Abstract
The coxsackie B virus and adenovirus receptor (CAR) is a member of the immunoglobulin superfamily. In addition to activity as a viral receptor, it may play a role in cellular adhesion. We asked what determines the cell membrane microdomain of CAR. We found that CAR is localized to a novel lipid-rich microdomain similar to that of the low-density lipoprotein receptor (LDLR) but distinct from that of a CAR variant that exhibited traditional lipid raft localization via fusion to a glycosylphosphatidylinositol (GPI) tail. The cytoplasmic tail determines its membrane localization, since deletion of this domain resulted in mislocalization. Results indicate that CAR, CAR-LDLR, and LDLR reside in a novel lipid raft that is distinct from caveolin-1-containing caveolae and GPI-linked proteins. Residence in a lipid-rich domain provides a mechanism that allows CAR to interact with other cell adhesion proteins and yet function as an adenovirus receptor.
The coxsackie B virus and adenovirus receptor (CAR) is the high-affinity receptor for the adenovirus (Ad) fiber protein (4). This receptor is expressed at variable levels on different tissues at distinct developmental stages and is primarily responsible for susceptibility to Ad infection (5, 40, 51). Although it is clear that this protein plays a role in viral infection, the endogenous function of CAR is still largely unknown. CAR is a member of the immunoglobulin (Ig) superfamily and has two extracellular Ig-like domains, a single-pass transmembrane (TM) domain, and a cytoplasmic domain. The most distal extracellular Ig loop (D1 domain) of CAR facilitates a homophilic interaction that allows aggregation of cells expressing CAR (10, 18, 47). Additionally, CAR localizes to the region of tight and adherens junctions in polarized epithelia, further suggesting a role in cell adhesion (10, 51).
The fiber knob of Ad interacts with the D1 domain of CAR with high affinity and may compete with the lower-affinity homophilic CAR interaction (6, 38, 47). This extracellular interaction may be sufficient to initiate the endocytosis of Ad, since the intracellular carboxy terminus of CAR is not essential for Ad infection (49, 54).
Two of the predominant cellular mechanisms for internalizing proteins are clathrin-coated pits (CCP) and caveolae. CCP are the membrane structures responsible for the majority of receptor-mediated endocytosis events (7). Caveolae are specialized lipid-rich rafts that control elements of cellular signal transduction, cholesterol transport, and transcytosis (12, 29). Despite functional differences, it has been difficult to distinguish between the routes of endocytosis mediated by these structures due to some redundancies in cellular machinery (17, 31).
It is currently accepted that Ad enters a host cell via CCP (14, 15, 26). However, Ad infection in cells expressing CAR modified with a glycosylphosphatidylinositol (GPI) linkage is indistinguishable from that in cells expressing wild-type (wt) CAR. Because GPI-linked proteins are known to enter the cell by non-clathrin-mediated pathways (8, 28), it is possible that Ad can also efficiently enter a cell by alternative pathways. Thus, CAR possibly plays a role only in fiber binding without being involved in endocytosis. If this were the case, how would all forms of CAR be able to interact efficiently with the appropriate coreceptor(s) required for infection? Alternatively, CAR-mediated endocytosis may occur by several different mechanisms, each leading to infection. To further understand the innate function of CAR and its role in viral infection, it is important to identify its specific cell membrane target or residence. Thus, we asked where CAR is localized and what determines its membrane localization.
MATERIALS AND METHODS
Materials.
Chemicals, including methyl-β-cyclodextrin (C4555), chlorpromazine (C8138), and FLAG antibody (Ab) (F3165), were purchased from Sigma (St. Louis, Mo.). Alexa 488-labeled transferrin (T-13342) and cholera toxin subunit B (C-22841) were from Molecular Probes (Eugene, Oreg.). Antibodies included caveolin-1 (610406), clathrin (610499), and flotillin (610820) from Becton Dickinson Transduction Laboratories (Lexington, Ky.), transferrin receptor (TR) (A11130) and goat anti-mouse Alexa 568 (A11019) from Molecular Probes, RmcB (CRL-2379; American Type Culture Collection, Manassas, Va.), and low-density lipoprotein receptor (LDLR) (PAB61099P; Maine Biotechnology Services, Inc., Portland, Maine). COS-7 cells were from the American Type Culture Collection and were maintained under standard culture conditions (Dulbecco's minimal essential medium with 10% fetal calf serum, penicillin, and streptomycin). Ad serotype 5 containing the β-galactosidase (Ad-β-Gal), enhanced green fluorescent protein (eGFP), red fluorescent protein (RFP) (DSRed1; Clontech, Palo Alto, Calif.), CAR, GPI-CAR, or Tailless-CAR gene has been described previously (51). All viruses were produced by the University of Iowa Gene Transfer Vector Core. Cells were virally transfected by calcium phosphate coprecipitation as previously described (11, 50).
TEM.
COS cells were seeded onto Millicell polycarbonate filters (Millipore Corp., Bedford, Mass.) at 5 × 104 cells per well. Cells were either transfected with Ad-wt CAR at 18 h or left in culture for a total of 2 days. Cells were then placed on ice for 10 min followed by 1 h of incubation with 5 × 109 particles of Ad. Cells were transferred to 37°C for either 5 or 20 min before removal of medium, gentle washing with phosphate-buffered saline, and submergence in transmission electron microscopy (TEM) fixative (2.5% glutaraldehyde in 0.1 M cacodylate buffer). Samples were postfixed in 1% osmium tetroxide followed by 2.5% aqueous uranyl acetate and then dehydrated in a graded series of ethanol washes. The samples were then transitioned to Eponate-12 resin (Ted Pella Inc., Redding, Calif.) and cured overnight at 65°C. Ultrathin sections were poststained with uranyl acetate and lead citrate and imaged in a Hitachi H-7000 TEM (Pleasanton, Calif.).
Effect of inhibition of endocytosis on Ad infection.
Cells were seeded at 2 × 104 cells per well in 24-well dishes. Cells were pretreated with 10 μM methyl-β-cyclodextrin or 100 μM chlorpromazine in Eagle's minimal essential medium for 20 min at 37°C. Ad-β-Gal was mixed with cyclodextrin, chlorpromazine, or Eagle's minimal essential medium alone immediately prior to infection at a multiplicity of infection (MOI) of 5. Cells were incubated with virus for 30 min at 37°C, washed twice with medium, and incubated overnight at 37°C. Total β-Gal activity was measured using a commercially available method (Galacto-Light; Tropix, Inc., Bedford, Mass.) as previously described (50).
Colocalization of CAR with a marker of lipid rafts.
COS cells grown on collagen-coated chamber slides were infected with Ad-CAR (MOI of 10) for 1 h at 37°C. Patching was performed at 48 h postinfection by incubating the cells for 20 min on ice and for 20 min at 12°C in the presence of FLAG Ab, followed by three washes and a second incubation for 20 min at 12°C in the presence of anti-mouse A568. Cells were washed, fixed with 4% formaldehyde, incubated with A488-cholera toxin (0.2 μg/ml) for 1 h at room temperature, washed, and coverslipped with Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, Calif.). Images were acquired with a Bio-Rad MRC-1024 laser scanning confocal microscope (Hercules, Calif.) mounted on a Nikon E600 microscope (Melville, N.Y.) using a 60× oil immersion lens.
Colocalization of Ad with markers of endocytosis.
COS cells grown on collagen-coated chamber slides were incubated with A488-transferrin (5 μg/ml) or A488-cholera toxin (0.2 μg/ml) and Cy3-labeled Ad (MOI of 200) for 15 min at 37°C before being washed, fixed, coverslipped, and imaged as above.
Cell lysis and sucrose gradients.
COS-7 cells were transfected with plasmids or Ad containing CAR, CAR-LDLR, GPI-CAR, Tailless-CAR, human LDLR, or eGFP as a control. Well-differentiated primary human airway epithelia were grown and infected with Ad-CAR as previously described (20, 51). At 3 days after transfection, approximately 7 × 106 cells per condition were lysed with 2 ml of 500 mM Na2CO3 (pH 11) supplemented with protease inhibitors (leupeptin, pepstatin, and aprotinin), scraped, and Dounce homogenized. Homogenate was mixed with 90% sucrose in MBS (25 mM MES [morpholinoethanesulfonic acid], 150 mM NaCl, pH 6.5) and placed at the bottom of an ultracentrifuge tube. Volumes of 4 ml each of 30% and 5% sucrose in MBS and 250 mM Na2CO3 were layered on top of the homogenate and spun at 39,000 rpm in a Beckman L8-70 ultracentrifuge with a SW40 Ti rotor for 60 h at 6°C. Fractions (1 ml each) were collected from the top of the ultracentrifuge tube by a fraction collector (Tube Piercer Model 184; ISCO, Lincoln, Nebr.). Triton lysis was performed by adding 2 ml of 1% Triton X-100 (10 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, protease inhibitors, phenylmethylsulfonyl fluoride [TNE buffer]) and allowing gentle rocking at 4°C for 20 min. Cells were scraped and sonicated 5 times. Sucrose gradients were prepared as described above, except that the sucrose solutions were made in TNE buffer and the ultracentrifugation time was reduced to 20 h.
Cloning of CAR-LDLR and GFP fusion proteins.
The CAR extracellular domain was connected with the LDLR TM and intracellular domains via fusion PCR (95°C for 5 min; 30 rounds of 94°C for 30 s, 58°C for 45 s, and 72°C for 45 s; and 72°C for 5 min). Briefly, the extracellular portion of CAR was cloned with primers to the 5′ end of CAR (CAR5′: 5′TGGAATTCCCAGGAGCGAGAG) and a primer that contained a 6-amino-acid sequence 5′ to the beginning of the putative TM domain of CAR and a 6-amino-acid sequence of the putative LDLR TM domain (CAR-LDLR: 5′GAGGACAATGGACAGAGCTTTATTTGAAGGAGGGAC). The CAR-LDLR primer, in the reverse direction, along with a primer flanking the 3′ end of the LDLR (LDLR3′: 5′GCTCTAGATCACGCCACGTCATCCTCCAG), was used to clone the LDLR TM and cytoplasmic domains. The fragments were fused by PCR with the CAR5′ and LDLR3′ primers, digested with EcoRI and XbaI, and cloned into pcDNA3.1(+). Sequencing and Western blotting confirmed the presence of the expected fusion protein. wt CAR and CAR-LDLR were subcloned into the vector pEGFP-C1 (Clontech) by standard methods, and the results were confirmed by sequencing.
Immunofluorescence.
COS cells grown in 4-well chamber slides were infected with Ad containing the various CAR constructs. Cells were fixed, permeabilized where indicated with 0.1% Triton X-100, incubated with primary FLAG monoclonal Ab and secondary goat anti-mouse Alexa 568, coverslipped, and imaged as described above.
CAR-complemented Ad infection.
CHO cells were plated in 24-well dishes (5 × 104 cells/well) and transfected the next day using Lipofectamine 2000 (Invitrogen, Carlsbad, Calif.). Transfected cells were infected the following day with Ad-eGFP (MOI of 5) or Ad-RFP at the MOI specified in the text. Cells were analyzed 36 to 48 h later by fluorescence-activated cell sorter (FACS) (Lysis II software; Becton Dickinson, San Jose, Calif.) after labeling of CAR with FLAG monoclonal Ab followed by goat anti-mouse Alexa 568 or directly with C-terminal CAR-GFP or CAR-LDLR-GFP fusions (pEGFP-C1 vector; Clontech). The fluorescence intensity was recorded from 10,000 events (n = 3 to 6).
RESULTS
TEM.
TEM was performed on COS-7 cells to visualize the compartmentalization of bound Ad, a surrogate marker for the localization of endogenously expressed CAR (Fig. 1). The Ad was allowed to bind to cells on ice and was then warmed to 37°C for either 5 or 20 min before fixation. At 5 min, some of the Ad bound to the cell membrane was associated with structures that resembled CCP or caveolae (Fig. 1A and B). Interestingly, virus was also present in areas of the cell membrane that lacked invaginations or clathrin coats, suggesting that the receptor for Ad is localized to areas of the membrane that are distinct from CCP or caveolae. Virus was not observed in closed or internalized vesicles at 5 min. In contrast, after 20 min at 37°C (Fig. 1C to G), virus was present in both non-clathrin- (Fig. 1C and E) and clathrin-coated vesicles (Fig. 1F) as well as free in the cytoplasm (data not shown). Virus could also be observed at the cell surface in non-clathrin- (Fig. 1D) and clathrin-coated (Fig. 1E and G) invaginations. At that time, viruses bound to other undefined regions of the membrane were often observed in clusters of two or more (Fig. 1C and F). The appearance of Ad in various types of endocytic structures suggests that its endocytosis can occur by both clathrin- and non-clathrin-mediated mechanisms. Despite the limitations inherent to TEM, including sectioning artifacts and the rapid removal of clathrin coats upon endocytosis, these data agree with previous TEM studies of HeLa cells that revealed the presence of bound and internalized Ad in both clathrin- and non-clathrin-coated vesicles (32).
FIG. 1.
TEM of Ad bound to COS cells. Ad was allowed to bind to COS cells for 1 h on ice followed by 5 min (panels A and B) or 20 min (panels C to G) of incubation at 37°C. Cells were then fixed and processed for TEM. Ad entering via CCP as well as caveolae can be observed. Arrows identify Ad associated with clathrin-containing structures, while arrowheads indicate nonclathrin structures. Size bar, 100 nm.
Ad entry by both clathrin- and non-clathrin-mediated endocytosis.
Endocytosis by caveolae or CCP may be inhibited by several methods, including chemical disruption. Chlorpromazine is a cationic, amphiphilic molecule that acts to shift clathrin and the AP-2 complex to the late endosomal compartment, thus inhibiting its ability to mediate CCP endocytosis (53). Cyclodextrin binds cholesterol, depleting it from the cell surface and disrupting endocytosis via cholesterol-sensitive caveolae and lipid rafts (2, 36). It may also concurrently reduce CCP endocytosis (44). Dose response studies revealed that at a concentration of 100 μM chlorpromazine and 10 mM cyclodextrin, a maximal effect was achieved without toxicity (data not shown). Treatment of cells with either chlorpromazine or cyclodextrin reduced Ad infection by approximately 40% (Fig. 2A). Pretreatment (−24 h) or posttreatment (+6 h) of cells had no effect on Ad-mediated gene transfer (data not shown). Treatment with both drugs combined resulted in significant cell toxicity and was not evaluated. These data suggest that Ad can enter and mediate gene expression in the cell by both clathrin- and non-clathrin-mediated events and that the disruption of lipid rafts only partially affects Ad infection. The magnitude of inhibition observed here was in a range similar to that of the inhibition of other endocytic mediators, including dynamin (52) and rab5 GTPase (33).
FIG. 2.
(A) Percent Ad-β-Gal infection in COS cells treated with 10 mM cyclodextrin (CD) or 100 μM chlorpromazine (CH) or left untreated (Ad Control). *, P < 0.01, n = 3 to 4 experiments. Confocal microscopy of endocytic events after coincubation of A488-transferrin (green) and Cy3-Ad (red) (B) or A488-cholera toxin (green) and Cy3-Ad (red) (C). Regions of green (marker alone), red (Ad alone), and yellow (both marker and Ad) were visible after treatment with either transferrin or cholera toxin. The lower insets in panels B and C show an XZ projection of the Ad particles associated with the cell. (D) Confocal image of wt CAR (red) patched at 12°C with FLAG Ab and anti-mouse A568 followed by fixation and staining with A488-cholera toxin as a marker for the cholera toxin receptor GM1 (green).
A distinction between the paths of endocytosis for Ad may also be observed by incubating cells with fluorescently tagged fluid-phase markers. Transferrin is a serum glycoprotein that enters a cell by CCP after binding to the TR and is a marker of early endosomes and lysosomes (13). Cholera toxin subunit B, which binds to the lipid raft protein ganglioside GM1, marks intracellular compartments formed by non-clathrin-mediated endocytosis (27). Cells were concurrently incubated with either A488-transferrin or A488-cholera toxin and infected with Cy3-labeled Ad (Fig. 2B and C). Colocalization by confocal microscopy showed distinct vesicular populations that were green (transferrin or cholera toxin alone), red (Ad alone), or yellow (containing both Ad and the fluid-phase marker). XZ projections of confocal images (shown in insets in Fig. 2B and C) show the large number of Ad particles associated with each cell. Colocalization with the respective markers was observed in individual confocal images.
Taken together, these data suggest that Ad entry is not limited to a single pathway. Thus, we asked where CAR, the primary Ad receptor, was localized such that many different endocytic processes might occur.
To evaluate the possibility that CAR resides in a lipid raft, we used a copatching technique to evaluate colocalization between CAR and GM1, a known lipid raft protein, as determined by the surrogate marker cholera toxin (16, 39). CAR and GM1 showed significant colocalization, suggesting an association with a lipid-rich microdomain (Fig. 2D).
CAR fractionates with markers of lipid rafts.
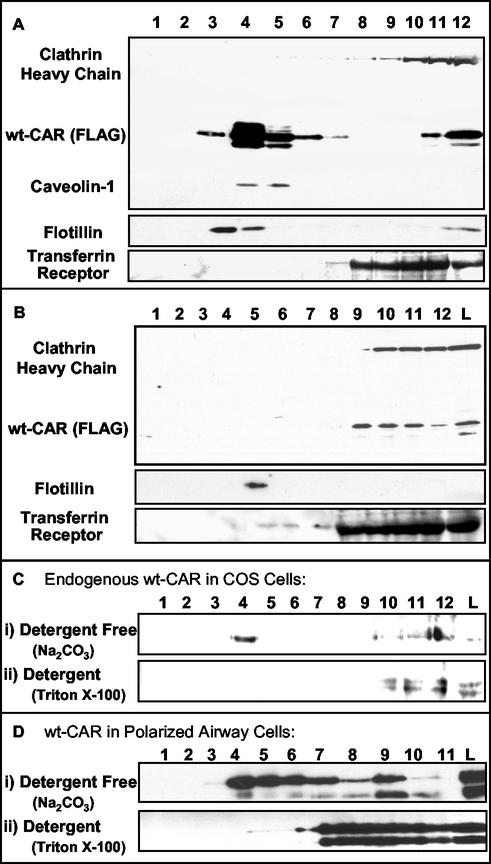
CAR is found in the tight junctions as well as the adherens junctions of epithelia (10), and these structures are known to be specialized lipid rafts (30). Additionally, Ad infection may proceed by both clathrin- and non-clathrin-mediated events, suggesting that CAR resides in an environment that can facilitate entry via both of these routes. CAR also colocalizes with GM1, a lipid raft protein. Thus, we investigated biochemically whether CAR resides in lipid-rich domains. To categorize the cellular location of CAR, we compared the flotation of CAR in sucrose gradients isolated under alkaline detergent-free (42, 43) or Triton X-100 detergent extraction conditions. We compared CAR flotation to the flotation of proteins that play a structural role in CCP (clathrin) and caveolae (caveolin-1). We also compared CAR flotation to those of the TR, which is known to be localized in CCP, and the lipid raft protein flotillin. Under detergent-free Na2CO3 extraction conditions, wt CAR was found in the light fractions after sucrose gradient ultracentrifugation. This flotation profile matched that of caveolin-1 and flotillin but not clathrin or the TR (Fig. 3A), suggesting a lipid raft localization. However, after Triton X-100 extraction, CAR was found in fractions that were different than flotillin and similar to clathrin and TR (Fig. 3B). That detergent solubility was detected suggests that CAR is in a different lipid raft than flotillin and other classically defined caveolae-associated proteins. However, the fact that CAR floats upon nondetergent extraction suggests that it localizes to a distinctive lipid-rich domain. To assess the possibility that the results can be explained by an overexpression of the protein or by the addition of a FLAG epitope, we repeated the experiment with endogenous CAR and the RmcB Ab and obtained similar results (Fig. 3C).
FIG. 3.
Western blotting results for clathrin, wt CAR, and caveolin-1 after nondetergent Na2CO3 (A) or Triton X-100 (B) extraction followed by sucrose gradient fractionation. Blots were routinely divided into three sections prior to immunoblotting. Additional flotillin and TR controls were analyzed on separate blots. (C) Endogenous CAR in control COS-7 cells analyzed by both nondetergent (i) and detergent (ii) extraction. (D) CAR in primary polarized human airway epithelia analyzed by both nondetergent (i) and detergent (ii) extraction.
To determine the physiological relevance of this localization to polarized cells, similar assays were performed on primary polarized human airway epithelia grown at the air-liquid interface and expressing recombinant FLAG-tagged CAR. CAR was found to follow an extraction profile similar to that described above (Fig. 3D).
CAR variants localize to different membrane domains.
To further understand the localization of CAR and to determine how the cytoplasmic domain dictates its localization, we compared wt CAR to three carboxy-terminal variants (diagrammatically shown in the insets of Fig. 4A, B, C, and D). The first variant contained a GPI tail that replaced both the TM and cytoplasmic tail (CT) of CAR (GPI-CAR). Since GPI-linked proteins reside in detergent-resistant lipid rafts, we hypothesized that GPI-CAR localizes to this type of lipid raft. The second variant contained the TM but not the CT of CAR (Tailless-CAR). The CT is a rich source of motifs that were all deleted from Tailless-CAR except for a putative dicysteine palmitoylation motif that could potentially localize CAR to a lipid raft if modified correctly (45). The third variant replaced the TM and CT of CAR with the TM and CT of the LDLR, a protein known to internalize through the CCP-mediated pathway (21). We hypothesized that CAR-LDLR would localize in a nonlipid raft domain.
FIG. 4.
Comparison of immunofluorescence of wt CAR (panels A and E), GPI-CAR (panels B and F), CAR-LDLR (panels C and G) and Tailless-CAR (panels D and H) in transiently transfected unpermeabilized (panels A to D) and Triton X-100-permeabilized (panels E to H) COS-7 cells. Three additional representative fields are included for each condition. A schematic diagram of each of the variants of CAR is inserted in the top right corner of panels A to D.
We first investigated (by indirect immunofluorescence followed by confocal microscopy) whether the wt CAR localization at the cell membrane differed from the localization of CAR-LDLR, GPI-linked CAR, and Tailless-CAR (Fig. 4). Each condition is shown in Fig. 4 with one large photograph and with three additional representative pictures included for comparison. CAR at the plasma membrane was clearly present over the entire cell surface in a punctate manner in unpermeabilized cells (Fig. 4A to D). Surprisingly, very little difference was evident between the different CAR constructs except for a slight increase in staining at the perimeter in CAR- and CAR-LDLR-transfected cells (Fig. 4A and B). Interestingly, upon permeabilization the differences among the CAR constructs became more apparent (Fig. 4E to H). Wt CAR remained present at the cell membrane in a punctate manner but was more intensely observed at the perimeter of the cells and at sites of contact between cells overexpressing CAR (Fig. 4E). Permeabilization may have increased the epitope accessibility of CAR residing either in cell adhesion complexes or in a hidden submembranous population. CAR-LDLR appeared to localize to the perimeter and between transfected cells in a similar fashion (Fig. 4F). In contrast, although a significant amount of Tailless-CAR or GPI-CAR was at the cell surface, permeabilization did not affect the staining, which revealed little preference for the edge or perimeter of the cell (Fig. 4G and H). However, a small amount of Tailless-CAR was found concentrated between cells. This suggests that GPI-linked CAR and Tailless-CAR localize to a different microdomain than wt CAR and CAR-LDLR.
Biochemical localization of CAR variants.
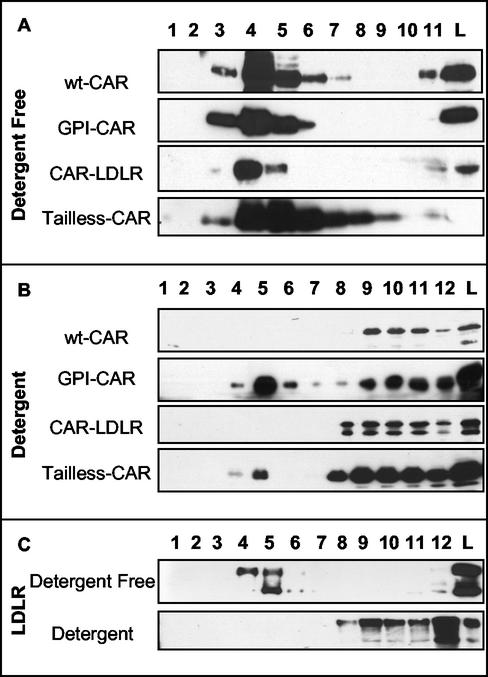
Wt CAR resembled CAR-LDLR to a greater extent than GPI- or Tailless-CAR, as revealed by immunofluorescence microscopy. To examine biochemical differences, we determined the extraction and sucrose gradient centrifugation profiles of the different CAR constructs. After detergent-free extraction, virtually all GPI-CAR protein resided in the light, lipid-rich fractions (Fig. 5A). Moreover, the GPI modification changed the localization of CAR under detergent extraction where a significant portion remained in the light fractions (Fig. 5B). This indicates that, as expected for a lipid-anchored protein, the GPI modification localizes the CAR ectodomain to a lipid raft that is similar to caveolin-1 and flotillin.
FIG. 5.
Extraction and ultracentrifugation of the variants of CAR by nondetergent Na2CO3 extraction (A) or detergent Triton X-100 extraction (B). Panel C shows a comparison of transfected human LDLR after nondetergent and detergent extractions.
Tailless-CAR failed to clearly colocalize with markers of either heavy or light fractions under either extraction technique (Fig. 5A and B). This suggests that the TM domain and remaining putative palmitoylation motif are not sufficient for targeting CAR to a lipid raft and implies that the C-terminal tail of CAR is required for proper membrane localization.
Surprisingly, the extraction profile of the CAR-LDLR chimera closely resembled that of wt CAR (Fig. 5A and B). CAR-LDLR resided in the buoyant, lipid-rich fractions after detergent-free extraction. CAR-LDLR was also detergent soluble and appeared in the heavy, clathrin-associated fractions after Triton X-100 treatment and ultracentrifugation. This similarity in extraction profiles suggests that the wt CAR cytoplasmic domain confers a membrane lipid microdomain localization or affinity similar to that of the LDLR CT. Analysis of the LDLR under both of these conditions produced an extraction profile identical to those of both CAR and CAR-LDLR (Fig. 5C). LDLR was found in light, lipid raft-associated fractions after detergent-free treatment and in the heavy, clathrin-associated fractions with detergent extraction. The LDLR is a receptor that has been shown to be internalized by CCP (21) and is commonly thought of as a CCP, nonlipid raft protein. However, it resides in a compartment that is associated with lipids and is distinct from the TR. This reveals a novel method of separating these proteins into subsets of membrane microdomains. Thus, CAR does not reside in classical caveolin-1-containing caveolae but is in a separate lipid raft that perhaps is similar to the LDLR.
CAR-LDLR mediates Ad infection.
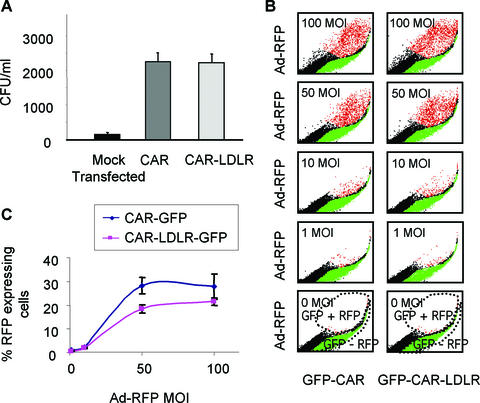
Due to the similar localization and extraction profiles, we hypothesized that fusing CAR to the LDLR CT would have no effect on its function as an Ad receptor. Transfected CHO cells expressing either wt CAR or CAR-LDLR were infected with Ad-eGFP at similar levels of efficiency, as detected by FACS (Fig. 6A). This experiment was repeated using CAR and CAR-LDLR with a C-terminal fusion to eGFP (CAR-GFP or CAR-LDLR-GFP). In this experiment, transfected cells were incubated with increasing MOI levels of Ad-RFP and analyzed by FACS. This revealed only a small difference between CAR and CAR-LDLR at an intermediate MOI (Fig. 6B and C). Fusion of the CAR ectodomain to the C terminus of LDLR did not alter the ability of CAR to mediate Ad infection.
FIG. 6.
Comparison of Ad infections in CAR-deficient CHO cells complemented with wt CAR or CAR-LDLR. (A) CAR-deficient cells complemented with wt CAR or CAR-LDLR result in identical Ad infections. Transfected cells were infected with Ad-GFP at an MOI of 5, immunostained for CAR or CAR-LDLR, and analyzed by FACS. Data shown are the numbers of events testing positive in both of the green and red channels out of 10,000 events (n = 3). (B) A representative experiment showing that CAR-deficient cells complemented with wt CAR-eGFP or CAR-LDLR-eGFP result in similar Ad infections. Cells were infected with Ad-RFP at various MOI and analyzed by FACS. The regions shown in green represent cells expressing CAR-GFP or CAR-LDLR-GFP and not infected with Ad-RFP. The regions in red show cells expressing CAR-GFP or CAR-LDLR-GFP and infected with Ad-RFP. The regions in black represent untransfected, uninfected cells. (C) Graphical depiction of data averaged from six experiments as represented by panel B. The y axis represents the percentage of cells testing positive in both of the green and red channels out of total transfected (green) cells.
DISCUSSION
Our data suggest that CAR and LDLR are localized in lipid-rich membrane microdomains. Traditionally, lipid raft proteins are detergent insoluble and are buoyant when run on a sucrose gradient. However, detergent solubilization can artificially extract lipid raft-associated proteins. It has become common to test flotation with several alternative methods to attempt to identify the particular characteristic environment in which a specific protein is residing (24, 34). Using detergent and nondetergent extraction techniques, we compared the extraction and flotation of CAR and several variants of CAR. Although CAR is highly detergent soluble, it is strongly lipid raft associated in the absence of detergent. This suggests that either CAR is loosely associated with lipid rafts or it resides in a distinct lipid-rich microdomain that is sensitive to detergent extraction. The same characteristics for CAR were also observed in polarized primary airway epithelia, extending this localization to a more physiologically relevant cell type. Additionally, CAR colocalizes with GM1, a marker of lipid rafts. These data are consistent with the finding that CAR resides in a distinct lipid raft microdomain.
GPI-linked proteins behave quite distinctly and are localized to lipid rafts (8, 28), while the effect of deleting the CT is unclear. Both modifications to CAR resulted in altered membrane localization and biochemical extraction in comparison to those of wt CAR. GPI-CAR behaved as expected for a conventional GPI-linked protein residing in a detergent-insoluble glycolipid raft. Whether the Tailless form was a lipid or nonlipid raft protein was not clear. This indicates that it is the CT that dictates the cellular localization of CAR to a distinct environment.
Fusion of the LDLR cytoplasmic and intracellular domains to the extracellular domain of proteins which are normally directed to caveolae, such as the folate receptor, alters the internalization properties of those proteins (35). However, fusion to the extracellular domain of CAR did not significantly alter Ad infection. Our data show that in contrast to the GPI and Tailless forms, CAR-LDLR localized and behaved in a manner biochemically similar to that of wt CAR but not that of the TR. LDLR is widely thought of as a nonlipid raft protein and is directly compared to the TR despite evidence suggesting that the LDLR may reside in a distinct environment from TR. Harder et al. performed complex Ab patching experiments suggesting that although LDLR is not located in a typical lipid raft, it also does not colocalize with the TR (16). Beyond containing a classical YxxØ clathrin-endocytosis motif (Ø represents a hydrophobic residue; x is any residue) (22) and basolateral localization signal (9, 23), there is little sequence similarity between the C termini of CAR and LDLR. Considering this diversity, LDL and Ad may follow distinct internalization pathways that partially overlap due to the redundancy of cellular machinery. However, our data suggest that LDLR, prior to LDL binding, resides in a novel cell membrane lipid-rich domain that has characteristics similar to those of the domain that contains CAR.
The intracellular carboxy terminus of CAR contains many potential signals for transport, clustering, and association with other proteins. The importance of a tyrosine-based motif has been shown in the trafficking of newly synthesized CAR to the appropriate basolateral sites in polarized epithelia (9). CAR also contains a dicysteine palmitoylation motif juxtaposed to the putative TM domain that could direct CAR to lipid-rich domains (48). The putative C-terminal PDZ motif in CAR may interact with scaffolding proteins and may be involved in the localization of CAR to sites of cell adhesion. This places CAR, along with several other proteins, such as the family of cadherins, in a complex network that forms specialized lipid raft domains (3, 10) that likely undergo endocytosis via non-clathrin-mediated mechanisms (1). It would be of great interest to determine whether altering the C terminus affects the native function of CAR.
The question remains how infection is efficiently mediated by all of the forms of CAR studied here. There are several possibilities. First, a coreceptor that responds to either a signal in CAR or a signal on the Ad particle may be required (26). It has been shown that the αν/β3 or αν/β5 integrins play a role in viral infection through an interaction with RGD motifs on the penton base of Ad. This interaction is not sufficient for efficient infection in the absence of CAR; however, integrin-mediated signaling and endocytosis have been well described previously (19, 55). Second, the Ad particle may direct its own endocytosis and trafficking by forming its own unique endocytic vesicle through the cross-linking of receptors, deformation of the membrane, and cooption of the cellular machinery. A third possibility is that Ad may not be restricted to one route of endocytosis and may be able to escape any endocytic vesicle that forms after receptor binding regardless of the mechanism of vesicle formation. The endosomal escape of Ad remains poorly understood. Although the effects of vesicle acidification on Ad infection remain controversial (37, 41), there are several other factors that play a role, such as the αν integrins, protein kinase C, and F-actin (25). There may be either a universal mechanism or several distinct mechanisms that allow Ad to escape from vesicles of diverse origins. The ability of Ad infection to proceed despite the inhibition of specific endocytic processes implies the existence of an innate flexibility that is necessary for viral survival.
The localization of plasma membrane proteins is of great importance for establishing function and modes of interaction with other proteins. The complex nature of lipid rafts is now starting to be appreciated. Mounting evidence suggests that lipid rafts of different function or composition exist that can cluster certain receptors and potentially exclude others (12, 29, 46). The presence of CAR in a lipid-rich domain provides a mechanism that explains the flexibility of Ad mobility and entry. CAR is present on both the cell surface and at sites of cell adhesion in nonpolarized cells, while in polarized cells, CAR is localized to the adhesion complex. The mode of Ad entry may depend in part on the proximity of CAR to endocytic machinery. Moreover, the CT dictates the cellular localization of CAR to a distinct and novel membrane environment. In the same way that Ad first taught us about oncogenesis and RNA splicing, we now have the ability to use the Ad receptor to unravel and track this novel yet unnamed domain.
Acknowledgments
We thank Michael Klotz, Michael Seiler, Pary Weber, Phil Karp, Theresa Mayhew, and Rosanna Smith for excellent assistance. We especially appreciate the help of Michael Welsh and Wouter van't Hof for stimulating discussions.
We appreciate the support of the Gene Transfer Vector Core (supported by the Roy J. Carver Charitable Trust, the NHLBI, CFF, and NIDDK [DK54759]), the In Vitro Cell Models Core (supported by the NHLBI, CFF, and NIDDK [DK54759]) and the Morphology Core of the Gene Therapy Center (supported by the NIDDK [DK 54759]) at the University of Iowa. This work was supported by the National Heart, Lung and Blood Institute.
REFERENCES
- 1.Akhtar, N., and N. A. Hotchin. 2001. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell 12:847-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R. G., B. A. Kamen, K. G. Rothberg, and S. W. Lacey. 1992. Potocytosis: sequestration and transport of small molecules by caveolae. Science 255:410-411. [DOI] [PubMed] [Google Scholar]
- 3.Angst, B. D., C. Marcozzi, and A. I. Magee. 2001. The cadherin superfamily: diversity in form and function. J. Cell Sci. 114:629-641. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler, and D. E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517-568. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee, S., E. R. Smith, K. Hanada, V. L. Stevens, and S. Mayor. 2001. GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. EMBO J. 20:1583-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, C. J., J. Gaetz, T. Ohman, and J. M. Bergelson. 2001. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J. Biol. Chem. 276:25392-25398. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, C. J., J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasbender, A., J. H. Lee, R. W. Walters, T. O. Moninger, J. Zabner, and M. J. Welsh. 1998. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J. Clin. Investig. 102:184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galbiati, F., B. Razani, and M. P. Lisanti. 2001. Emerging themes in lipid rafts and caveolae. Cell 106:403-411. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, R. N., D. L. Gelman, and F. R. Maxfield. 1994. Quantification of low density lipoprotein and transferrin endocytic sorting HEp2 cells using confocal microscopy. J. Cell Sci. 107:2177-2189. [DOI] [PubMed] [Google Scholar]
- 14.Greber, U. F. 1998. Virus assembly and disassembly: the adenovirus cysteine protease as a trigger factor. Rev. Med. Virol. 8:213-222. [DOI] [PubMed] [Google Scholar]
- 15.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477-486. [DOI] [PubMed] [Google Scholar]
- 16.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda, T., H. Saitoh, M. Masuko, T. Katagiri-Abe, K. Tominaga, I. Kozakai, K. Kobayashi, T. Kumanishi, Y. G. Watanabe, S. Odani, and R. Kuwano. 2000. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Mol. Brain Res. 77:19-28. [DOI] [PubMed] [Google Scholar]
- 19.Hynes, R. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 20.Karp, P. H., T. O. Moninger, S. P. Weber, T. S. Nesselhauf, J. Launspach, J. Zabner, and M. Welsh. 2002. Developing an in vitro model of differentiated human airway epithelia: methods for establishing primary cultures, p. 115-137. In C. Wise (ed.), Epithelial cell culture protocols. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed]
- 21.Kibbey, R. G., J. Rizo, L. M. Gierasch, and R. G. Anderson. 1998. The LDL receptor clustering motif interacts with the clathrin terminal domain in a reverse turn conformation. J. Cell Biol. 142:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh, M., and H. T. McMahon. 1999. The structural era of endocytosis. Science 285:215-220. [DOI] [PubMed] [Google Scholar]
- 23.Matter, K., W. Hunziker, and I. Mellman. 1992. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71:741-753. [DOI] [PubMed] [Google Scholar]
- 24.McCabe, J. B., and L. G. Berthiaume. 2001. N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol. Biol. Cell 12:3601-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemerow, G. R. 2000. Cell receptors involved in adenovirus entry. Virology 274:1-4. [DOI] [PubMed] [Google Scholar]
- 27.Nichols, B. J. 2002. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4:374-378. [DOI] [PubMed] [Google Scholar]
- 28.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 30.Nusrat, A., C. A. Parkos, P. Verkade, C. S. Foley, T. W. Liang, W. Innis-Whitehouse, K. K. Eastburn, and J. L. Madara. 2000. Tight junctions are membrane microdomains. J. Cell Sci. 113:1771-1781. [DOI] [PubMed] [Google Scholar]
- 31.Oh, P., D. P. McIntosh, and J. E. Schnitzer. 1998. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson, S., and W. C. Russell. 1983. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J. Gen. Virol. 64:1091-1099. [DOI] [PubMed] [Google Scholar]
- 33.Rauma, T., J. Tuukkanen, J. M. Bergelson, G. Denning, and T. Hautala. 1999. rab5 GTPase regulates adenovirus endocytosis. J. Virol. 73:9664-9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddell, D. R., G. Christie, I. Hussain, and C. Dingwall. 2001. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr. Biol. 11:1288-1293. [DOI] [PubMed] [Google Scholar]
- 35.Ritter, T. E., O. Fajardo, H. Matsue, R. G. Anderson, and S. W. Lacey. 1995. Folate receptors targeted to clathrin-coated pits cannot regulate vitamin uptake. Proc. Natl. Acad. Sci. USA 92:3824-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez, E., and E. Everitt. 1996. Adenovirus uncoating and nuclear establishment are not affected by weak base amines. J. Virol. 70:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 39.Roepstorff, K., P. Thomsen, K. Sandvig, and B. van Deurs. 2002. Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. J. Biol. Chem. 277:18954-18960. [DOI] [PubMed] [Google Scholar]
- 40.Seidman, M. A., S. M. Hogan, R. L. Wendland, S. Worgall, R. G. Crystal, and P. L. Leopold. 2001. Variation in adenovirus receptor expression and adenovirus vector-mediated transgene expression at defined stages of the cell cycle. Mol. Ther. 4:13-21. [DOI] [PubMed] [Google Scholar]
- 41.Seth, P., M. C. Willingham, and I. Pastan. 1984. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J. Biol. Chem. 259:14350-14353. [PubMed] [Google Scholar]
- 42.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 43.Song, K. S., S. Li, T. Okamoto, L. A. Quilliam, M. Sargiacomo, and M. P. Lisanti. 1996. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271:9690-9697. [DOI] [PubMed] [Google Scholar]
- 44.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ten Brinke, A., A. B. Vaandrager, H. P. Haagsman, A. N. Ridder, L. M. van Golde, and J. J. Batenburg. 2002. Structural requirements for palmitoylation of surfactant protein C precursor. Biochem. J. 361:663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vainio, S., S. Heino, J. E. Mansson, P. Fredman, E. Kuismanen, O. Vaarala, and E. Ikonen. 2002. Dynamic association of human insulin receptor with lipid rafts in cells lacking caveolae. EMBO Rep. 3:95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Raaij, M. J., E. Chouin, H. van der Zandt, J. M. Bergelson, and S. Cusack. 2000. Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7 A resolution. Structure Folding Design 8:1147-1155. [DOI] [PubMed] [Google Scholar]
- 48.van't Hof, W., and R. G. Crystal. 2002. Fatty acid modification of the coxsackievirus and adenovirus receptor. J. Virol. 76:6382-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van't Hof, W., and R. G. Crystal. 2001. Manipulation of the cytoplasmic and transmembrane domains alters the cell surface levels of the coxsackie-adenovirus receptor and changes the efficiency of adenovirus infection. Hum. Gene Ther. 12:25-34. [DOI] [PubMed] [Google Scholar]
- 50.Walters, R., and M. Welsh. 1999. Mechanism by which calcium phosphate coprecipitation enhances adenovirus-mediated gene transfer. Gene Ther. 6:1845-1850. [DOI] [PubMed] [Google Scholar]
- 51.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 52.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, X., and J. M. Bergelson. 1999. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]