Abstract
The fundamental question of whether a primed immune system is capable of preventing latent gammaherpesvirus infection remains unanswered. Recent studies showing that vaccination can reduce acute replication and short-term latency but cannot alter long-term latency further call into question the possibility of achieving sterilizing immunity against gammaherpesviruses. Using the murine gammaherpesvirus 68 (γHV68) system, we demonstrate that it is possible to effectively vaccinate against long-term latency. By immunizing mice with a γHV68 mutant virus that is deficient in its ability to reactivate from latency, we reduced latent infection of wild-type challenge virus to a level below the limit of detection. Establishment of latency was inhibited by vaccination regardless of whether mice were challenged intraperitoneally or intranasally. Passive transfer of antibody from vaccinated mice could partially reconstitute the effect, demonstrating that antibody is an important component of vaccination. These results demonstrate the potential of a memory immune response against gammaherpesviruses to alter long-term latency and suggest that limiting long-term latent infection in a clinically relevant situation is an attainable goal.
The human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus pose significant health risks worldwide, including the induction of cancer and lymphoproliferative diseases. Although acute gammaherpesvirus infection is rapidly cleared by a strong immune response, the establishment of latent infection in hematopoietic cells allows these viruses to successfully evade the host immune response and maintain lifelong infection. Therefore, one of the great challenges in the field is prevention of latent infection. However, despite concerted efforts to understand gammaherpesvirus pathogenesis and immunity, a convincing strategy to prevent long-term latency has not yet been realized.
Due to the strict host restrictions of the human gammaherpesviruses, strategies for vaccinating against human gammaherpesviruses have been difficult to study. Therefore, several groups have used murine gammaherpesvirus 68 (γHV68) infection of mice to examine whether protection against gammaherpesvirus latency can be achieved by single-protein vaccine approaches (reviewed in references 7 and 41). Although the physiology of gammaherpesvirus infection is not completely understood, it appears that latency has multiple forms in vivo, with early forms transitioning to stable long-term latency (2, 37). Several previous vaccination studies have demonstrated effective reduction in both acute infection and the early forms of latency. Vaccination against the lytic cycle protein peptide epitopes ORF6487-495/Db and ORF61425-531/Kb induces strong CD8 T-cell responses and significantly reduces acute infection and early latency (3, 16, 25). Vaccination against the cell membrane and virus particle antigen gp150 induces neutralizing antibody and reduces acute infection and the early stages of latency (16, 27). Vaccination against the latency-associated antigen M2 also induces CD8 T-cell responses and reduces early latency (33). Despite the effectiveness of these strategies against acute infection and/or early latency, none was effective against γHV68 latency beyond 20 days of infection.
These findings raise the question of whether vaccination against long-term gammaherpesvirus latency can be achieved. Although single-protein vaccine strategies have been unsuccessful, it is possible that a live-attenuated vaccine could be more effective. The utility of vaccination with live-attenuated viruses has been demonstrated for both alphaherpesviruses and betaherpesviruses. For example, replication-defective viruses have been used to vaccinate against herpes simplex virus in mice (1, 6, 15, 20, 21), and a live-attenuated varicella zoster virus vaccine is useful in humans (30, 39). Vaccination with a live-attenuated murine cytomegalovirus can limit, but not eliminate, long-term latency (17). Use of a live-attenuated virus for vaccination against a gammaherpesvirus has not been reported.
In this report, we present the use of a mutant reactivation-deficient virus as a vaccination agent for gammaherpesviruses. The γHV68.v-cyclin.LacZ mutant virus, like the γHV68 v-bcl-2 mutant viruses (10), establishes a normal acute infection and a normal latent infection, but has a severe defect in reactivation from latency (35). Three factors influenced the choice of this vaccine virus. First a live-attenuated virus might offer better protection against latency than single-protein vaccination. Second, the mutant vaccine virus and the wild-type challenge virus are genetically distinguishable, allowing us to use a sensitive nested PCR assay to determine whether the challenge virus establishes and maintains latent infection. Finally, because the vaccine virus is reactivation deficient, we could determine whether the challenge virus reactivates from latency in the presence of a primed immune response.
In this study, we show that vaccination with a reactivation-deficient virus reduced long-term latent infection of wild-type challenge virus to an undetectable level. Vaccination was effective whether the challenge virus entered the host via mucosal (intranasal [i.n.]) or systemic (intraperitoneal [i.p.]) routes. Immune antibody could partially reconstitute the vaccination effect, and, surprisingly, CD8 T cells were not required.
MATERIALS AND METHODS
Viruses and mice.
γHV68 clone WUMS (ATCC VR1465) and γHV68.v-cyclin.LacZ (35) were passaged and titered by plaque assay on NIH 3T12 cells (36). γHV68.v-cyclin.LacZ contains a LacZ expression cassette (the β-galactosidase gene driven by the human cytomegalovirus immediate-early promoter-enhancer) in place of the first 475 bp of the v-cyclin gene (35). Vaccinia virus (WR strain) was obtained from Bill Joklik (Duke University). Mice of the C57BL/6J (B6) and CD8α−/− lines on a B6 background were obtained from Jackson Laboratories (Bar Harbor, Maine) and were bred at Washington University School of Medicine in accordance with all federal and university guidelines. Eight- to 12-week-old animals were used for all experiments.
Vaccination and challenge virus infections.
Mice were vaccinated i.p. with 106 PFU of γHV68.v-cyclin.LacZ in 0.5 ml of Dulbecco's modified Eagle's medium (DMEM) or were mock vaccinated with DMEM alone or DMEM containing an equivalent volume of 3T12 cell lysate (prepared identically to virus stock samples). Twenty-eight days later, mice were challenged i.p. with 100 PFU (unless otherwise stated) of γHV68 in 0.5 ml of DMEM or i.n. with 400 PFU of γHV68 in 40 μl of DMEM. At the end of the challenge period, mice were sacrificed, and organs from five mice per group were harvested and pooled as described previously (11, 37).
Ex vivo limiting dilution reactivation analysis.
The frequency of cells reactivating from latency was assayed as described previously (37). Serial twofold dilutions of harvested cells (24 wells per dilution, starting at 1 × 105 cells per well for splenocytes and 4 × 104 cells per well for peritoneal cells) were plated onto permissive mouse embryonic fibroblast (MEF) monolayers for 21 days and then scored for cytopathic effect due to reactivating virus. To verify that cell samples contained latently infected cells and not preformed infectious virus as a result of persistent replication, replicate cell aliquots were mechanically disrupted in 1/3× DMEM in the presence of 0.5-mm-diameter silica beads. This procedure kills >99% of cells, but has at most a twofold effect on viral titer (37), thus allowing an experimental distinction between reactivation from latency (which requires live cells) and persistent replication. The highest frequencies of persistent replication observed in mock-vaccinated mice were <1 in 40,000 for peritoneal cells and <1 in 100,000 for splenocytes. As previously described (31), this level of persistent replication does not significantly impact the frequency of reactivation from latency. Persistent replication was not detected in samples from vaccinated mice. In samples from mice that were both vaccinated and challenged, the identity of reactivating virus was determined by staining for the presence of β-galactosidase. (γHV68.v-cyclin.LacZ, but not γHV68, encodes β-galactosidase.) Individual wells containing single reactivation events (cell dilutions that yielded ≤63.2% cytopathic effect for that dilution) were replated onto fresh MEF monolayers, fixed with 2% paraformaldehyde, and stained overnight at 37°C with a mixture of 2.2 mM X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), 2 mM MgCl2, 5 mM K3Fe(CN)6, and 5 mM K4Fe(CN)6 · H2O (4). The presence of a white or blue monolayer staining was evaluated microscopically.
Limiting dilution PCR analysis.
To determine the frequency of cells carrying the γHV68 genome, single-copy sensitivity nested PCR for γHV68 v-cyclin (gene 72), LacZ, or gene 50 was performed on serial dilutions of cells by a previously published method (31, 35, 37). Briefly, frozen test cells were thawed, washed, resuspended in an isotonic solution, and counted. Starting at 104 cells per reaction, cells were serially diluted threefold in a background of uninfected NIH 3T12 cells such that 104 total cells were present in each well (10 μl total) and plated in a 96-well PCR plate at 12 wells per dilution. Single copies of a plasmid containing γHV68 v-cyclin and LacZ genes as well as gene 50 in a background of 3T12 cells were included as positive controls. 3T12 samples with no plasmid were included as negative controls. After overnight lysis of cells with proteinase K, round 1 of PCR was performed with 20 μl per reaction. Nested PCR was performed following addition of 10 μl of round 2 reaction buffer to the same well, and nested products were visualized on a 1.5% agarose gel. The primers for v-cyclin were GAGATCTGTACTCAGGCACCTGT and GGATTTCTTGACAGCTCCCTGT for round 1 and TGTCAGCTGTTGTTGCTCCT and CTCCGTCAGGATAACAACGTCT for round 2. The primers for LacZ were CTGGTTTCCATCAGTTGCTG and GACATTGGCGTAAGTGAAGC for round 1 and ACTGGTGTGGGCCATAATTC and AGTGCACGGCAGATACACTT for round 2. The primers for gene 50 were as published previously (37). False positives were detected in 0.4% of all reactions. Positive control reaction mixtures containing 10, 1, or 0.1 copy of v-cyclin plasmid DNA were positive in 94, 63, and 9% of all reactions, respectively. Positive control reaction mixtures containing 10, 1, or 0.1 copy of LacZ plasmid DNA were positive in 96, 51, and 7% of all reactions, respectively. Positive control reaction mixtures containing 10, 1, or 0.1 copy of gene 50 plasmid DNA were positive in 83, 58, and 8% of all reactions, respectively.
Plaque assays.
Plaque assays were performed as previously described (34, 35). Briefly, organs were harvested into sterile, screw-cap 2-ml tubes containing 1 ml of DMEM and 100 μl of 1-mm-diameter zirconia-silica beads (BioSpec Products, Inc., Bartlesville, Okla.) and stored at −80°C. Samples were thawed on ice and homogenized with a Mini BeadBeater (BioSpec) and then further diluted in DMEM prior to infecting NIH 3T12 monolayers. Infected monolayers were overlaid with Noble agar, and plaques were visualized at day 7 by neutral red staining. The limit of detection was 50 PFU.
Passive antibody transfer.
Blood from 10 mock-vaccinated or vaccinated B6 mice was harvested from the retro-orbital sinus at day 28, pooled, and allowed to stand at room temperature for 5 to 10 min. Serum separation was performed with Microtainer serum separator tubes (Becton Dickinson, Franklin Lakes, N.J.), and serum was aliquoted and stored at −20°C until use. One day prior to challenge and 7 days after challenge, 100 μl of serum was transferred i.p. to each of five naive B6 mice for each experimental group. Sixteen days postchallenge, peritoneal cells and splenocytes were harvested and assayed as described above.
Statistical analysis.
All data points represent the mean of all experiments ± standard error for two to three experiments, with 5 mice per condition per experiment. To quantify the number of cells from which the virus reactivated or which carry latent viral genome, data were subjected to nonlinear regression (sigmoidal dose curve with nonvariable slope) by using GraphPad Prism (GraphPad, San Diego, Calif.). Frequencies of reactivation events or genome-positive cells were based on a Poisson distribution of the cell number at which 63.2% of the wells scored positive for reactivation or the viral genome, respectively. To calculate significance, unless otherwise noted, data were statistically analyzed by paired t test over the range of dilutions.
RESULTS
Vaccination severely limits reactivation of challenge virus from latency.
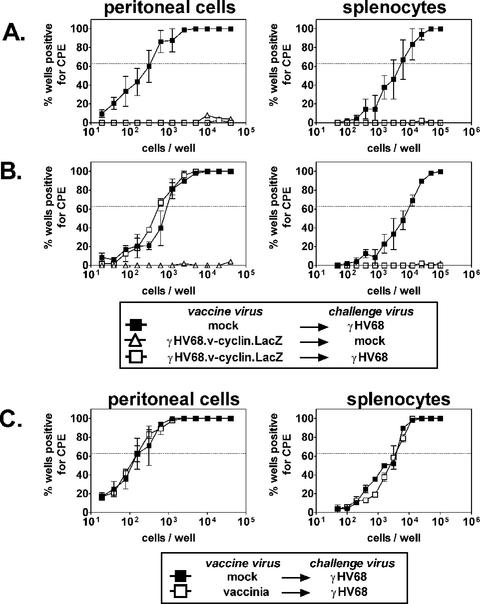
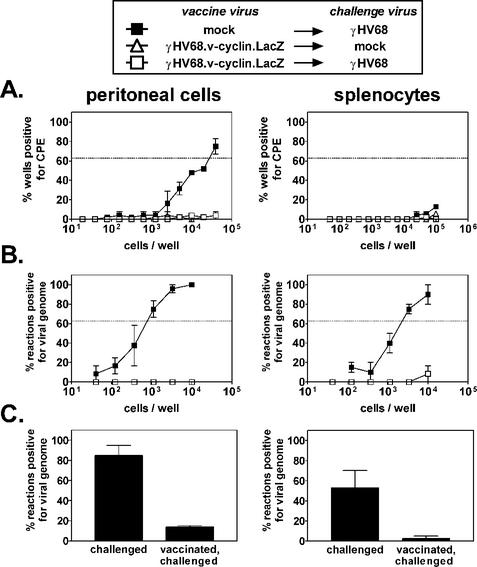
To determine if the immune system is capable of preventing latent infection, we vaccinated mice against γHV68 infection by using a reactivation-deficient γHV68 mutant. B6 mice were either mock vaccinated or vaccinated with γHV68.v-cyclin.LacZ for 28 days and then challenged with γHV68 for 16 days. Based on the results of dosing experiments (Tibbetts et al., unpublished data), we used a γHV68 challenge dose of 100 PFU, because this dose is 1,000-fold above the minimum dose (0.1 PFU) required to establish maximal levels of latency in B6 mice. It is notable that establishment of full levels of latency by infection with 0.1 PFU (as detected by plaque assay on 3T12 cells) likely reflects the fact that mice provide a more sensitive readout for infection with γHV68 than 3T12 cells. This is also seen when viral stocks are serially diluted on MEFs, an assay that detects 0.1 to 0.2 PFU of γHV68 (36, 37). A control group was vaccinated and mock challenged. The effect of vaccination on latency was assessed by ex vivo limiting dilution reactivation analysis of cells from two major sites of latency (29, 37, 38), the peritoneum and the spleen (Fig. 1A). In mice that were mock vaccinated and challenged, the frequencies of peritoneal cells and splenocytes reactivating from latency 16 days postchallenge were 1 in 300 and 1 in 5,200, respectively. In contrast, little or no reactivation was detected in mice vaccinated prior to challenge, demonstrating that previous exposure to γHV68.v-cyclin.LacZ decreased latent γHV68 infection in peritoneal cells by >130-fold and in splenocytes by >19-fold. Reduction of latency below an undetectable level was not due to saturation of the potential cellular reservoir for latency, since challenge with a higher dose (106 PFU) of γHV68 could overcome the vaccination effect in peritoneal cells (Fig. 1B). Notably, vaccination had a partial effect even at this high dose, suggesting that the mechanism of vaccination involves more than a complete block of infection by neutralizing antibody. Vaccination was not due to nonspecific effects induced by previous viral vaccination, since vaccination with vaccinia virus had no effect on latent γHV68 infection (Fig. 1C).
FIG. 1.
Vaccination prevents reactivation from latency. Limiting dilution reactivation analysis was used to quantify latent infection in B6 mice challenged only, vaccinated only, or challenged and vaccinated 16 days after i.p. challenge. CPE, cytopathic effect. The horizontal line indicates the 63.2% Poisson distribution line. (A) Vaccination with γHV68.v-cyclin.LacZ, challenge with 100 PFU of γHV68. Statistical differences between mock-vaccinated and vaccinated mice were P < 0.0001 and P = 0.002 for peritoneal cells and splenocytes, respectively. (B) Vaccination with γHV68.v-cyclin.LacZ and challenge with 106 PFU of γHV68. The statistical difference between mock-vaccinated and vaccinated mice was P = 0.004 for splenocytes. (C) Vaccination with vaccinia virus and challenge with 100 PFU of γHV68.
Vaccination severely limits the establishment of latency by challenge virus.
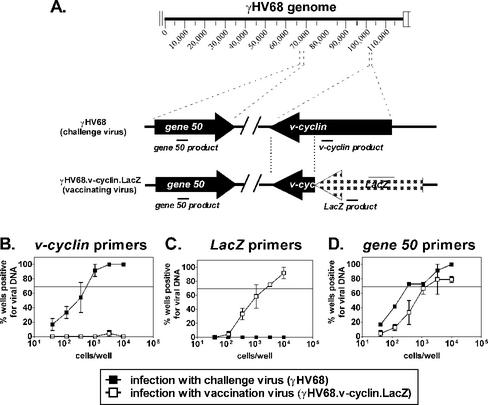
The frequency of ex vivo reactivation reflects both the number of latently infected cells and the ability of those cells to reactivate from latency (reactivation phenotype) (31, 37). Thus, any decrease in the frequency of reactivation could result from an overall decrease in the number of latently infected cells or an alteration in the reactivation phenotype of latently infected cells (31). To distinguish between these possibilities, we developed nested PCR assays to specifically detect either the vaccine or challenge virus. Due to the difference between the γHV68.v-cyclin.LacZ and γHV68 genomes at the v-cyclin locus (Fig. 2A), we developed a PCR assay for v-cyclin that specifically detects the challenge virus (wild-type γHV68), but does not detect the vaccine virus (γHV68.v-cyclin.LacZ) (Fig. 2B). Likewise, we developed a PCR assay for LacZ that detects the vaccine virus, but not the challenge virus (Fig. 2C). Both assays detect single copies of viral genome (see Materials and Methods), and thus the sensitivity of the PCR assays for v-cyclin or LacZ is comparable to that of the previously established gene 50 limiting dilution PCR assay (Fig. 2D) (37).
FIG. 2.
Limiting dilution PCR assays to detect either challenge virus or vaccine virus. (A) Location of sites of amplification from challenge virus (γHV68) or vaccine virus (γHV68.v-cyclin.LacZ) genome by using PCR primers specific for v-cyclin, LacZ, or gene 50. (B) Limiting dilution PCR with primers specific for v-cyclin detects challenge virus only. PCR analysis of serial dilutions of splenocytes harvested from mice infected with challenge virus for 16 days or vaccine virus for 44 days (28-day vaccination plus 16-day challenge period). The horizontal line indicates the 63.2% Poisson distribution line. (C) Limiting dilution PCR with primers specific for LacZ detects vaccine virus only. (D) Limiting dilution PCR with primers specific for gene 50 detects both challenge virus and vaccine virus.
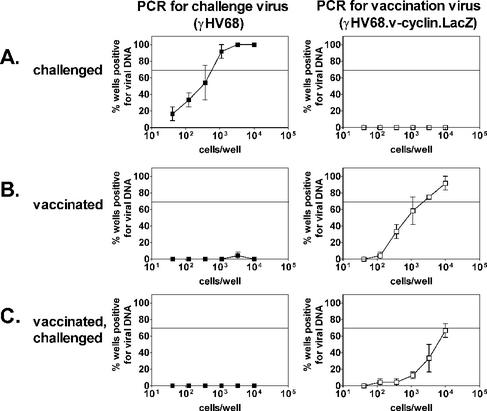
To determine whether vaccination alters the number of latently infected cells, we used v-cyclin or LacZ limiting dilution PCR to determine the frequency of cells carrying the wild-type γHV68 or γHV68.v-cyclin.LacZ genome. In mice that were mock vaccinated and challenged, 1 in 400 splenocytes contained the γHV68 genome, while the γHV68.v-cyclin.LacZ genome was not detected (Fig. 3A). In mice that were vaccinated with γHV68.v-cyclin.LacZ but not challenged, we detected γHV68.v-cyclin.LacZ, but not γHV68 (Fig. 3B). γHV68.v-cyclin.LacZ was similarly detected in mice that had been both vaccinated and challenged, but γHV68 was not (Fig. 3C). Similarly, the γHV68 genome was not detected in peritoneal cells from mice that had been vaccinated and challenged (data not shown). These results show that vaccination inhibited establishment of the early forms of latency, at least to a level below 1 in 10,000 cells. Although these data indicate a significant decrease in latent infection, they do not rule out the possibility that latency was established below a detectable level or at a different site. It should be noted that, surprisingly, the number of γHV68.v-cyclin.LacZ-infected cells decreased by 85% in challenged samples, suggesting that the memory immune response provoked by the challenge virus altered latency previously established by γHV68.v-cyclin.LacZ.
FIG. 3.
Vaccination prevents establishment of latency. Limiting dilution PCR specific for challenge virus (v-cyclin primers) or vaccine virus (LacZ primers) was used to detect viral genome in splenocytes from B6 mice challenged only (A), vaccinated only (B), or vaccinated and challenged (C) 16 days after i.p. challenge with 100 PFU of γHV68. The horizontal line indicates the 63.2% Poisson distribution line. For challenge virus, the difference between mock-vaccinated and vaccinated mice was statistically significant (P = 0.007). The frequency of cells containing the vaccine virus genome decreased by 84% in challenged mice (1 in 1,400 mock-vaccinated versus 1 in 9,000 vaccinated [P = 0.03]).
Vaccination severely limits latent infection after i.n. challenge.
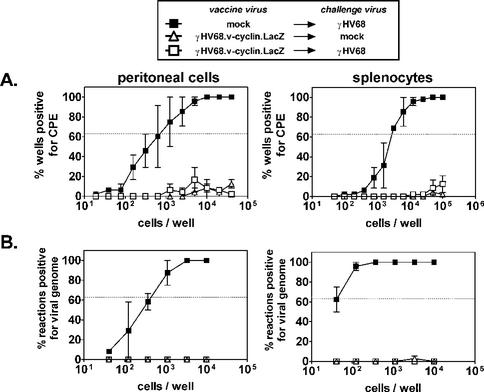
To determine if vaccination is also effective against mucosal γHV68 infection, we challenged vaccinated and mock-vaccinated mice i.n. with 400 PFU of γHV68. Sixteen days later, latent infection in peritoneal cells and splenocytes was examined. Efficient reactivation of wild-type γHV68 was observed in both peritoneal cells (1 in 680) and splenocytes (1 in 3,400) from mock-vaccinated mice (Fig. 4A). In contrast, reactivation was low or undetectable in cells from vaccinated mice. This effect was due to a decrease in the number of latently infected cells, since we did not detect peritoneal cells or splenocytes harboring the γHV68 genome in vaccinated mice (Fig. 4B). Although the presence of genome-bearing cells at a frequency below 1 in 10,000 cells would not be detected with this assay, viral genome was readily detected in mock-vaccinated animals (>1 in 40 splenocytes, 1 in 420 peritoneal cells), and thus these results indicate a >250-fold decrease in splenic latency and a >24-fold decrease in latency in peritoneal cells. Therefore, vaccination achieved by systemic administration of a reactivation-deficient virus reduced latency to undetectable levels by virus entering the host via either systemic (Fig. 1) or mucosal (Fig. 4) routes.
FIG. 4.
Vaccination prevents latent infection after i.n. challenge. Limiting dilution reactivation analysis and limiting dilution PCR were used to examine latency in B6 mice challenged only, vaccinated only, or challenged and vaccinated 16 days after i.n. challenge with 400 PFU of γHV68. CPE, cytopathic effect. (A) Limiting dilution reactivation analysis. Differences between mock-vaccinated and vaccinated mice were statistically significant (P = 0.0003 and P = 0.002 for peritoneal cells and splenocytes, respectively). (B) Limiting dilution PCR specific for challenge virus (v-cyclin primers). Differences between mock-vaccinated and vaccinated mice were statistically significant (P = 0.01 and P < 0.0001 for peritoneal cells and splenocytes, respectively).
Vaccination limits long-term challenge virus latency.
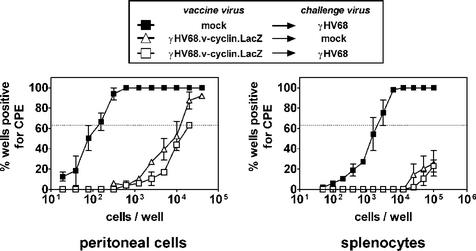
As previously noted, other vaccination approaches can decrease early forms of latency (14 to 17 days postchallenge), but fail to control long-term latency (after 20 to 28 days) (16, 25, 27, 33). We therefore determined whether the effect of γHV68.v-cyclin.LacZ vaccination observed 16 days after infection was transient. Vaccinated or mock-vaccinated mice were challenged i.p. with 100 PFU of γHV68, and latency was assessed 42 days later. Even at this late time, little or no reactivation was observable from peritoneal cells or splenocytes of vaccinated mice (Fig. 5A). Furthermore, samples from vaccinated mice contained no detectable challenge virus genome (Fig. 5B). These data demonstrate that vaccination with a reactivation-defective mutant virus was effective against long-term latent γHV68 infection.
FIG. 5.
Vaccination is effective against long-term latent infection. Limiting dilution reactivation and limiting dilution PCR analysis were used to examine long-term latency in B6 mice after i.p. challenge with 100 PFU of γHV68. CPE, cytopathic effect. (A) Limiting dilution reactivation analysis was used to determine the status of the latent infection in cells harvested 42 days postchallenge. The difference between mock-vaccinated and vaccinated mice was statistically significant (P = 0.01 for peritoneal cells over the five highest cell dilutions). (B) Limiting dilution PCR specific for challenge virus (v-cyclin primers) was used to detect viral genome in cells harvested 42 days postchallenge. Differences between mock-vaccinated and vaccinated mice were statistically significant (P = 0.02 and P = 0.04 for peritoneal cells and splenocytes, respectively). (C) PCR specific for challenge virus for cells harvested 125 days postchallenge. Bars indicate the percentage of reactions positive for viral genome at 10,000 cells per reaction. Differences between mock-vaccinated and vaccinated mice were statistically significant (P = 0.02 and P = 0.10 for peritoneal cells and splenocytes, respectively).
Despite the effectiveness of vaccination against long-term γHV68 infection, we further questioned whether this effect was enduring. Due to the extraordinary ability of gammaherpesviruses to establish latent infection, we reasoned that if virus was not permanently cleared from the host that viral genome might eventually be detectable at some level in peritoneal cells and splenocytes. To provide a stringent test of long-term protection, we assessed latency in vaccinated or mock-vaccinated mice 125 days after challenge with wild-type γHV68 (Fig. 5C). Although viral genome was detected in vaccinated mice at a frequency of less than 1 in 10,000 cells, this was significantly lower than the frequency in mice that had not been vaccinated. Thus, although vaccination was not 100% sterilizing, the effects of vaccination on the frequency of latently infected cells were evident more than 4 months postchallenge. Furthermore, these data demonstrate that vaccination was effective at decreasing long-term latency without blocking infection, indicating that vaccination involves mechanisms in addition to blockade of infection.
Vaccination reduces acute infection of challenge virus.
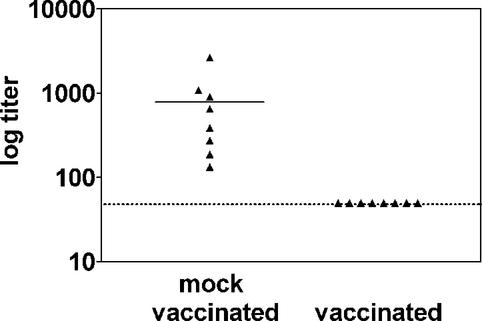
To determine if the reduction in long-term latency correlated with reduction in acute infection, we assessed viral titers 13 days after challenge (Fig. 6). This time point represents the peak of acute titer for a low-dose i.p. infection (Tibbetts et al., unpublished). In B6 mice that were mock vaccinated and challenged, the splenic titer corresponded to 784 ± 293 PFU, whereas the titer in all vaccinated animals was below the limit of detection (<50 PFU). Thus, vaccination reduced both acute and latent infection to an undetectable level.
FIG. 6.
Vaccination is effective against acute infection. Plaque assays were used to determine the acute viral titers in splenocytes from B6 mice after i.p. challenge with 100 PFU of γHV68. The mean titer was 784 ± 293 PFU for mock-vaccinated mice (n = 8). Titers in vaccinated mice were below the 50-PFU level of detection (n = 8). The difference between mock-vaccinated and vaccinated mice was statistically significant (P = 0.02).
Antibody, but not CD8 T cells, is critical for vaccination against latency.
We next sought to determine the immunologic mechanisms responsible for vaccination against latency. Based on the important role of CD8 T cells in viral infections and their ability to alter latent EBV infection (12, 24), one hypothesis was that a CD8 T-cell-dependent antiviral immune response was an essential component of vaccination against gammaherpesvirus latency. Furthermore, we and others have demonstrated an important role for CD8 T cells in restricting latent γHV68 infection (8, 26, 31, 32), and adoptive transfer of CD8 T cells directed against the latent M2 protein of γHV68 effectively limits early forms of γHV68 latency (32).
To determine whether CD8 T cells were required for the vaccination effect, CD8−/− mice were vaccinated and then challenged with 100 PFU of γHV68 for 16 days. In CD8−/− mice that were not vaccinated, γHV68 established latent infection and reactivated normally (Fig. 7). Vaccination with γHV68.v-cyclin.LacZ reduced wild-type γHV68 latent infection to an undetectable level in CD8−/− mice, as shown by decreased reactivation from latency and a decreased frequency of cells containing wild-type viral genome (Fig. 7) (data not shown). The low level of reactivating virus detected in mice vaccinated only and mice vaccinated and challenged was γHV68.v-cyclin.LacZ, as determined by staining for β-galactosidase. Wells containing single reactivation events (wells in cell dilutions that yielded ≤63.2% cytopathic effect for that dilution) in vaccinated samples stained positive for the presence of β-galactosidase (46 of 46 wells), indicating that reactivation was due to the presence of the vaccine virus rather than the challenge virus. Wells from mice that were vaccinated only were positive for β-galactosidase activity (12 of 12), whereas wells from mice that were challenged with wild-type virus but mock vaccinated were all negative (12 of 12). Detection of a low frequency of γHV68.v-cyclin.LacZ reactivation in CD8−/− mice is consistent with results previously described for infection of immunodeficient animals with this virus (37). These results demonstrate effective vaccination against latency in the absence of CD8 T cells.
FIG. 7.
CD8 T cells are not required for vaccination. Limiting dilution reactivation analysis was used to determine the status of the latent infection in CD8−/− mice challenged only, vaccinated only, or challenged and vaccinated 16 days after i.p. challenge with 100 PFU of γHV68. Differences between mock-vaccinated and vaccinated mice were statistically significant (P < 0.0001 and P = 0.0005 for peritoneal cells and splenocytes, respectively). All 46 wells containing single reactivation events (wells in cell dilutions that yielded a cytopathic effect [CPE] for that dilution of ≤63.2%) in vaccinated samples stained positive for the presence of β-galactosidase, indicating that reactivation was due to the presence of the vaccine virus rather than the challenge virus.
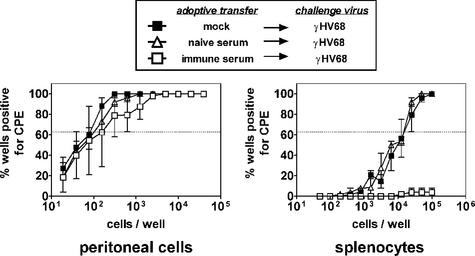
We and others have demonstrated that B cells regulate γHV68 latency in vivo (28, 37). Furthermore, generation of antibody is an important determinant for protection against diseases caused by the alphaherpesviruses herpes simplex virus type 1 (HSV-1) and -2 (1, 5, 21-23). In our experiments, antiviral antibody was not detectable in serum from naive mice, but was readily detectable in mice vaccinated with γHV68.v-cyclin.LacZ (24 μg of antiviral immunoglobulin G per ml [data not shown]). Thus, we questioned whether antiviral antibody could account for vaccination against γHV68 latency. To test this, naive B6 mice were injected with serum from either mock-vaccinated or vaccinated mice, and challenged 1 day later with γHV68. Mice were treated with antibody again at day 8. Naive serum had no effect on the ability of γHV68 to establish latency (Fig. 8). In contrast, immune serum reduced wild-type virus latency to an undetectable level in the spleen after i.p. infection. However, immune serum at this dose had no effect on the ability of γHV68 to establish latency in peritoneal cells. This effect was not due to a resistance of peritoneal cell infection to antibody, since passive transfer of antibody reduced latency in peritoneal cells when mice were challenged i.n. (data not shown). These results demonstrate that antibody can play an important role in reducing latent infection and suggest that antibody is one important component of the vaccination-induced memory immune response to gammaherpesvirus infection.
FIG. 8.
Antibody from vaccinated mice can prevent splenic latency. Limiting dilution reactivation analysis was used to determine the status of the latent infection in B6 mice after mock transfer or passive transfer of naive serum or immune serum from vaccinated mice 1 day prior to and 7 days after challenge. Mice were analyzed 16 days after i.p. challenge with 100 PFU of γHV68. CPE, cytopathic effect. The difference between mock-vaccinated and vaccinated mice was statistically significant (P = 0.008 for splenocytes).
DISCUSSION
Utilizing a reactivation-deficient mutant virus as a live-attenuated vaccine, we demonstrate that it is possible to vaccinate against long-term gammaherpesvirus latency. Importantly, vaccination was effective against either systemic or mucosal viral challenge. In addition to decreasing the early form of latency, vaccination decreased latent infection to below detectable levels 42 days after challenge and had significant effects as late as 125 days postchallenge. Thus, despite the remarkable ability of γHV68 to establish maximal latency at doses as low as 0.1 PFU (Tibbetts et al., unpublished), vaccination with a live-attenuated virus was effective at altering long-term latency. Due to the limitations of the assays to detect latent infection, we cannot conclude and do not speculate that vaccination completely prevented latent infection. In fact, at 125 days postchallenge, the viral genome was detectable at a very low level, indicating that vaccination did not induce sterilizing immunity in 100% of the animals. However, these results do indicate that vaccination is effective at reducing short- and long-term latency to an undetectable level as detected by the most sensitive assays that are currently available. This demonstrates the potential of immunization to severely limit long-term gammaherpesvirus latency.
Because this strategy was partially effective against high-dose challenge (107-fold greater than the maximum dose required to establish latency), it is unlikely that the effectiveness of vaccination is due to a simple block in primary infection. Further work will be needed to clarify at which point in infection vaccination acts. It is possible that vaccination acts on virus or virus-infected cells during acute infection, during the transition from acute infection to latency, during latency itself, or, conceivably, during all of these phases.
As described above, previous attempts to vaccinate against γHV68 latency by using single-protein vaccines have been unsuccessful despite their ability to induce strong virus-specific immunity (16, 25, 27, 33). Despite impressive control of acute productive infection in these previous experiments, γHV68 was able to establish latency at normal levels by 21 to 28 days of infection. It is not clear why these strategies alter the early stages of latency, but have no effect on long-term latency. It is unlikely that reduction of the acute titer results in a delay in the ability of the virus to establish the early forms of latency, since we have not observed differences in early latency over doses ranging from 0.1 × 106 to 1 × 106 PFU for i.p. infection and from 40 × 105 to 4 × 105 PFU for i.n. infection (Tibbetts et al., unpublished).
Alternatively, it is possible that the early forms of latent infection differ in viral gene expression from the later forms, thereby allowing the presentation of antigens that can be targeted by a primed immune system while simultaneously allowing the escape of latently infected cells that do not express those antigens. Consistent with this hypothesis, during the early stage of latency (e.g., 16 days), a higher proportion of γHV68 genome-bearing cells reactivate ex vivo than during long-term latency (e.g., 42 days), suggesting that these stages of infection are distinct (37). Furthermore, M2 is transiently expressed during the early stages of latency (13), and M2-specific CD8 T cells can significantly limit early latency, but have no effect on long-term latency (16). Thus, other vaccination strategies may be effective against early but not long-term latency because viral gene expression or the types of latently infected cells differ during these stages of infection.
We have utilized a reactivation-deficient virus as a tool to examine the potential of a memory immune response to protect against latent infection following secondary virus challenge. In this case, it is unlikely that effective vaccination is the specific result of the use of a cyclin-deficient virus. Rather, these results likely reflect the presence of a memory response to the initial viral infection, and we speculate that many other live-attenuated viruses could function equivalently to γHV68.v-cyclin.LacZ. The use of a reactivation-deficient virus is a novel and intriguing possibility for work in the clinical setting. However, for this to be a viable strategy, a virus completely incapable of reactivation would be optimal. In this case, γHV68.v-cyclin.LacZ serves as a useful instrument to study the potential of an anti-gammaherpesvirus immune response and a standard to measure the utility of other more feasible vaccination strategies.
The mechanism of vaccination by γHV68.v-cyclin.LacZ has yet to be fully elucidated. The facts that vaccination is partially effective even at a high dose of challenge, that vaccinia virus vaccination has no effect on establishment of γHV68 latency, and that antiviral antibody is induced by γHV68.v-cyclin.LacZ vaccination and contributes to reducing latency are consistent with an antigen-specific, immune-mediated mechanism. The ability of passively transferred antiviral antibody to reduce latency in the spleen is consistent with previous work demonstrating an important role for antibody for partial vaccination against HSV-1 and -2 (1, 5, 21-23). It is possible that antibody acts to neutralize viral particles and to prevent trafficking of the virus to secondary sites or that it acts directly on infected cells. Consistent with a role for antibody in viral trafficking, passive transfer of antibody could reduce latency in the spleen but not peritoneal cells following i.p. challenge, but could reduce latency in peritoneal cells following i.n. challenge. Furthermore, recent work has demonstrated that immune antibody can alter established latent infection (10a, 14), suggesting that antibody can function at stages of chronic infection in addition to its widely recognized role in acute infection.
Although our data suggest an important role for antibody in vaccination with γHV68.v-cyclin.LacZ, antibody alone was not sufficient to reduce latency in peritoneal cells. It is possible that this result is a reflection of direct infection of the peritoneal cavity. While it is conceivable that the passively transferred antibody did not sustain high enough levels in the peritoneal cavity to alter latency after i.p. challenge, this result is also consistent with the need to induce multiple types of immune responses (e.g., CD4 T cells and antibody) in order to establish effective vaccination. This argument would be consistent with findings that neutralizing antibody is not always sufficient to protect against the development of EBV-related lymphomas in cottontop tamarins (9, 19, 40).
Because restricting acute infection is not sufficient to reduce long-term latency (described above), it is likely that some of the critical regulators of γHV68.v-cyclin.LacZ vaccination target latency-associated antigens. Although CD8 T cells were not essential for vaccination at either low- or high-dose challenge, more work will be needed to determine whether other immune components such as CD4 T cells are involved in γHV68.v-cyclin.LacZ vaccination or whether CD4 and CD8 T cells have redundant roles. In addition, it is formally possible that class I-dependent, CD8-independent T cells develop in the CD8−/− mouse, as has been described for the development of class II-dependent T cells in CD4−/− mice (18).
In the clinically relevant situation of human gammaherpesvirus-related lymphoproliferative disorders and malignancies, vaccination against latency is key to improving clinical outcome. Work presented here establishes that it is possible to generate immune responses that effectively limit gammaherpesvirus latency and suggests that priming an immune response can alter an established latent infection. Furthermore, these data provide an initial step toward understanding the mechanisms involved in effective vaccination.
Acknowledgments
H.W.V. was supported by NIH grants CA96511, CA74730, and HL60090, and S.H.S. was supported by NIH grants CA43143, CA52004, CA58524, and CA74730. S.A.T. was supported by grant 5 T32 CA09547-14 from the NIH and is a Leukemia & Lymphoma Society Fellow (no. 5609-01).
We thank members of the Speck and Virgin laboratories and members of the laboratories of David Leib, Lynda Morrison, and Paul Olivo for helpful discussions. We thank Darren Kreamalmeyer for expert assistance with mouse strains and Douglas Braaten for helpful comments on the manuscript.
REFERENCES
- 1.Asher, L. V., M. A. Walz, and A. L. Notkins. 1978. Effect of immunization on the development of latent ganglionic infection in mice challenged intravaginally with herpes simplex virus types 1 and 2. Am. J. Obstet. Gynecol. 131:788-791. [DOI] [PubMed] [Google Scholar]
- 2.Babcock, G. J., D. Hochberg, and D. A. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. [DOI] [PubMed] [Google Scholar]
- 3.Belz, G. T., P. G. Stevenson, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 2000. Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc. Natl. Acad. Sci. USA 97:2725-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, J. Y., E. M. Johnson, Jr., and P. D. Olivo. 1991. A gene delivery/recall system for neurons which utilizes ribonucleotide reductase-negative herpes simplex viruses. Virology 185:437-440. [DOI] [PubMed] [Google Scholar]
- 5.Cremer, K. J., M. Mackett, C. Wohlenberg, A. L. Notkins, and B. Moss. 1985. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science 228:737-740. [DOI] [PubMed] [Google Scholar]
- 6.Da Costa, X. J., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. USA 96:6994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty, P. C., J. P. Christensen, G. T. Belz, P. G. Stevenson, and M. Y. Sangster. 2001. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:581-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein, M. A., B. J. Randle, S. Finerty, and J. K. Kirkwood. 1986. Not all potently neutralizing, vaccine-induced antibodies to Epstein-Barr virus ensure protection of susceptible experimental animals. Clin. Exp. Immunol. 63:485-490. [PMC free article] [PubMed] [Google Scholar]
- 10.Gangappa, S., L. F. Van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin IV. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Gangappa, S., S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2002. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 76:11460-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heise, M. T., and H. W. Virgin IV. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heslop, H., C. Y. C. Ng, C. Li, C. A. Smith, S. K. Loftin, R. A. Krance, M. K. Brenner, and C. M. Rooney. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2:551-555. [DOI] [PubMed] [Google Scholar]
- 13.Husain, S. M., E. J. Usherwood, H. Dyson, C. Coleclough, M. A. Coppola, D. L. Woodland, M. A. Blackman, J. P. Stewart, and J. T. Sample. 1999. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8(+) T lymphocytes. Proc. Natl. Acad. Sci. USA 96:7508-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, I. J., E. Flano, D. L. Woodland, and M. A. Blackman. 2002. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 168:3958-3964. [DOI] [PubMed] [Google Scholar]
- 15.Klein, R. J., L. A. Kaley, and A. E. Friedman-Kien. 1984. Protection against establishment of latent infections in mice immunized with a non-pathogenic herpes simplex virus mutant and reinfected with the pathogenic parental strain. Vaccine 2:219-223. [DOI] [PubMed] [Google Scholar]
- 16.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald, M. R., X.-Y. Li, R. M. Stenberg, A. E. Campbell, and H. W. Virgin IV. 1998. Mucosal and parenteral vaccination against acute and latent murine cytomegalovirus (MCMV) infection by using an attenuated MCMV mutant. J. Virol. 72:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matechak, E. O., N. Killeen, S. M. Hedrick, and B. J. Fowlkes. 1996. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity 4:337-347. [DOI] [PubMed] [Google Scholar]
- 19.Morgan, A. J., M. Mackett, S. Finerty, J. R. Arrand, F. T. Scullion, and M. A. Epstein. 1988. Recombinant vaccinia virus expressing Epstein-Barr virus glycoprotein gp340 protects cottontop tamarins against EB virus-induced malignant lymphomas. J. Med. Virol. 25:189-195. [DOI] [PubMed] [Google Scholar]
- 20.Morrison, L. A., and D. M. Knipe. 1994. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J. Virol. 68:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, L. A., and D. M. Knipe. 1997. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology 239:315-326. [DOI] [PubMed] [Google Scholar]
- 22.Price, R. W., M. A. Walz, C. Wohlenberg, and A. L. Notkins. 1975. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science 188:938-940. [DOI] [PubMed] [Google Scholar]
- 23.Richards, C. M., C. Shimeld, N. A. Williams, and T. J. Hill. 1998. Induction of mucosal immunity against herpes simplex virus type 1 in the mouse protects against ocular infection and establishment of latency. J. Infect. Dis. 177:1451-1457. [DOI] [PubMed] [Google Scholar]
- 24.Rooney, C. M., C. A. Smith, C. Y. Ng, S. Loftin, C. Li, R. A. Krance, M. K. Brenner, and H. E. Heslop. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 345:9-13. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson, P. G., G. T. Belz, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 1999. A gamma-herpesvirus sneaks through a CD8(+) T cell response primed to a lytic-phase epitope. Proc. Natl. Acad. Sci. USA 96:9281-9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, P. G., R. D. Cardin, J. P. Christensen, and P. C. Doherty. 1999. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J. Gen. Virol. 80:477-483. [DOI] [PubMed] [Google Scholar]
- 27.Stewart, J. P., N. Micali, E. J. Usherwood, L. Bonina, and A. A. Nash. 1999. Murine gamma-herpesvirus 68 glycoprotein 150 protects against virus-induced mononucleosis: a model system for gamma-herpesvirus vaccination. Vaccine 17:152-157. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi, M., T. Otsuka, Y. Okuno, Y. Asano, and T. Yazaki. 1974. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 2:1288-1290. [DOI] [PubMed] [Google Scholar]
- 31.Tibbetts, S. A., L. F. van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usherwood, E. J., K. A. Ward, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75:8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Berkel, V., B. Levine, S. B. Kapadia, J. E. Goldman, S. H. Speck, and H. W. Virgin IV. 2002. Critical role for a high affinity chemokine binding protein in lethal meningitis caused by a γ-herpesvirus. J. Clin. Investig. 109:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin IV. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weibel, R. E., B. J. Neff, B. J. Kuter, H. A. Guess, C. A. Rothenberger, A. J. Fitzgerald, K. A. Connor, A. A. McLean, M. R. Hilleman, and E. B. Buynak. 1984. Live attenuated varicella virus vaccine. Efficacy trial in healthy children. N. Engl. J. Med. 310:1409-1415. [DOI] [PubMed] [Google Scholar]
- 40.Wilson, A. D., M. Shooshstari, S. Finerty, P. Watkins, and A. J. Morgan. 1996. Virus-specific cytotoxic T cell responses are associated with immunity of the cottontop tamarin to Epstein-Barr virus (EBV). Clin. Exp. Immunol. 103:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodland, D. L., E. J. Usherwood, L. Liu, E. Flano, I. J. Kim, and M. A. Blackman. 2001. Vaccination against murine gamma-herpesvirus infection. Viral Immunol. 14:217-226. [DOI] [PubMed] [Google Scholar]