Abstract
The proper folding and assembly of viral envelope proteins are mediated by host chaperones. In this study, we demonstrated that an endoplasmic reticulum luminal chaperone GRP78/BiP bound specifically to the pre-S1 domain of the L protein in vitro and in vivo where complete viral particles were secreted, suggesting that GRP78/BiP plays an essential role in the proper folding of the L protein and/or assembly of viral envelope proteins.
The hepatitis B virus (HBV) is a noncytopathic double-stranded DNA virus of the hepadnavirus family that causes acute and chronic liver disease. Chronic HBV infection can lead to cirrhosis and finally to hepatocellular carcinoma. HBV is an enveloped virus whose envelope consists of three related proteins, designated the small (S), middle (M), and large (L) surface proteins (27). All of these proteins have a common carboxy-terminal sequence of 226 amino acid residues (S protein). The M protein has an additional sequence (pre-S2) of 55 amino acid residues located at the amino terminus. The L protein contains an additional sequence (pre-S1) of 108 or 119 amino acid residues (depending on the presence of subtype ay or ad, respectively) at the amino terminus of the M protein. All three proteins are found either unglycosylated or glycosylated at Asn146 of the S protein. The M protein is additionally glycosylated at Asn4 within its pre-S2 region (15). The M and S proteins are cotranslationally inserted into the endoplasmic reticulum (ER) membrane with their amino termini translocated in the ER lumen (9). Conversely, the pre-S region (pre-S1 plus pre-S2) of the L protein fails to be cotranslationally translocated and remains on the cytosolic side of the ER membrane. During maturation, approximately half of the L protein molecules posttranslationally translocate their pre-S region into the luminal space, thereby generating a dual topology that is maintained in the secreted viral particles (3, 28, 35). The dual transmembrane topology, disposing the pre-S region at either a luminal (external) or a cytosolic (internal) site, may provide such crucial functions as hepatocyte receptor binding or capsid envelopment, respectively, in the viral life cycle (3, 28, 35). The envelope proteins synthesized as transmembrane proteins of rough ER oligomerize rapidly. Virus assembly is thought to occur at post-ER/pre-Golgi membranes where preformed cytosolic nucleocapsids are packaged by transmembrane envelope proteins (2, 18, 40). Virions then bud into intraluminal cisternae and leave the cell via the constitutive pathway of secretion. An excess of M and S proteins, however, is not incorporated into virion envelopes but self-assembles into secreted subviral particles (15, 26).
Eucaryotic viruses usually use host cell factors during their entire life cycle. Given the known importance of cellular chaperones for correct folding and assembly of host proteins, it is likely that the proper folding and assembly of viral structural proteins are mediated by host chaperones. So far, two host chaperones are known to be involved in HBV morphogenesis. It is suggested that the cytosolic chaperone Hsc70 plays a role in maintaining the dual topology of the pre-S region of the L protein, while the ER transmembrane chaperone calnexin promotes proper folding and trafficking of the M protein and is involved also in intracellular retention of the L protein that is expressed in the absence of the M and S proteins (22, 35, 36, 41). Thus, calnexin is suggested to play a crucial role in folding and assembly of the HBV proteins.
GRP78/BiP, which is a member of the hsp70 family of proteins and is found in the lumen of the ER, is known to be associated with a variety of folding and assembly intermediates of cellular and viral membrane proteins (1, 5, 6, 8, 10, 11, 19, 24). In this study, we demonstrated that GRP78/BiP binds to the L protein in the cells where the infectious viral particles are secreted, suggesting that GRP78/BiP is involved in folding of the L protein and/or assembly of HBV.
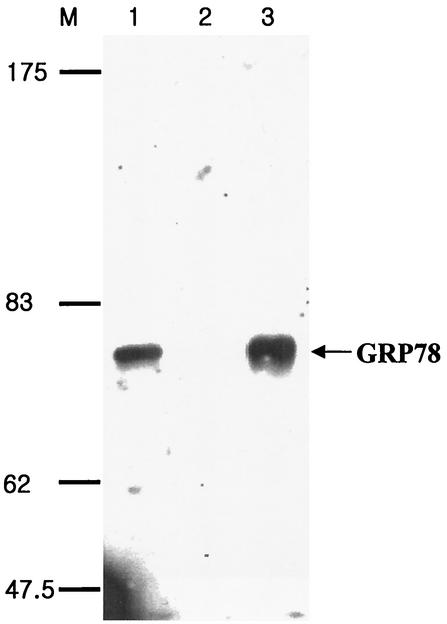
In a previous study (39), Ryu et al. found that an 80-kDa protein (p80) on the surface of the hepatocyte specifically bound to the pre-S1 domain of HBV and proposed that the p80 protein is involved in the viral entry. For further characterization of p80, we sought to purify the protein. To begin with, the purified glutathione S-transferase (GST) or GST-pre-S1 protein (39) was coupled to agarose resin in an AminoLink column (Pierce) to prepare a GST or GST-pre-S1 affinity column, as recommended by the manufacturer. A large-scale preparation of nonbiotinylated HepG2 cell lysates was incubated with a GST affinity column, and the unbound proteins (precleared lysate) were incubated with the GST-pre-S1 column and washed with lysis buffer (25 mM Tris-HCl [pH 7.4], 250 mM NaCl, 5 mM EDTA [pH 8.0], 1% Nonidet P-40, 2 μg of aprotinin/ml, 100 μg of phenylmethylsulfonyl fluoride/ml, 5 μg of leupeptin/ml). The bound proteins were eluted with 0.1 M glycine (pH 2.5) and subjected to sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-7.5% PAGE) followed by silver staining. Expectedly, a protein of approximately 80 kDa was detected (data not shown). Next, the N-terminal amino acid sequence of the purified protein was determined with a Procise 49I automatic sequencer (Applied Biosystems) according to the supplier's recommendations. The result showed that the 22 amino acid residues of the N-terminal sequence (N-EEEDKKEDVGTVVGIDLGTTYS-C) were the same as those of the glucose-regulated protein/immunoglobulin (Ig) heavy-chain binding protein (GRP78/BiP), which is a molecular chaperone of the HSP70 family and is found in the lumen of the ER (13, 14). To validate the identity of the protein, Western blot analysis was carried out using goat anti-BiP antibody (Santa Cruz) (specific to the N terminus of human BiP) and anti-goat IgG-horseradish peroxidase (HRP) conjugates. After rinsing with PBS-T (phosphate-buffered saline [PBS] containing 0.1% Tween 20), the blots were visualized by the enhanced chemiluminescence procedure as recommended by the supplier (Amersham). As shown in Fig. 1, the p80 protein reacted strongly with the anti-BiP antibody (lane 3). To further confirm that the protein was GRP78/BiP, the immunoblot was probed with another anti-BiP antibody (Santa Cruz) (specific to the C terminus of human BiP; data not shown), which clearly showed that GRP78 bound to the pre-S1 region.
FIG. 1.
Purification and Western blot analysis of GRP78/BiP. Nonbiotinylated HepG2 lysates (lane 1), proteins bound to a GST-Sepharose column (lane 2), and proteins bound to a GST-pre-S1-Sepharose column (lane 3) were subjected to Western blotting using goat anti-BiP and HRP-conjugated anti-goat IgG antibodies. Molecular size markers (in kilodaltons) (lane M) are shown on the left. GRP78/BiP is indicated with an arrow.
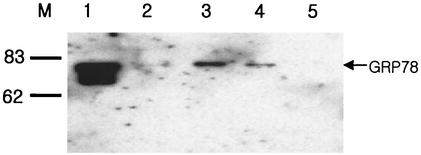
Although it was confirmed that GRP78/BiP binds to the pre-S1 region of HBV, we were unable to rule out the possibility that GRP78/BiP nonspecifically binds to the pre-S1 region. It has been reported that the binding and release of GRP78/BiP are controlled by the ATPase activity of the chaperone (25, 30). To test whether ATP is able to dissociate the GRP78/BiP-pre-S1 complex in vitro, the biotinylated HepG2 lysates were incubated with GST-pre-S1 bound to glutathione-Sepharose beads. After extensive washing, the bound GRP78/BiP was eluted once with PBS (Fig. 2, lane 2) and once (lane 3) or twice (lane 4) with ATP elution buffer (2.5 mM ATP, 10 mM Mg2+ in PBS) and was finally eluted by boiling in the protein sample buffer (lane 5). The eluted proteins were subjected to Western blot analysis, using anti-GRP78/BiP polyclonal antibody (Fig. 2). The results showed that the GRP78/BiP bound to the GST-pre-S1 beads was completely eluted with ATP and thus was not detected even after boiling the GST-pre-S1 beads (Fig. 2, lane 5). HepG2 cell lysates, included as a control, themselves gave a strong signal in the Western blot analysis (Fig. 2, lane 1), suggesting that GRP78/BiP is a housekeeping protein and is present in abundance. The data indicate that the binding of GRP78/BiP to the pre-S1 domain is reversible by ATP hydrolysis, supporting the idea of a specific interaction between GRP78/BiP and the pre-S1 domain.
FIG. 2.
GRP78/BiP binding is ATP dependent. Biotinylated HepG2 lysates were incubated with GST and further incubated with glutathione-Sepharose beads. The unbound proteins (precleared lysate; lane 1) were incubated with GST-pre-S1 fusion protein that bound to glutathione-Sepharose beads. After the beads were extensively washed with lysis buffer, the bound proteins were eluted with PBS (lane 2), ATP elution buffer (lane 3), and ATP elution buffer again (lane 4), in that order, before they were eluted by boiling in the protein sample buffer (lane 5). All eluates were divided into two parts and subjected to SDS-PAGE and Western blotting using goat anti-BiP and HRP-conjugated anti-goat IgG antibodies. Molecular size markers (in kilodaltons) (lane M) are shown on the left.
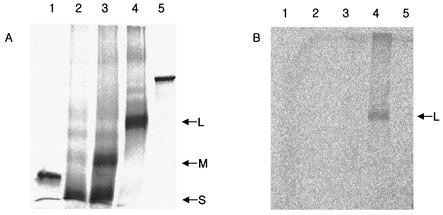
To investigate whether GRP78/BiP binds to the L protein of HBV, we carried out cell-free expression of the L protein in a coupled in vitro transcription-translation-translocation system and coimmunoprecipitated the product using anti-GRP78/BiP antibody. To begin with, the coding sequence of the S, M, or L protein was synthesized from pHBV315 (adr subtype [21]) by PCR and subcloned into the BamHI-SacI, PstI-SacI, or SalI-SacI sites of pSP64(polyA) (Promega) to yield pSP64-S, pSP64-M, or pSP64-L, respectively. The coding sequences of GST were also cloned into pSP64(polyA) on a SalI-SacI fragment to yield pSP64-GST. The nucleotide sequences of the cloned genes were checked by DNA sequencing for fidelity. Next, in vitro transcription and translation were performed using a TnT quick-coupled transcription-translation system (Promega) according to the protocol recommended by the supplier, with slight modifications (35). The L gene was transcribed in vitro and translated in rabbit reticulocyte lysate supplemented with [35S]methionine and dog pancreas microsomes. The M and S genes of HBV and non-HBV proteins (GST and luciferase) were included as negative controls. After the in vitro reaction was run for 90 min, microsomes were recovered by sedimenting the reaction mixture on 1 ml of 250 mM sucrose-TBS (10 mM Tris-HCl [pH 7.4], 150 mM NaCl) at 15,000 × g for 30 min and were diluted 20-fold in ice-cold NET buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Nonidet P-40, 1 mM EDTA [pH 8.0], 0.25% gelatin) to prepare the microsomal extract. Equal amounts of the microsomal proteins were subjected to SDS-PAGE directly (Fig. 3A) or after immunoprecipitation with the anti-GRP78/BiP antibody (Fig. 3B). The SDS-PAGE analysis of the immunoprecipitates revealed that only the L protein (Fig. 3B, lane 4) and not the S (lane 2) or M (lane 3) protein was coimmunoprecipitated with the anti-GRP78/BiP antibody (Fig. 3B). This result suggests that GRP78/BiP binds to the pre-S1 domain of the L protein in vitro.
FIG. 3.
GRP78/BiP binds to the L protein in vitro. The S (lane 2), M (lane 3), and L (lane 4) proteins of the HBV envelope, along with GST (lane 1) and luciferase (lane 5) as negative controls, were synthesized in vitro using a TnT quick-coupled transcription-translation system and canine microsomes. Equal amounts of proteins were subjected to SDS-PAGE either directly (A) or after coimmunoprecipitation with anti-BiP antibody (B).
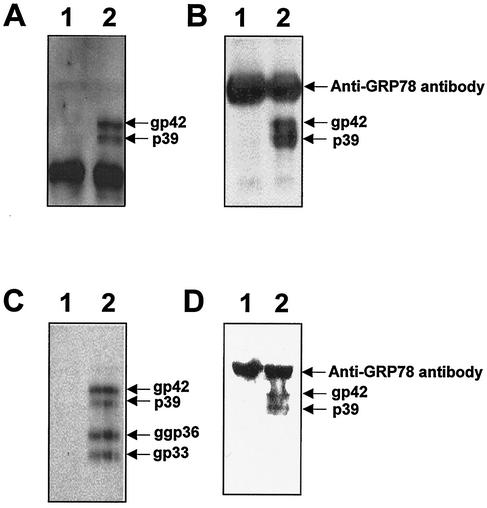
To study whether GRP78/BiP interacts with the L protein in vivo, the L protein was expressed under the control of a human cytomegalovirus promoter in COS7 cells. The coding sequence of the L protein was cloned into pcDNA3 (Invitrogen) on a BamHI-EcoRV fragment to yield pCDNAL. This plasmid, or pcDNA3 as a negative control, was transfected with LipofectAMINE reagent (Life Technologies) into COS7 cells according to the protocol recommended by the supplier. After culturing for 2 days, the transfected cells were harvested and incubated in lysis buffer (50 mM Tris-HCl [pH 7.4], 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 1 μg of aprotinin/ml) at 4°C for 30 min. After further lysis by sonication, the supernatant was harvested by centrifugation at 8,000 × g at 4°C for 10 min. The cell lysate was subjected to Western blot analysis with the humanized version of an anti-pre-S1 monoclonal antibody, KR127 (23); the analysis showed that the nonglycosylated (p39) and the glycosylated forms (gp42) of the L protein were expressed in the cells (Fig. 4A, lane 2). The two glycosylated forms (gp33 and ggp36) and the nonglycosylated form (p30) of the M protein were also detected with anti-pre-S2 antibody (17) (data not shown). Subsequently, the cell lysates were coimmunoprecipitated with anti-GRP78/BiP antibody and the immunoprecipitates were analyzed by Western blotting with anti-pre-S1 murine monoclonal antibody as the primary antibody. As shown in Fig. 4B, the anti-GRP78/BiP antibody coimmunoprecipitated the two forms of the L protein (lane 2), while goat IgG used as a negative control did not (lane 1), suggesting that the GRP78/BiP interacts with the L protein in vivo, irrespective of the state of glycosylation.
FIG. 4.
GRP78/BiP binds to the L protein in vivo. (A) The COS7 cell lysates, transfected with pCDNA3 as a control vector (lane 1) or the L protein expression plasmid, pCDNAL (lane 2), were subjected to Western blotting with the humanized version of anti-pre-S1 antibody. (B) The COS7 cell lysates, transfected with pCDNA3 (lane 1) or pCDNAL (lane 2), were immunoprecipitated with anti-BiP antibody. The immunoprecipitates were subjected to Western blot analysis using the humanized version of anti-pre-S1 antibody and HRP-conjugated anti-human IgG antibody. (C) The HepG2 cell lysates, transfected with mock DNA (lane 1) or pHBV5.2 (lane 2), were subjected to Western blotting with anti-pre-S2 antibody. (D) The HepG2 cell lysates, transfected with mock DNA (lane 1) or pHBV5.2 (lane 2), were immunoprecipitated with anti-BiP antibody, and the immunoprecipitates were subjected to Western blot analysis using anti-pre-S2 monoclonal antibody and HRP-conjugated anti-murine IgG antibody. The L and M proteins and the heavy chain of anti-BiP antibody that was reacted with HRP-conjugated anti-murine IgG antibody or anti-human IgG antibody are indicated. Molecular size markers (in kilodaltons) (lane M) are shown on the right.
To examine whether GRP78/BiP interacts with the L protein in the cells where the production and secretion of complete virus particles are actually taking place, HepG2 cells were transfected with a replication-competent plasmid, pHBV5.2 (38), by using LipofectAMINE reagent (Life Technologies). Using the HBV genome as a probe, the production of the HBV particles was confirmed by Southern blot analysis of the culture supernatant of the transfected HepG2 cells (data not shown) as described previously (38). The expression of the viral surface proteins in the cells was also confirmed by Western blot analysis, using the anti-pre-S2 monoclonal antibody (which, unlike most anti-S monoclonal antibodies, does not recognize conformation-dependent epitopes), of the transfected HepG2 cell lysates (17). As expected, nonglycosylated and glycosylated forms of the L protein (p39 and gp42) and two glycosylated forms of the M protein (gp33 and ggp36) were detected (Fig. 4C, lane 2). Next, to investigate the interaction between GRP78/BiP and the L protein, the cell lysates were coimmunoprecipitated with anti-GRP78/BiP antibody and the immunoprecipitates were analyzed by Western blotting with anti-pre-S2 antibody as the primary antibody. As shown in Fig. 4D, the L protein was coimmunoprecipitated with anti-GRP78/BiP antibody (lane 2), while the M protein was not. A minor amount of the M protein (gp33 and ggp36) was also detected when the film was exposed for a long time (data not shown). This might have been due to the intermolecular disulfide bond formation between the L and M proteins during the viral assembly. Taken together, these findings led to our conclusion that GRP78/BiP interacts with the L protein of HBV in vitro and in vivo.
It has been shown that the L protein expressed in the absence of the S and M proteins is retained in the lumen of the post-ER and thus is not secreted (4, 29, 34, 41). In this case, calnexin was shown to interact with the L protein particles (41). However, the study showed that the retained L particles were also found in the center of the ER lumen, implying that an intraluminal factor(s) may also be involved in the retention of the L protein particles (41). GRP78/BiP has been postulated to prevent aggregation by binding to folding intermediates that are incompletely folded, disulfide bonded, or assembled, both to help solubilize aggregates and to promote folding and oligomerization (7, 20, 31-33, 37). It has also been suggested that its function is to prevent transport of incompletely assembled, misfolded, or aggregated proteins from the ER (12, 16, 19). Therefore, it is likely that GRP78/BiP interacts with the incompletely assembled L protein particles and retains them in the lumen of ER to prevent their secretion.
Virus assembly is thought to occur at post-ER/pre-Golgi membranes where preformed cytosolic nucleocapsids are packaged by transmembrane envelope proteins (2, 18, 40). Virions then bud into intraluminal cisternae and leave the cell via the constitutive pathway of secretion. We observed that GRP78/BiP interacted with both the glycosylated and nonglycosylated forms of the L protein in the cells where viral replication actually took place and complete viral particles were secreted (Fig. 4D). Therefore, GRP78/BiP may play an important role in HBV morphogenesis by regulating proper folding of the L protein and/or assembly of the envelope proteins. The exact function of GRP78/BiP remains to be established.
Acknowledgments
This work was supported by a grant (NLM0030224) from the Ministry of Science and Technology of Korea.
REFERENCES
- 1.Brodsky, J. L., and R. Schekman. 1994. Heat shock cognate proteins and polypeptide translocation across the endoplasmic reticulum membrane, p. 85-109. In R. I. Marimoto, A. Tissieres, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 2.Bruss, V., and D. Ganem. 1991. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 88:1059-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruss, V., X. Lu, R. Thomssen, and W. H. Gerlich. 1994. Post-translational alterations in transmembrane topology of the hepatitis B virus large envelope protein. EMBO J. 13:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, K. C., G. L. Smith, and B. Moss. 1986. Hepatitis B virus large surface antigen is not secreted but is immunogenic when selectively expressed by recombinant vaccinia virus. J. Virol. 60:337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choukhi, A., S. Ung, C. Wychowski, and J. Dubuisson. 1998. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 72:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig, E. A., B. D. Gambill, and R. J. Nelson. 1993. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol. Rev. 57:402-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshaies, R. J., B. D. Koch, and R. Schekman. 1988. The role of stress proteins in membrane biogenesis. Trends Biochem. Sci. 13:384-388. [DOI] [PubMed] [Google Scholar]
- 8.Earl, P. L., B. Moss, and R. W. Doms. 1991. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J. Virol. 65:2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eble, B. E., D. R. MacRae, V. R. Lingappa, and D. Ganem. 1987. Multiple topogenic sequences determine the transmembrane orientation of hepatitis B surface antigen. Mol. Cell. Biol. 7:3591-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudin, Y. 1997. Folding of rabies virus glycoprotein: epitope acquisition and interaction with endoplasmic reticulum chaperones. J. Virol. 71:3742-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gething, M. J., S. Blond-Elguindi, K. Mori, and J. F. Sambrook. 1994. Structure, function, and regulation of the endoplasmic reticulum chaperone, BiP, p. 111-135. In R. I. Marimoto, A. Tissieres, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Plainview, N. Y.
- 12.Gething, M. J., K. McCammon, and J. Sambrook. 1986. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell 46:939-950. [DOI] [PubMed] [Google Scholar]
- 13.Gething, M. J., and J. Shambrook. 1992. Protein folding in the cell. Nature 355:33-45. [DOI] [PubMed] [Google Scholar]
- 14.Haas, I. G. 1991. BiP—a heat shock protein involved in immunoglobulin chain assembly. Curr. Top. Microbiol. Immunol. 167:71-82. [DOI] [PubMed] [Google Scholar]
- 15.Heermann, K. H., and W. H. Gerlich. 1991. Surface proteins of hepatitis B viruses, p. 109-143. In A. McLachlan (ed.), Molecular biology of the hepatitis B virus. CRC Press, Inc., Boca Raton, Fla.
- 16.Hendershot, L., D. Bole, G. Kohler, and J. F. Kearney. 1987. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J. Cell Biol. 104:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong, H. J., A. K. Kim, C. J. Ryu, S. S. Park, H. K. Chung, K. S. Kwon, K. L. Kim, J. Kim, and M. H. Han. 1992. Cloning and characterization of cDNAs coding for heavy and light chains of a monoclonal antibody specific for pre-S2 antigen of hepatitis B virus. Gene 121:331-335. [DOI] [PubMed] [Google Scholar]
- 18.Huovila, A. J., A. M. Eder, and S. D. Fuller. 1992. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 118:1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurtley, S. M., D. G. Bole, H. Hoover-Litty, A. Helenius, and C. S. Copeland. 1989. Interaction of misfolded influenza virus hemagglutinin with binding protein (BiP). J. Cell Biol. 108:2117-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurtley, S. M., and A. Helenius. 1989. A protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol. 5:277-307. [DOI] [PubMed] [Google Scholar]
- 21.Kim, Y. S., and H. S. Kang. 1984. Cloning and expression of hepatitis B virus surface antigen gene. Korean Biochem. J. 17:70-79. [Google Scholar]
- 22.Loffler-Marry, H., M. Werr, and R. Prange. 1997. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology 235:144-152. [DOI] [PubMed] [Google Scholar]
- 23.Maeng, C.-Y., C. J. Ryu, P. Gripon, C. Guguen-Guillouzo, and H. J. Hong. 2000. Fine mapping of virus-neutralizing epitopes on hepatitis B virus preS1. Virology 270:9-16. [DOI] [PubMed] [Google Scholar]
- 24.Mulvey, M., and D. T. Brown. 1995. Involvement of the molecular chaperone BiP in maturation of Sindbis virus envelope glycoproteins. J. Virol. 69:1621-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro, S., and H. R. B. Pelham. 1986. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46:291-300. [DOI] [PubMed] [Google Scholar]
- 26.Nassal, M. 1996. Hepatitis B virus morphogenesis. Curr. Top. Microbiol. Immunol. 214:297-337. [DOI] [PubMed] [Google Scholar]
- 27.Neurath, A. R., and S. B. H. Kent. 1988. The preS region of hepadnavirus envelope proteins. Adv. Virus Res. 34:65-142. [DOI] [PubMed] [Google Scholar]
- 28.Ostapchuk, P., P. Hearing, and D. Ganem. 1994. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 13:1048-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou, J.-H., and W. J. Rutter. 1987. Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J. Virol. 61:782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palleros, D. R., K. L. Reid, L. Shi, W. J. Welch, and A. L. Fink. 1993. ATP-induced protein-HSP70 complex dissociation requires K+ but not ATP hydrolysis. Nature 365:664-666. [DOI] [PubMed] [Google Scholar]
- 31.Pelham, H. R. 1986. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46:959-961. [DOI] [PubMed] [Google Scholar]
- 32.Pelham, H. R. 1988. Heat-shock proteins. Coming in from the cold. Nature 332:776-777. [DOI] [PubMed] [Google Scholar]
- 33.Pelham, H. R. 1989. Control of protein exit from the endoplasmic reticulum. Annu. Rev. Cell Biol. 5:1-23. [DOI] [PubMed] [Google Scholar]
- 34.Persing, D. H., H. E. Varmus, and D. Ganem. 1986. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science 234:1388-1391. [DOI] [PubMed] [Google Scholar]
- 35.Prange, R., and R. E. Streeck. 1995. Novel transmembrane topology of the hepatitis B virus envelope proteins. EMBO J. 14:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prange, R., M. Werr, and H. Loffler-Mary. 1999. Chaperones involved in hepatitis B virus morphogenesis. Biol. Chem. 380:305-314. [DOI] [PubMed] [Google Scholar]
- 37.Rothman, J. E. 1989. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell 59:591-601. [DOI] [PubMed] [Google Scholar]
- 38.Ryu, C. J., P. Gripon, H. R. Park, S. S. Park, Y. K. Kim, C. Guguen-Guillouzo, O. J. Yoo, and H. J. Hong. 1997. In vitro neutralization of hepatitis B virus by monoclonal antibodies against the viral surface antigen. J. Med. Virol. 52:226-233. [DOI] [PubMed] [Google Scholar]
- 39.Ryu, C. J., D.-Y. Cho, P. Gripon, H. S. Kim, C. Guguen-Guillouzo, and H. J. Hong. 2000. An 80-kilodalton protein that binds to the pre-S1 domain of hepatitis B virus. J. Virol. 74:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueda, K., T. Tsurimoto, and K. Matsubara. 1991. Three envelope proteins of hepatitis B virus: large S, middle S, and major S proteins needed for the formation of Dane particles. J. Virol. 65:3521-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, Z., V. Bruss, and T. S. B. Yen. 1997. Formation of intracellular particles by hepatitis B virus large surface protein. J. Virol. 71:5487-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]