 2002b;66:103–107. doi: 10.4269/ajtmh.2002.66.103. [DOI] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Meek SR, Macdonald WW. Crossing relationships among seven members of the group of Aedes scutellaris (Walker) (Diptera: Culicidae) Bull Entomol Res. 1984;74:65–78. [Google Scholar]
- Moore CG, Mitchell CJ. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg Infect Dis. 1997;3:329–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, A.C., 1997. Microinjection of mosquito embryos. In: Crampton, J.M., Beard, C.B., Louis, C. (Eds.), Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman & Hall, 2-6 Boundary Row, London SE1 8HN, UK, pp. 423–429.
- O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill, S.L., Hoffmann, A.A., Werren, J.H., 1997a. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press, Oxford.
- O’Neill SL, Pettigrew MM, Sinkins SP, Braig HR, Andreadis TG, Tesh RB. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol. 1997b;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Takaoka H. Elimination of Wolbachia pipientis from Aedes albopictus. Med Entomol Zool. 1997;48:257–260. [Google Scholar]
- Perrot-Minnot MJ, Guo LR, Werren JH. Single and double infections with Wolbachia in the parasitic wasp Nasonia vitripennis: effects on compatibility. Genetics. 1996;143:961–972. doi: 10.1093/genetics/143.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsot D, Mercot H. Wolbachia injection from usual to naive host in Drosophila simulans(Diptera: Drosophilidae) Eur J Entomol. 2001;98:25–30. [Google Scholar]
- Poinsot D, Charlat S, Mercot H. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: confronting the models with the facts. Bioessays. 2003;25:259–265. doi: 10.1002/bies.10234. [DOI] [PubMed] [Google Scholar]
- Reiter P. Aedes albopictus and the world trade in used tires, 1988–1995: the shape of things to come? J Am Mosq Control Assoc. 1998;14:83–94. [PubMed] [Google Scholar]
- Reynolds KT, Thomson LJ, Hoffmann AA. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics. 2003;164:1027–1034. doi: 10.1093/genetics/164.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler M, Charlat S, Stauffer C, Mercot H. Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: investigating the outcomes of host–symbiont coevolution. Appl Environ Microbiol. 2004;70:273–279. doi: 10.1128/AEM.70.1.273-279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F, Raymond M, Kjellberg F. Cytoplasmic incompatibilities in the mosquito culex pipiens: how to explain a cytotype polymorphism? J Evol Biol. 1991;4:69–81. [Google Scholar]
- Rousset F, Braig HR, O’Neill SL. A stable triple Wolbachia infection in Drosophila with nearly additive incompatibility effects. Heredity. 1999;82:620–627. doi: 10.1046/j.1365-2540.1999.00501.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kubo T, Ishikawa H. Interspecific transfer of Wolbachia between two lepidopteran insects expressing cytoplasmic incompatibility: a Wolbachia variant naturally infecting Cadra cautella causes male killing in Ephestia kuehniella. Genetics. 2002;162:1313–1319. doi: 10.1093/genetics/162.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherron DA, Rai KS. Genetics of speciation in the Aedes (Stegomyia) scutellaris group (Diptera: Culicidae) J Med Entomol. 1983;20:520. doi: 10.1093/jmedent/21.5.540. [DOI] [PubMed] [Google Scholar]
- Singh KR, Curtis CF, Krishnamurthy BS. Partial loss of cytoplasmic incompatibility with age in males of Culex fatigans. Ann Trop Med Parasitol. 1976;70:463–466. doi: 10.1080/00034983.1976.11687148. [DOI] [PubMed] [Google Scholar]
- Sinkins, S.P., O’Neill, S.L., 2000. Wolbachia as a vehicle to modify insect populations. In: James, A.M.H.A.A. (Ed.), Insect Transgenesis: Methods and Applications. CRC Press, Boca Raton, FL, pp. 271–287.
- Sinkins SP, Braig HR, O’Neill SL. Wolbachia super-infections and the expression of cytoplasmic incompatibility. Proc R Soc London B Biol Sci. 1995;261:325–330. doi: 10.1098/rspb.1995.0154. [DOI] [PubMed] [Google Scholar]
- Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc London B Biol Sci. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Dobson SL. Characterization of Wolbachia transfection efficiency using microinjection of embryonic cytoplasm and embryo homogenate. Appl Environ Microbiol. 2005;71(6) doi: 10.1128/AEM.71.6.3199-3204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O’Neil S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc London B Biol Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]


