Abstract
Human immunodeficiency virus (HIV) gp120 induces multiple cellular signaling pathways, including the phosphatidylinositol 3-kinase (PI3-kinase) pathway. The role of the PI3-kinase pathway in HIV-1 replication is not understood. Here we examined whether HIV-1 gp120 upregulates the PI3-kinase pathway and whether PI3-kinase activity plays a role in virus replication in primary human CD4+ T cells and macrophages. Soluble and virion-associated HIV-1 gp120 induced calcium mobilization and phosphorylation of the PI3-kinase downstream effectors PKB/Akt and p70 S6 kinase. gp120-induced PI3-kinase activity and calcium mobilization were inhibited by pertussis toxin and blocking antibodies directed against CCR5 and CXCR4, suggesting that the signaling is mediated through the chemokine receptor. The PI3-kinase inhibitor LY294002 inhibited infection of CD4+ T cells and macrophages with X4 and R5 HIV-1-pseudotyped viruses at concentrations that did not induce cell toxicity or downregulate HIV-1 coreceptor expression. When gp120-induced signaling was bypassed with the vesicular stomatitis virus G envelope protein, infection was still sensitive to PI3-kinase inhibition, suggesting that basal PI3-kinase activity is required for infection. LY294002 inhibited HIV-1 infection when added after viral entry and did not affect formation of the HIV-1 reverse transcriptase products R/U5 and long terminal repeat/Gag in the presence of the inhibitor. However, when the inhibitor was added after viral integration had occurred, no inhibition of HIV infection was observed. Our studies show that inhibition of the PI3-kinase signaling pathway suppresses virus infection post-viral entry and post-reverse transcription but prior to HIV gene expression. This type of host-virus interaction has implications for anti-HIV therapeutics that target cellular signaling machinery.
Human immunodeficiency virus type 1 (HIV-1) can activate multiple signaling pathways within a target cell to facilitate viral entry and replication. A number of signal transduction pathways may be activated during engagement of the HIV-1 envelope with CD4 and/or the chemokine coreceptor. Binding to CD4 causes phosphorylation of receptor tyrosine kinases such as p56Lck, which activate the Raf/MEK/ERK and phosphatidylinositol 3-kinase (PI3-kinase) pathways and indirectly activate calcium channels (6, 15, 33, 34, 38). The chemokine receptor is coupled to G-proteins, which, depending on the subunit composition, can activate adenyl cyclase, protein tyrosine kinases such as Pyk2, and phospholipase C, which catalyzes the formation of inositol 1,4,5-triphosphate, subsequently opening calcium channels on the endoplasmic reticulum (reviewed in references 18 and 37). Because these pathways ultimately regulate functions such as cytoskeletal rearrangement, cell survival, differentiation, and activation of transcription, HIV gp120-induced signal transduction may facilitate virus infection.
Previously, HIV signaling through the chemokine receptor has been considered dispensable for infection. Under conditions where signaling through the CCR5 receptor is blocked either by mutagenesis or G-protein inactivation with pertussis toxin, tumor cells are still capable of supporting viral entry and replication (3, 21). However, the phenotypes and functions of these tumor cell lines differ from those of the primary targets of HIV infection in vivo, primary macrophages and CD4+ lymphocytes, so the requirements for infection differ substantially. Only a relatively small fraction of T cells are productively infected in HIV-positive patients despite the presence of the relevant receptors, and activation of CD4+ T cells is critical for efficient viral reverse transcription and replication in these cells (29). Although HIV-1 entry can occur in quiescent T cells, there is a preintegration postentry block in replication (8). In addition, signaling through chemokine receptors by their natural ligands can enhance or suppress HIV replication in T cells and macrophages (12, 27, 28). Recent studies suggest that signal transduction by HIV envelope glycoprotein gp120 may affect host cell susceptibility to virus entry and infection in primary cells (2, 4, 10, 11, 23).
In the case of HIV-1, only viruses competent to induce signaling through the CCR5 coreceptor are able to establish productive infection within macrophages (4). Primary viral isolates and laboratory-adapted strains with gp120 envelopes that do not induce calcium mobilization enter macrophages but are unable to complete replication. This postentry block can be overcome by stimulating signaling through CCR5 with its natural ligand, MIP-1α. In addition, perturbation of coreceptor signaling with pertussis toxin markedly decreases infection of peripheral blood mononuclear cells with CXCR4-utilizing (X4) and CCR5-utilizing (R5) HIV-1 viruses (2, 23). It has been proposed that coreceptor function is important for both entry and postentry events during HIV infection (9). A role for the Raf/MEK/ERK pathway has been demonstrated for nuclear import of the HIV reverse transcriptase complex (7) and in NF-κB-driven transcription from the HIV long terminal repeat promoter (34).
The role of PI3-kinase signal transduction in HIV infection has not been fully studied. PI3-kinases are a cellular family of heterodimeric enzymes that consist of a regulatory subunit (p85) activated by tyrosine phosphorylation, which recruits inositol phospholipids, which are phosphorylated by the catalytic subunit (p110). These lipids serve as second messengers which regulate the phosphorylation of other kinases such as Akt/PKB, cyclic AMP-dependent protein kinase A, some protein kinase C isoforms, and the ribosomal S6 kinases p70 and p85 (reviewed in reference 13). Because PI3-kinase controls the activation of many different pathways, it is a critical mediator of various cellular processes such as cell migration, survival, and changes in morphology. The ability of PI3-kinase to regulate multiple cellular pathways, coupled with the need for HIV to induce an environment favorable for viral replication, prompted us to examine the role of PI3-kinase signaling in HIV-1 infection of CD4+ T cells and macrophages.
MATERIALS AND METHODS
Cell culture and signaling experiments.
Peripheral blood mononuclear cells from healthy donors were isolated by Ficoll-Hypaque gradient centrifugation. Monocyte-derived macrophages were obtained by plastic adherence for 7 to 14 days. Macrophages were >98% negative for CD3 and 80% positive for CD14 by fluorescence-activated cell sorting analysis. CD4+ T lymphocytes were magnetically separated from human peripheral blood mononuclear cells with a negative selection system (Miltenyi Biotech, Auburn, Calif.). Cells were >98% CD4 positive as determined by fluorescence-activated cell sorting analysis. Cells were activated for 3 days with 5 μg of phytohemagglutinin (Sigma, St. Louis, Mo.) and 50 U of interleukin-2 (R & D Systems, Minneapolis, Minn.) per ml. CD4+ T cells were washed in phosphate-buffered saline and resuspended in Hanks' buffered salt solution (HBSS; 10 mM HEPES [pH 7.45], 145 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 11 mM glucose) prior to treatment.
Cells were treated with 10 nM or 20 nM purified recombinant oligomeric IIIB gp120 (Immunodiagnostics Inc., Woburn, Mass.) or monomeric BaL gp120 (AIDS Research & Reference Reagent Program, Divisions of AIDS, National Institute of Allergy and Infectious Disease) at 37°C at the indicated times. For signal blockade experiments cells were treated with 200 ng of pertussis toxin per ml for 1 h at 37°C (List Biologicals, Campbell, Calif.). Cell pellets were collected by centrifugation and frozen at −70°C until detergent lysis and protein extraction.
Virus pseudotype production.
HIV-1-pseudotyped viruses were produced by transfection of 107 293T cells with 25 μg of pNL4-3.Luc.R-E- (AIDS Research & Reference Reagent Program, National Institute of Allergy and Infectious Disease, National Institutes of Health, from N. Landau, as described in reference 14) and 12.5 μg of HIV-1 envelope plasmid (either JRFL or HXB2; gift of D. Littman, New York University) or vesicular stomatitis virus envelope plasmid (pMD.G.; gift of D. Trono, University of Geneva, Geneva, Switzerland) with Lipofectamine 2000 reagent (Life Technologies, Inc., Rockville, Md.).
In the pNL4-3 construct, the luciferase gene is inserted in nef, and Nef is considered nonfunctional (14). For luciferase viruses used to infect macrophages, an additional plasmid, pCMVΔR8.2 (gift of D. Trono; as described in reference 31), was used to complement the vpr deficiency. Mock virus control was produced as above but with omission of the viral envelope plasmid. Virus production was boosted with 8 mM sodium butyrate for 4 h at 37°C, and viral supernatants were harvested 2 days later. Viral supernatants were centrifuged at 2,000 rpm for 3 min, filtered through 0.45-μm filter, DNase I treated, and concentrated with a Centricon Plus-20 membrane with a 100,000 molecular weight cutoff (Millipore Corporation, Bedford, Mass.) to remove contaminating cytokines and growth factors which might interfere with signal transduction analysis. Concentrated virus was stored in aliquots at −70°C until use. Stock virus titers were determined with a colorimetric reverse transcriptase activity assay (Roche Molecular Biochemicals, Indianapolis, Ind.).
Calcium mobilization assays.
Calcium mobilization in response to chemokine and HIV-1 gp120 stimulation in T cells was measured according to the method of Rabin et al. (35). Briefly, CD4+ T lymphocytes were loaded with 20 μM Indo-1-AM (Molecular Probes, Inc., Eugene, Oreg.) for 45 min at 37°C. Cells were washed and resuspended in HBSS and incubated at 37°C for 15 min prior to stimulation. Approximately 2 × 106 cells were analyzed per treatment. Indo-1 fluorescence was collected and analyzed with the Summit Software program and the MoFlo high speed cell sorter (Cytomation, Inc., Fort Collins, Colo.) at 390/320 nm and 530/520 nm for bound and free probe, respectively. Cells were collected for 30 s prior to stimulation and treated with HBSS and the calcium ionophore ionomycin (Calbiochem Corporation, San Diego, Calif.) as negative and positive controls, respectively, for calcium flux.
Functional CCR5 and CXCR4 receptor expression was confirmed by calcium mobilization in response to 50 nM of their respective ligands RANTES and SDF-1α (R & D Systems). To demonstrate specificity, cells were treated with 10 μg of anti-CCR5 or anti-CXCR4 blocking antibodies (R & D Systems) or mouse isotype immunoglobulin G2b (IgG2b) control per ml for 30 min at 37°C. Data were gated on live activated CD4-positive T cells on the basis of forward and side scatter profiles and surface marker staining with phycoerythrin-conjugated CD4 antibodies (BD Pharmingen, San Diego, Calif.). Data were analyzed with the kinetics platform of FlowJo (Tree Star Inc., San Carlos, Calif.), and percent response was computed by subtracting baseline values prior to stimulation.
Western blot analysis.
Protein concentration was determined with the Bio-Rad (Hercules, Calif.) DC protein assay, and 40 μg of protein per lane was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nylon-reinforced nitrocellulose membrane. Samples were analyzed by immunoblotting with antibodies against phosphorylation-specific and total signaling proteins. All antibodies were from Cell Signaling Technology (Beverly, Mass.) except for the phospho-Pyk2-specific antibody (Calbiochem Corp.) and the antibody against total Pyk2 (Transduction Laboratories, Lexington, Ky.). All immunoblots were visualized with enhanced chemiluminescence Western blotting detection reagents (Amersham, Buckinghamshire, United Kingdom). Densitometry was performed with Un-Scan-It Gel 3.4 software (Silk Scientific Corp., Orem, Utah). Density values for phosphorylated proteins were expressed as a ratio relative to the total amount of protein loaded.
Infection assays.
Experiments were performed under serum-free conditions in AIM V medium (Life Technologies Inc.) for activated lymphocytes and RPMI 1640 medium (Sigma) for macrophages. Cells were treated for 1 h at 37°C with 5 to 20 μM LY294002 (Sigma), 100 nM wortmannin (Sigma), 50 μM benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-FMK) (Calbiochem) or dimethyl sulfoxide (DMSO) control prior to infection with HIV-pseudotyped luciferase reporter virus. Unless otherwise noted, inhibitors were present during the entire infection. Triplicates of 7 × 104 CD4+ T cells were infected with 7.25 ng of reverse transcriptase from HIV-1 HXB2- or JRFL-pseudotyped virus at 37°C. Triplicates of 3 × 105 macrophages were infected with 0.28 ng of reverse transcriptase of JRFL-pseudotyped virus (with Vpr) at 37°C. Cells were lysed 24 h postinfection, and luciferase expression was measured with the Promega (Madison, Wis.) luciferase assay system. Units were normalized relative to total protein concentration for each sample.
Specific inhibition by the added inhibitors was determined by immunoblotting for their phospho-specific target. For experiments to test the effects of LY294002 on postentry events, cells were either treated for 1 h at 37°C with 20 μM LY294002, 100 nM TAK779, a CCR5 inhibitor (AIDS Research & Reference Reagent Program, National Institute of Allergy and Infectious Disease, National Institutes of Health, as described in reference 5), 1 μM AMD3100, a CXCR4 inhibitor (gift of J. P. Moore, Weill Medical College of Cornell University, New York, as described in reference 19), or 1 mM zidovudine (Sigma) prior to infection, or inhibitors were added following 2 h of incubation with virus and washed to remove unbound virus. In some experiments, 20 μM LY294002 was added 24 h postinfection, and cells were harvested after an additional 24 h of incubation at 37°C. Statistical P values were calculated for comparing two independent sample means with Student's t test.
Cell viability and coreceptor expression analysis.
Cell toxicity in response to inhibitors after 24 h of incubation was determined in CD4+ T cells and macrophages with the CellTiter96 aqueous nonradioactive proliferation assay (Promega), which measures reduction of MTS to formazan by dehydrogenase enzymes in metabolically active cells. The effect of inhibitors on expression levels of cell surface receptors was determined by staining with allophycyanin-, fluorescein isothiocyanate-, and phycoerythrin-conjugated antibodies against CD4, CCR5, and CXCR4 and their corresponding isotype controls (BD Pharmingen). Fluorescence staining was measured and analyzed with the FACSCalibur analytical flow cytometer (BD Immunocytometry Systems, San Jose, Calif.) with CellQuest software.
Semiquantitative PCR analysis.
DNA was extracted from cells with the QIAamp DNA blood minikit (Qiagen Inc., Valencia, Calif.), and 50 ng of template DNA was used per PCR amplification. Each PCR mix contained a 200 μM concentration of each of the four deoxynucleoside triphosphates, a 0.2 μM concentration of each primer, 2 mM MgCl2, 1× GeneAmp PCR buffer II, and 1.25 U of Amplitaq DNA polymerase (Applied Biosystems). Reaction mixes were subjected to an initial 2-min denaturation step at 95°C and then 35 cycles consisting of 1 min at 91°C, 2 min at 65°C, and 1 min at 72°C in a DNA thermal cycler (Perkin Elmer).
The primers used to detect each sequence (Invitrogen) were as follows: R/U5 forward M667, 5′-GGC TAA CTA GGG AAC CCA CTG-3′; R/U5 reverse AA55, 5′-CTG CTA GAG ATT TTC CAC ACT GAC-3′ (41); long terminal repeat/gag forward M667 (described above); long terminal repeat/gag reverse M661, 5′-CCT GCC TCG AGA GAG CTC CTC TGG-3′, derived from the sequence of HIV-1 pNL4-3 (1); GAPDH forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′; and GAPDH reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′ (20).
To control for HIV DNA in the virus inoculum, PCR amplification was performed on viral preparations heated at 70°C for 10 min to inactivate DNase I and lysed in 0.1% Triton X-100 for 1 h at 37°C. Quantification of HIV-1 DNA during PCR amplification was performed by analyzing a standard curve of dilutions of plasmid containing DNA sequence of HIV-1 pNL4-3 (pNL4-3:d1443:EGFP:Δenv from M. Husain, Mt. Sinai School of Medicine [MSSM], New York, N.Y.; as described in reference 24). PCR products were separated by electrophoresis on a 2% agarose gel and analyzed with the FluorChem 8800 imaging system (Alpha Innotech, San Leandro, Calif.).
RESULTS
HIV-1 gp120 activates multiple signal transduction pathways, including the PI3-kinase pathway.
We tested the ability of HIV-1 gp120 to upregulate various signal transduction pathways in primary activated CD4+ T cells. When HIV-1 gp120 or chemokines, such as MIP-1α or SDF-1α, bind to CCR5 or CXCR4 receptors, G-proteins are activated which catalyze calcium mobilization from intracellular stores (37). Approximately 10 to 15% of CD4+ cells mobilized calcium within 1 min in response to 20 nM recombinant oligomeric IIIB (X4) gp120 or 20 nM BaL (R5) gp120 (Fig. 1A and B). The magnitude of the signaling responses were consistent with those induced by chemokine receptor stimulation with SDF-1α, RANTES, and MIP-1α (data not shown).
FIG. 1.
HIV-1 gp120 induction of calcium mobilization in primary CD4+ T lymphocytes. (A) Phytohemagglutinin-activated CD4+ T lymphocytes were loaded with the calcium indicator dye indo-1 AM (20 μM), and the ratio of FL5/FL6 fluorescence was analyzed by flow cytometry immediately following treatment with 10 μM ionomycin and HBSS as positive and negative controls, respectively (left panel), 20 nM oligomeric IIIB (X4) g120 (middle panel), or 20 nM BaL (R5) gp120 (right panel). Acquisition data shown are representative of four independentexperiments. (B) Data acquired in A were analyzed with the FlowJo kinetics platform. The percentage of cells responding to gp120 in A is shown. (C) Calcium mobilization in activated CD4+ T cells in response to either control mock virus preparation lacking envelope, 15 ng of reverse transcriptase of JRFL (R5) HIV-1-pseudotyped virus per ml, or 50 nM MIP-1α. Calcium flux was measured as described above (A and B), with percentages of cells responding to each stimulation shown. To demonstrate specificity, T cells were treated with anti-CCR5 (gray bars) or mouse isotype control IgG2b (black bars) antibodies for 15 min at 37°C before stimulation. The anti-CCR5 antibody completely blocked calcium flux in response to JRFL. Data are representative of two independent experiments.
Recombinant protein may not be an accurate representation of the in vivo conformation of gp120 presented on the viral envelope; therefore, signaling studies were repeated in this context. JRFL-pseudotyped HIV-1 (R5) and HXB2-pseudotyped HIV-1 (X4) replication-defective virions induced calcium mobilization in activated CD4+ T cells (Fig. 1C and data not shown). Specific blocking antibodies against the CCR5 receptor abolished R5 HIV-1-induced calcium mobilization the same way that they blocked induction by MIP-1α, a natural ligand of CCR5 (Fig. 1C). Antibodies against CXCR4 also reduced the X4 HIV-1-induced response (data not shown).
To rule out effects of nonspecific contaminants in the viral preparation, a mock virus produced in the same manner as the HIV-pseudotyped viruses was used as a control and failed to induce calcium mobilization in CD4+ T cells (Fig. 1C).
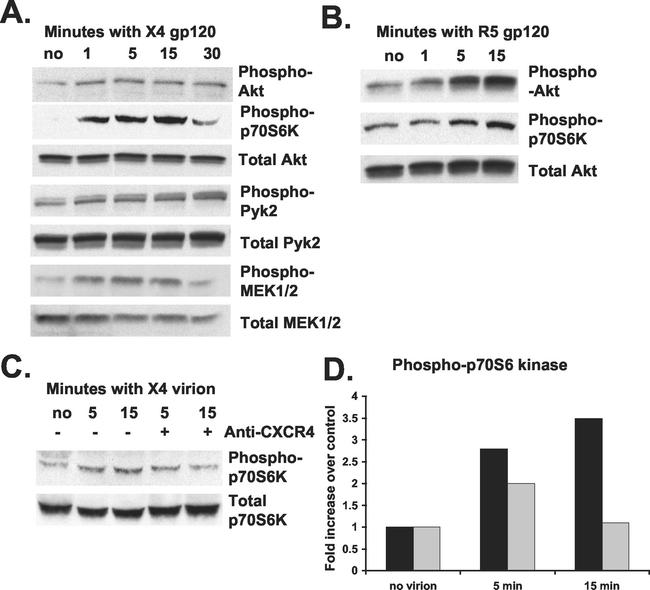
Recombinant oligomeric IIIB (X4) gp120 at 10 nM upregulated phosphorylation of PI3-kinase downstream targets Akt and phospho-p70 S6 kinase within 1 min of treatment (Fig. 2A). Maximum phosphorylation of Akt was obtained 1 to 5 min after gp120 treatment, and activation was sustained for 30 min following treatment (Fig. 2A). Furthermore, nonreceptor tyrosine kinase Pyk2 and mitogen-activated protein kinase pathway kinases MEK1 and MEK2 were also phosphorylated between 1 and 30 min of gp120 treatment (Fig. 2A). The magnitude of these phosphorylation responses was consistent with only a small percentage of cells responding to gp120, as shown by the calcium mobilization experiment (Fig. 1A and B).
FIG. 2.
HIV-1 gp120 induction of PI3-kinase activity in CD4+ T lymphocytes. (A) Phytohemagglutinin-activated CD4+ T lymphocytes were treated with 10 nM oligomeric IIIB (X4) gp120 for the times indicated at 37°C. Total protein was extracted and analyzed by immunoblotting the same blot with antibodies specific for the proteins indicated at the right of the panels. Data are representative of five independent experiments. (B) Activated CD4+ T cells were treated with 10 nM BaL (R5) gp120 for the times indicated at 37°C. Total protein was extracted and analyzed as in A. Data are representative of three independent experiments. (C) Activated CD4+ T cells were treated with anti-CXCR4 antibody (+) or mouse IgG2b isotype control (−) for 30 min at 37°C prior to treatment with 15 ng of reverse transcriptase of concentrated HXB2 (X4) HIV-1-pseudotyped luciferase virus per ml for 5 and 15 min at 37°C. (D) Phosphorylation of p70 S6 kinase in C was analyzed by immunoblotting, and densitometry results are expressed as the increase in the levels of phosphorylated protein over that in the untreated control. Results are shown for cells treated with anti-CXCR4 antibody (gray bars) or IgG2b isotype control (black bars). Data are representative of two independent experiments.
Although there was some donor-to-donor variability in the level of constitutive Akt phosphorylation prior to gp120 stimulation, an increase of Akt phosphorylation in response to gp120 from 1.5- to 3-fold was consistently observed. In general, CD4+ T cells from donors that showed high levels of basal Akt phosphorylation had relatively lower upregulation elicited by gp120. These observations were similar to those reported for Pyk2 phosphorylation in primary macrophages (16). Phosphorylation levels of Akt, p70 S6 kinase, Pyk2, and MEK1/2 were increased 1 to 15 min after treatment with 10 nM BaL (R5) gp120 (Fig. 2B and data not shown).
CCR5 and CXCR4 are coupled to pertussis-sensitive G proteins (reviewed in reference 37), so calcium flux and protein kinase phosphorylation in response to gp120 were measured following treatment with 200 ng of pertussis toxin per ml. Pertussis toxin completely prevented calcium mobilization, and Pyk2 and Akt phosphorylation in response to oligomeric IIIB gp120 (data not shown), implicating the chemokine coreceptor in this signaling.
Signaling studies were also performed with HXB2 (X4) HIV-1-pseudotyped viruses, and these were able to upregulate phosphorylation of Pyk2 and p70 S6 kinase approximately threefold (Fig. 2C and D and data not shown). Pretreatment of cells with anti-CXCR4 antibodies decreased virion-induced p70 S6 kinase phosphorylation to basal levels (Fig. 2C and D). These studies demonstrate that PI3-kinase is activated by soluble as well as virion-associated HIV-1 gp120 in primary CD4+ T lymphocytes and that this activation is mediated via the coreceptor.
PI3-kinase activity is required for productive HIV infection of CD4+ T cells.
Since we have established that the PI3-kinase pathway is upregulated in primary CD4+ T cells in response to HIV, we wanted to see whether PI3-kinase activity is required for productive HIV-1 infection. Cells were treated with the PI3-kinase inhibitor LY294002 and infected with HIV-pseudotyped single-pass luciferase reporter viruses. Pretreatment of CD4+ T cells with 20 μM LY294002 inhibited infection by HXB2-HIV-1 (X4) approximately 70% and by JRFL-HIV-1 (R5) approximately 80% compared to DMSO-treated controls (Fig. 3A). Pretreatment with 100 nM wortmannin, a chemically distinct pharmacological inhibitor of PI3-kinase, also inhibited HIV-1 infection of CD4+ T cells (data not shown).
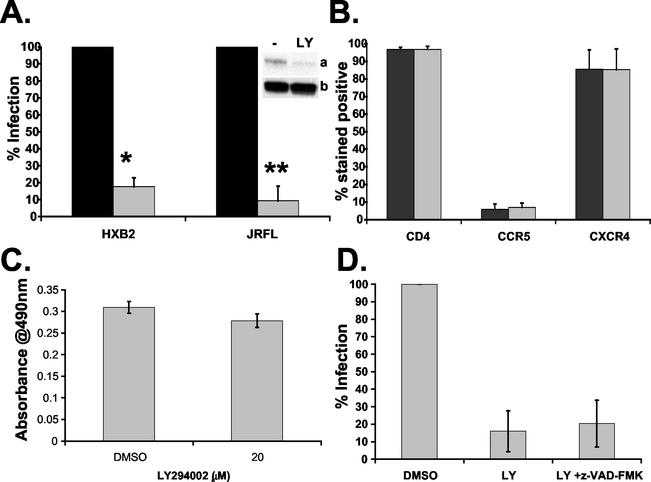
FIG. 3.
Effect of LY294002 on HIV-1 infection of CD4+ T lymphocytes. (A) Activated CD4+ T cells were treated with 20 μM LY294002 (gray bars) or DMSO control (black bars) for 1 h at 37°C prior to infection with either HXB2 (X4) or JRFL (R5) HIV-1-pseudotyped luciferase virus. Luciferase activity was measured 24 h postinfection and normalized to total protein concentration. Uninfected background levels of luciferase were subtracted from total luciferase activity. Results are expressed as a percentage of the DMSO control infection. Data are averages from four or more independent experiments, where * and ** indicate P < 0.0001. Inset, confirmation of PI3-kinase inhibition by immunoblotting for phosphorylated Akt (a) and total Akt protein (b) following LY294002 treatment. (B) Activated CD4+ T cells were treated with DMSO (gray bars) or 20 μM LY294002 (black bars) for 60 min at 37°C prior to staining with allophycyanin-, fluorescein isothiocyanate-, and phycoerythrin-conjugated antibodies against CD4, CCR5, and CXCR4 and their corresponding isotype controls. Fluorescence staining was measured by flow cytometry, and data are averages of two independent experiments, with the ranges indicated. (C) Cell viability of activated CD4+ T lymphocytes incubated with 20 μM LY294002 or DMSO control for 24 h at 37°C measured with the Promega CellTiter96 aqueous MTS assay. Over 90% of cells were viable after 24 h of incubation with 20 μM LY294002 (P > 0.05). (D) Activated CD4+ T cells were treated with 20 μM LY294002 in the absence or presence of 50 μM z-VAD-FMK for 1 h at 37°C prior to infection with HXB2-HIV-1-pseudotyped luciferase virus. Percenta infection was determined as described for A. Data are averages of two independent experiments, with the range indicated by bars.
LY294002-mediated inhibition of PI3-kinase activity was confirmed by immunoblotting for phosphorylated Akt (Fig. 3A, inset). Pretreatment of activated CD4+ T cells with 20 μM LY294002 inhibited both basal and gp120-induced Akt phosphorylation, confirming that this event was PI3-kinase dependent (data not shown). Nonspecific inhibition of other signaling pathways by 20 μM LY294002 was not observed in these cells when we examined other phosphorylated signaling intermediates, including Pyk2 and MEK1/2 (data not shown). To determine if the inhibition of infection by LY294002 was due to downregulation of surface expression of HIV-1 receptors, CD4, CCR5, and CXCR4 expression was analyzed on CD4+ T cells. LY294002 at 20 μM did not cause any changes in the levels of CD4, CCR5, or CXCR4 expression in CD4+ T cells compared to the DMSO-treated control (Fig. 3B).
To ensure that the concentration range and duration of LY294002 treatment that inhibits PI3-kinase activity has no apparent cellular toxicity, dose-response studies were carried out with the inhibitor on activated CD4+ T cells. LY294002 at concentrations of 1 to 20 μM did not induce significant cell toxicity in primary CD4+ T cells compared to the DMSO-treated control (Fig. 3C and data not shown).
Inhibition of PI3-kinase is a potent inducer of apoptosis in many cell types (40). Even though the concentration of LY294002 used did not induce significant cell toxicity in the MTS assay, it was important to ascertain that the inhibitory effect on infection was not due to apoptotic cell death. To examine the role of LY294002-induced apoptosis in the observed inhibition, cells were treated with the cell permeable broad-spectrum caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-FMK) (50 μM). z-VAD-FMK inhibits caspase-mediated apoptosis in a wide variety of mammalian cell types (36). If apoptosis was the inhibitory mechanism of LY294002 on HIV infection, one would expect that z-VAD-FMK would rescue HIV infection of T cells. However, z-VAD-FMK did not rescue X4- or R5-mediated HIV infection of CD4+ T cells (Fig. 3D and data not shown).
Taken together, these results show that PI3-kinase activity is involved in HIV infection of primary CD4+ lymphocytes by both X4 and R5 viruses. The inhibition of HIV infection was not due to downregulation of coreceptor, cellular toxicity, or apoptotic cell death.
PI3-kinase activity is required for productive HIV-1 infection of macrophages.
We have shown that PI3-kinase activity was required for infection of proliferating T cells. However, the requirement may be different for infection of nondividing cells, such as macrophages. Thus, we tested the effect of PI3-kinase inhibition on HIV-1 infection in primary macrophages. LY294002 inhibited JRFL-HIV-1 infection in macrophages in a dose-dependent manner (Fig. 4A). LY294002 at 20 μM caused over a 75% decrease in HIV infection (Fig. 4B). Similarly, pretreatment with another PI3-kinase inhibitor, 100 nM wortmannin, also inhibited HIV-1 infection in macrophages (data not shown). LY294002 did not cause significant cell toxicity in macrophages (data not shown), and the caspase inhibitor z-VAD-FMK did not significantly affect infection inhibition by 20 μM LY294002 (Fig. 4C), suggesting that cell toxicity and apoptosis were not involved. Thus, PI3-kinase activity appears to be involved in infection of nondividing macrophages in addition to proliferating T cells.
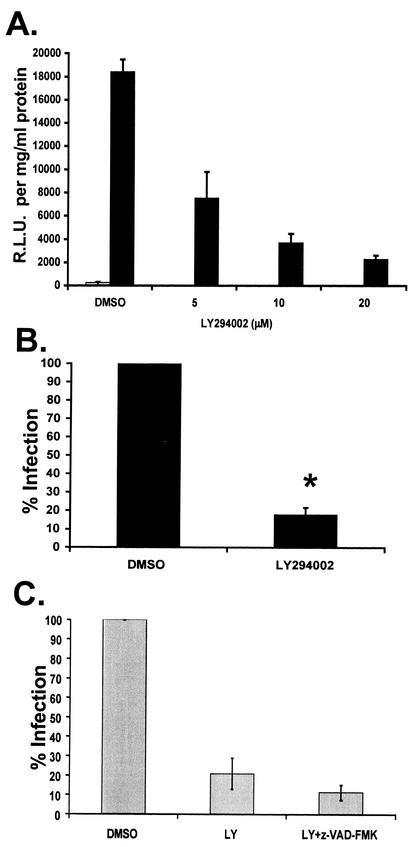
FIG. 4.
Effect of LY294002 on HIV-1 infection of macrophages. (A) Primary human macrophages were treated with 5 to 20 μM LY294002 or the DMSO control for 1 h at 37°C prior to infection with JRFL-HIV-1-pseudotyped luciferase virus. Twenty-four hours postinfection, luciferase activity and total protein concentrations were measured. Infection is expressed as relative luciferase units (R.L.U.), normalized to total protein concentration minus background. Infected samples (black bars) and uninfected control samples (gray bar) are indicated. Data are representative of two independent experiments. Error bars represent standard deviations of triplicate samples. (B) Macrophages were treated with 20 μM LY294002 or DMSO control for 1 h at 37°C prior to infection with JRFL-HIV-1-pseudotyped luciferase virus. Twenty-four hours postinfection, luciferase activity was measured as in A. Results are expressed as percentage of infection of the DMSO-treated control. Data are averages from three or more independent experiments (*, P < 0.0001). (C) Macrophages were treated with 20 μM LY294002 in the absence or presence of 50 μM z-VAD-FMK for 1 h at 37°C prior to infection with JRFL-HIV-1-pseudotyped luciferase virus. Percent infection was determined as in B. Data are averages of three independent experiments.
PI3-kinase activity regulates HIV-1 infection in T cells post-viral entry.
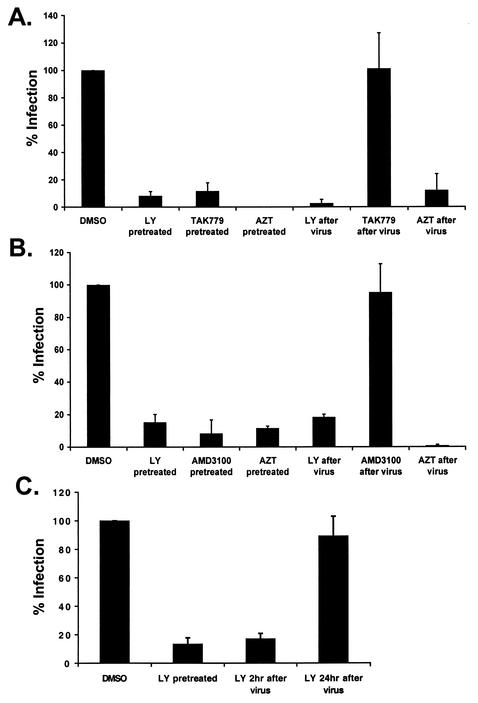
To delineate which steps of HIV replication are regulated by PI3-kinase, cells were treated with inhibitors either before or after a 2-h incubation with virus and removal of unbound virus. The R5 and X4 entry inhibitors TAK779 and AMD3100, respectively, and the reverse transcriptase inhibitor zidovudine were included as controls. LY294002 and zidovudine decreased infection of CD4+ T cells with both R5- and X4-pseudotyped HIV-1 luciferase viruses regardless of whether they were added before or after virus entry (Fig. 5A and B). In contrast, the HIV-1 entry inhibitors TAK779 and AMD3100 lost their ability to protect CD4+ T cells from infection with R5 and X4 viruses, respectively, when added after virus entry.
FIG. 5.
LY294002 inhibits HIV-1 infection of CD4+ T cells post-viral entry. Activated CD4+ T cells were treated either for 1 h before infection or immediately after virus entry with DMSO, 20 μM LY294002, 100 nM TAK779, 1 μM AMD3100, or 1 mM zidovudine. Twenty-four hours postinfection, cells were harvested, and percent infection was determined as described for Fig. 3. Results are averages of two or more independent experiments for infection with HIV-1 luciferase viruses pseudotyped with JRFL (A), HXB2 (B), or vesicular stomatitis virus (C). For vesicular stomatitis virus (C), LY294002 was also added 24 h postinfection, and cells were harvested after an additional 24 h of incubation.
LY294002 also inhibited infection of T cells with a vesicular stomatitis virus-pseudotyped HIV-1 luciferase virus after virus entry (Fig. 5C). However, when LY294002 was added after viral integration at 24 h postinfection, it failed to inhibit HIV infection (Fig. 5C). These results suggest that PI3-kinase activity is involved in HIV-1 infection following viral entry but prior to viral integration and gene expression. Furthermore, inhibition of basal PI3-kinase activity, as seen with the vesicular stomatitis virus-pseudotyped virus, results in significant inhibition of infection.
PI3-kinase activity regulates HIV-1 infection in T cells post-reverse transcription.
We further determined whether LY294002 inhibited infection before or after reverse transcription. Semiquantitative PCR was performed to amplify HIV-1 strong-stop (R/U5) and full-length (long terminal repeat/Gag) reverse transcriptase products, which represent early and late reverse transcriptase transcripts, respectively. LY294002 at 20 μM did not decrease the amount of HIV-1 R/U5 and long terminal repeat/Gag products obtained following infection with HXB2- or JRFL-pseudotyped viruses (Fig. 6A and B). The reverse transcriptase products detected represent mainly de novo synthesis, because less than 15 copies of the long terminal repeat/Gag and R/U5 reverse-transcribed DNA products were detected in the viral preparations (data not shown). Taken together, these results suggest that PI3-kinase activity regulates HIV infection not only post-viral entry but also post-reverse transcription.
FIG. 6.
LY294002 inhibits HIV-1 infection of CD4+ T cells post-viral reverse transcription. (A) Semiquantitative PCR was performed on DNA extracted from CD4+ T cells 24 h postinfection with HXB2- and JRFL-pseudotyped HIV-1 luciferase viruses following treatment with DMSO or 20 μM LY294002. Primers were used to amplify the HIV-1 reverse transcriptase products R/U5 (top panel) and long terminal repeat (LTR)/Gag (middle panel) as well as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (bottom panel) as a control. In parallel, PCR was performed on an HIV-1 sequence-containing plasmid with defined copy number to construct a standard curve. Data are representative of three independent experiments. (B) Quantification of data shown in panel A was performed with the Alpha Innotech FluorChem 8800 imaging system. Copy numbers of HIV-1 R/U5 (topgraph) and HIV-1 long terminal repeat/Gag (bottom graph) are shown for LY294002-treated samples (gray bars) and DMSO controls (black bars). There was no significant difference in long terminal repeat/Gag copies between LY294002- and DMSO-treated infected cells (P > 0.5) and that in R/U5 copies between LY294002- and DMSO-treated infected cells (P > 0.1).
DISCUSSION
We demonstrate for the first time that PI3-kinase activity is required for a robust productive infection of two primary physiological targets of HIV-1 infection, CD4+ T lymphocytes and macrophages. Our study shows that PI3-kinase-mediated signal transduction plays a crucial role in HIV replication at post-viral entry and post-reverse transcription stages but prior to viral integration and gene expression. Signal transduction events can affect many of the downstream events required for HIV-1 infection, such as the cytoskeletal rearrangement involved in virus entry and nuclear import. Cytochalasin D, a specific inhibitor of F-actin polymerization, inhibits viral entry in peripheral blood mononuclear cells (25) and inhibits association of the reverse transcriptase complex with the cytoskeleton, thus decreasing the formation of HIV-1 reverse transcriptase products in infected HeLa cells (7).
Other studies demonstrated a role for signal transduction through the chemokine receptor in postentry events during infection (4, 9). Different HIV-1 isolates are blocked postentry, at either the reverse transcriptase or nuclear import stage of viral replication in CD4-expressing macaque cells, and this restriction can be overcome by expressing and stimulating the human coreceptor (9). HIV-1 isolates with envelopes unable to signal through CCR5 fail to replicate in macrophages due to a postentry block that can be overcome by alternatively stimulating signaling through the receptor with CC chemokines (4). Studies are currently under way to determine whether PI3-kinase activity is required for nuclear import during HIV-1 infection.
Other pathogens also exploit the host signaling machinery to establish productive infections. Vaccinia virus is able to mimic the Src family kinase signaling pathways involved in the control of actin polymerization at the plasma membrane in order to favor its own actin-based motility (22). Influenza A virus activates the Raf/MEK/ERK cascade during infection, a process which is essential for virus production and viral ribonucleoprotein complex export from the nucleus during the viral life cycle (32). The human cytomegalovirus upregulates the PI3-kinase pathway, and this activation is required for viral DNA synthesis (26).
We also show that the PI3-kinase effectors Akt and p70 S6 kinase are phosphorylated in response to both soluble and virion-associated R5 and X4 gp120 in primary CD4+ T lymphocytes. Previously it has been demonstrated, in the human T-lymphoid cell line CEM-T4, that binding of HIV-1 NL4-3 (X4) virus results in rapid and strong tyrosine phosphorylation of the CD4-associated PI3-kinase p85α regulatory subunit (6). However, these studies were limited to tumor cell lines and did not address the role of the chemokine coreceptor in this signaling. We found that some of the PI3-kinase activation could be inhibited by treatment with pertussis toxin or by antibodies directed against the chemokine receptors, thus implicating signal transduction from the chemokine receptor in this process. In addition, others have shown that pertussis toxin, a G-protein inhibitor, can inhibit infection of activated peripheral blood mononuclear cells with R5 HIV-1 at the stage of viral entry and infection with X4 virus downstream of entry (2). It is interesting to speculate whether this inhibitory effect on X4 HIV-1 infection can be attributed to disruption of downstream chemokine receptor signaling pathways such as the PI3-kinase pathway examined in our study.
We propose a model in which HIV infection activates cellular PI3-kinase pathways and these cell signaling pathways promote viral replication. The gp120-induced PI3-kinase could act in an autocrine fashion to enhance replication and infection, probably with a contribution from additional viral or cell-associated inducers of PI3-kinase activity. HIV-1 gp120 only induced calcium mobilization and PI3-kinase activity in a small subset of CD4+ T lymphocytes. However, the PI3-kinase inhibitor would affect a much higher percentage of cells in that it would inhibit both basal and induced PI3-kinase. The effect of the inhibitor on infection could reflect inhibition of both basal and gp120-induced PI3-kinase activity. In fact, when we bypassed gp120-induced PI3-kinase activation with a vesicular stomatitis virus G protein-pseudotyped virus, HIV infection was still sensitive to the PI3-kinase inhibitor. This suggests that basal or non-gp120-induced PI3-kinase activity is required for HIV infection but does not rule out an additional effect of gp120-induced PI3-kinase on infection because LY294002 inhibited both gp120-induced and basal PI3-kinase activity in our experiments.
In addition, other studies have shown that blockade of signaling induced by HIV envelope inhibits infection (2, 4, 23). Other HIV-1 proteins, such as Tat and Nef, have been shown to activate PI3-kinase/Akt-dependent survival pathways (17, 39). Recently, PI3-kinase has been implicated in the activation of Nef-associated p21-activated kinase through binding of the regulatory subunit p85 to Nef (30). In that study, inhibition of PI3-kinase decreased viral particle production in COS and Jurkat cells, an effect which was Nef dependent. This is in contrast to our work, where PI3-kinase inhibition decreased infection of primary macrophages and T cells with Nef-defective virus prior to integration. These differences may reflect differences between primary and transformed cells.
Identifying the physiological targets of gp120-induced signaling in the immune system will yield valuable insights into the mechanisms of disease pathogenesis as well as identify potential cellular targets for anti-HIV therapeutics. An understanding of the role of chemokine receptor signaling, especially in response to virally presented gp120, is particularly important given the development of small-molecule inhibitors currently under study as potential anti-HIV therapeutics.
Acknowledgments
This work was supported by National Institutes of Health/NIDDK grant PO1 DK56492-01.
We thank Theresa Chang for helpful discussions and critical reading of the manuscript and the Mt. Sinai Flow Cytometry Shared Research Facility for assistance with T-lymphocyte calcium assays and analysis of cell surface receptors.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano, M., H. Schmidtmayeriva, C. A. Amella, T. Pushkarsky, and M. Bukrinsky. 1999. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J. Exp. Med. 190:597-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhatib, G., M. Locati, P. E. Kennedy, P. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. [DOI] [PubMed] [Google Scholar]
- 4.Arthos, J., A. Rubbert, R. L. Rabin, et al. 2000. CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J. Virol. 74:6418-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba, M., O. Nishimura, N. Kanzaki, et al. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briand, G., B. Barbeau, and M. Tremblay. 1997. Binding of HIV-1 to its receptor induces tyrosine phosphorylation of several CD4-associated proteins including the phosphatidylinositol 3-kinase. Virology 228:171-179. [DOI] [PubMed] [Google Scholar]
- 7.Bukrinskaya, A., B. Brichacek, A. Mann, and M. Stevenson. 1998. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J. Exp. Med. 188:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky, M. I., T. L. Stanwick, M. P. Dempsey, and M. Stevenson. 1991. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chackerian, B., E. M. Long, P. A. Luciw, and J. Overbaugh. 1997. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 71:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicala, C., J. Arthos, M. Ruiz, M. Vaccarezza, A. Rubbert, A. Riva, K. Wildt, O. Cohen, and A. S. Fauci. 1999. Induction of phosphorylation and intracellular association of CC chemokine receptor 5 and focal adhesion kinase in primary human CD4+ T cells by macrophage-tropic HIV envelope. J. Immunol. 163:420-426. [PubMed] [Google Scholar]
- 11.Cicala, C., J. Arthos, S. M. Selig, et al. 2002. HIV envelope induces a cascade of cell signals in nonproliferating target cells that favor virus replication. Proc. Natl. Acad. Sci. USA 99:9380-9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocchi, F., A. L. Devico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Luso. 1995. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 13.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 335:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor, R. I., B. K. Chen, S. Choe, and N. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 15.Davis, C. B., I. Dikic, D. Unutmaz, C. M. Hill, J. Arthos, M. A. Siani, D. A. Thompson, J. Schlessingeer, and D. R. Littman. 1997. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J. Exp. Med. 186:1793-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Corno, M., Q.-H. Liu, D. Schols, E. de Clerq, S. Gessani, B. D. Freedman, and R. G. Collman. 2001. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pretussis toxin-insensitive chemokine receptor signaling. Blood 98:2909-2916. [DOI] [PubMed] [Google Scholar]
- 17.Deregibus, M. C., V. Cantaluppi, S. Doublier, M. F. Brizzi, I. Deambrosis, A. Albini, and G. Camussi. 2002. HIV-1-Tat protein activates phosphatidylinositol 3-kinase/Akt-dependent survival pathways in Kaposi's sarcoma cells. J. Biol. Chem. 277:25195-25202. [DOI] [PubMed] [Google Scholar]
- 18.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 19.Donzella, G. A., D. Schols, S. W. Lin, et al. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 20.Ercolani, L., B. Florence, M. Denaro, and M. Alexander. 1988. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J. Biol. Chem. 30:15335-15341. [PubMed] [Google Scholar]
- 21.Farzan, M., H. Choe, K. A. Martin, Y. Sun, M. Sidelko, C. R. Mackay, N. P. Gerard, J. Sodroski, and C. Gerard. 1997. HIV-1 entry and macrophage inflammatory-1β-mediated signaling are independent functions of the chemokine receptor CCR5. J. Biol. Chem. 272:6854-6857. [DOI] [PubMed] [Google Scholar]
- 22.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signaling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 23.Guntermann, C., B. J. Murphy, R. Zheng, A. Qureshi, P. A. Eagles, and K. E. Nye. 1999. Human immunodeficiency virus-1 infection requires pertussis toxin sensitive G-protein coupled signaling and mediates cAMP downregulation. Biochem. Biophys. Res. Commun. 256:429-435. [DOI] [PubMed] [Google Scholar]
- 24.Husain, M., G. L. Gusella, M. E. Klotman, I. H. Gelman, M. D. Ross, E. J. Schwartz, A. Cara, and P. E. Klotman. 2002. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J. Am. Soc. Nephrol. 13:1806-1815. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar, S., J. E. Hildreth, and D. H. Schwartz. 1998. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J. Virol. 72:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, R. A., X. Wang, X.-L. Ma, S.-M. Huong, and E.-S. Huang. 2001. Human cytomegalovirus upregulates the phosphatidylinositol 3-kinase (PI3-kinase) pathway: inhibition of PI3-kinase activity inhibits viral replication and virus-induced signaling. J. Virol. 75:6022-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly, M. P., H. M. Naif, S. L. Adams, A. L. Cunningham, and A. R. Lloyd. 1998. Dichotomous effects of β-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J. Immunol. 160:3091-3095. [PubMed] [Google Scholar]
- 28.Kinter, A., A. Catanzaro, J. Monaco, et al. 1998. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4+ T cells: role of signal transduction. Proc. Natl. Acad. Sci. USA 95:11880-11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korin, Y. D., and J. A. Zack. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type I reverse transcription in T cells. J. Virol. 72:3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linneman, T., Y.-H. Zheng, R. Mandic, and B. Peterlin. 2002. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology 294:246-255. [DOI] [PubMed] [Google Scholar]
- 31.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and P. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 32.Pleschka, S., T. Wolf, C. Erhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signaling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 33.Popik, W., J. E. Hesselger, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates the expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6404-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popik, W., and P. M. Pitha. 1996. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol. Cell. Biol. 16:6532-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabin, R. L., M. K. Park, F. Liao, R. Swofford, D. Stephany, and J. M. Farber. 1999. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J. Immunol. 162:3840-3850. [PubMed] [Google Scholar]
- 36.Slee, E. A., H. Zhu, S. C. Chow, M. MacFarlane, D. W. Nicholson, and G. M. Cohen. 1996. Benzyloxycarbonyl-Val-Ala-Asp-(OMe)fluoromethylketone (z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem. J. 315:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thelen, M. 2001. Dancing to the tune of chemokines. Nat. Immunol. 2:129-134. [DOI] [PubMed] [Google Scholar]
- 38.Veillete, A., M. A. Bookman, E. M. Horak, L. Samelson, and J. B. Bolen. 1989. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine protein kinase p56Lck. Nature 338:257-259. [DOI] [PubMed] [Google Scholar]
- 39.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, and A. Schurmann. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 40.Yao, R., and G. M. Cooper. 1995. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267:2003-2006. [DOI] [PubMed] [Google Scholar]
- 41.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]