Abstract
T-cell costimulation molecules B7-1 and B7-2 play an important role in activation of T cells to cytolytic effector function and production of cytokines. Interaction with B7 also causes T cells to upregulate surface molecules, such as CD40L, that effectively stimulate antibody responses in conjunction with cytokines. We have shown that mice lacking both B7-1 and B7-2 (B7KO mice), when infected intravaginally with virulent herpes simplex virus type 2 (HSV-2), developed more severe disease and higher mortality than their wild-type counterparts. We have now investigated the effects of B7 costimulation deficiency on induction of immune responses to HSV-2 infection of the genital tract. Fewer gamma interferon (IFN-γ)-producing T cells were present in the genital lymph nodes of B7KO mice compared to wild-type mice, either acutely after primary infection or in recall responses. Less IFN-γ and especially interleukin-10 were produced by B7KO mice, and cytolytic T-lymphocyte activity was also attenuated. Reduced expression of CD25 on CD4+ T cells after infection of B7KO mice was consistent with deficits in T-cell activation to effector functions. Although HSV-specific immunoglobulin M (IgM) titers were comparable for both B7KO mice and wild-type mice, B7KO mice had significant deficits in HSV-specific serum IgG responses, with markedly reduced levels of IgG2a and IgG1. In addition, significantly less IgG was detected in the vaginal secretions of B7KO mice than in those from wild-type mice. CD4+ T-cell expression of CD40L was depressed in B7KO mice in vivo and in vitro. Together with reduced cytokine production, these results suggest a mechanism for decreased IgG class switching or production. Thus, in the absence of B7 costimulation, naïve T cells fail to undergo proper activation in response to HSV-2, which limits T-cell cytokine production, cytotoxic T lymphocyte activity, and provision of help for class-switched antibody responses.
T-cell activation is the central event in the evolution of antigen-specific cellular and most humoral immune responses. Activation is dependent upon engagement of an appropriate antigen-major histocompatibility complex (MHC) complex and a signal mediated by engagement of costimulation molecules. Numerous T-cell costimulation partners have now been described (8). Each appears to have its own niche in regulation of primary and memory immune responses. The B7-1 and B7-2 costimulation molecules were the first described and have been the best characterized. Interaction between B7 costimulation molecules B7-1 and B7-2 with their T-cell ligands CD28 and CTLA-4 is central to T-cell expansion (26) and induction of primary T-cell helper and cytotoxic T-lymphocyte (CTL) responses (18, 25, 51). Costimulation via B7 molecules also influences the development of class-switched antibody responses (4, 27).
The milieu in which immune responses develop during virus infection is more complex than that to a single foreign protein. This may be especially true of immune responses to large, complex viruses such as herpes simplex virus (HSV), which expresses more than 80 viral proteins in infected cells. The importance of B7 costimulation for T-cell activation and function in the context of virus infections has been an area of intense investigation. The effector T-cell response to virus infection has two principal facets, gamma interferon (IFN-γ) secretion and CTL activity. Blockade of B7 interactions by use of mice infused with or expressing CTLA-4-immunoglobulin (Ig) fusion protein, a soluble form of the B7 ligand, revealed an adverse effect on IFN-γ production by CD8+ T cells during the response to influenza virus (28) and adenovirus (61). Expansion and activation of primary CD8+ CTL is reduced in response to influenza virus and vesicular stomatitis virus infections (28, 63), but not to lymphocytic choriomeningitis virus (63). Memory CTL responses to lymphocytic choriomeningitis virus are unaffected (56, 63). Using mice with disruptions of the B7-1 and B7-2 loci (B7KO mice), McAdam et al. (29) confirmed a deficit in CTL induction in response to vesicular stomatitis virus infection and provided new evidence for a deficiency in memory CTL responses.
Lack of B7 costimulation also has marked effects on the antibody response to virus infection. Whereas the initial IgM response is normal (29, 63), IgG responses to several viruses are decreased (28, 29, 61, 63), suggesting a defect in class switching. B7KO mice infected with vesicular stomatitis virus have lower titers of virus-specific IgG2a response and reportedly lack an IgG1 response (29). The level of antigen replication does not influence the extent of immune alteration caused by B7 deficiency. Interestingly, lack of CD40-CD40 ligand (CD40L) interaction, another form of costimulatory signal influential in B-cell responses, results in the same class-switched antibody deficiency as seen when B7 costimulatory pathways are interrupted (5, 42, 60, 61). These observations have led to speculation that the two pathways are related. Despite extensive investigations into specific immune deficits caused by loss of B7 costimulation, the mechanisms underlying them are not completely understood.
Immune responses to HSV infection have provided a useful model system with which to investigate the importance of B7 costimulation in response to virus infection. In mice infused with CTLA-4-Ig and infected in the footpad with HSV-1, CD8+ CTL responses ex vivo are diminished and, as was seen with vesicular stomatitis virus, memory CTL maintenance is depressed (13). The first significant observations regarding the influence of B7 costimulation on antiviral CD4+ T-cell responses were also made. A transient decrease in the expansion of IFN-γ-producing CD4+ T cells in draining lymph nodes during the acute response to HSV-1 infection was observed. Lower interleukin (IL)-2 production by virus-responsive T cells was also noted and was theorized to be responsible for the limited expansion of IFN-γ-producing cells (13).
CTLA-4-Ig administration causes a reduction in IFN-γ synthesis during the recall response to UV-inactivated HSV (15), suggesting an inhibitory effect on HSV-specific CD4+ T-cell activation. The impact of disrupted B7-ligand interactions on humoral responses to HSV is less clear. HSV-specific IgG levels are lower in mice infused with CTLA-4-Ig (15). However, whereas IgG class-switched antibody responses to influenza virus (28) and vesicular stomatitis virus (29) show decreased IgG2a and especially IgG1, a surprising increase in the amount of IgG1 produced in response to HSV infection was previously noted (15).
In a mouse model of genital infection with HSV-2, virus replicates in defined locations at the site of mucosal inoculation and in sensory neurons innervating the mucosa. We have previously shown that mice genetically deficient in the B7-1 and B7-2-mediated costimulation pathways are more susceptible than wild-type mice to severe infection after intravaginal inoculation of HSV-2 (57). T cells (30, 33, 43) and IFN-γ produced by them (32, 44) play important roles in clearance of HSV infection from the genital tract, and HSV-specific antibody helps protect the nervous system from infection (12, 38, 49). We therefore hypothesized that the increased pathogenicity of HSV-2 in mice that lack the B7 costimulation molecules was related to decreased primary T-cell responses in the genital lymph nodes and a deficiency in T-cell help for antibody production.
In this study, we examined T-cell effector functions and antibody responses to intravaginal infection with HSV-2 in B7KO mice. Our results expand upon previous observations of deficits in T-cell activation to effector functions, and by examining soluble and cell-associated forms of help for immune response induction, we elucidate a mechanism by which deficits in class-switched antibody responses in the absence of B7 costimulation likely occur.
MATERIALS AND METHODS
Viruses.
An attenuated, thymidine kinase-deficient strain of HSV-2, 186tkΔKpn (10), was used in immune response studies to promote survival of infected mice. An infectious but nonreplicating strain of HSV-2, 5BlacZ (9), was used to infect stimulator cells in vitro. Virus stocks were grown and virus titer was determined in Vero cells and S2 cells (16), respectively, as previously described (23, 37). UV inactivation of 5BlacZ (36) reduced its titer on S2 cells by >4 log10 PFU.
Mice.
Female mice genetically deficient in B7-1 and B7-2 (B7KO mice) (4), backcrossed for 10 generations onto a BALB/c background, were used at 6 weeks of age. B7KO and age-matched BALB/c mice (National Cancer Institute, Frederick, Md.) were housed under specific-pathogen-free conditions in accordance with institutional and federal guidelines.
Infection.
Female mice were injected subcutaneously in the neck ruff with medroxyprogesterone acetate (Depo-Provera; Pharmacia and UpJohn) (3 mg/mouse in a 100-μl volume) 5 days prior to intravaginal infection. On the day of infection, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (1.6 mg/mouse), swabbed with a type 1 calcium alginate swab (Puritan, Guilford, Maine), and inoculated intravaginally with a 5-μl volume containing 3 × 105 PFU of HSV-2 strain 186tkΔKpn.
CTL assays.
Spleens and genital lymph nodes were harvested 5 days after infection of mice with 186tkΔKpn. Single-cell suspensions were prepared, and splenic mononuclear cells were isolated by centrifugation over lymphocyte separation medium (ICN, Costa Mesa, Calif.). Cells were suspended at a concentration of 107 cells/ml in RPMI containing 5% fetal calf serum and 50 μM β-mercaptoethanol and stimulated in vitro for 5 days with syngeneic splenocytes infected at a multiplicity of infection of 5 with 186tkΔKpn and then γ-irradiated (2,000 rads). A20-1.11 or P815 syngeneic target cells were uninfected or infected for 15 h with HSV-2 186tkΔKpn before use.
Effector and target cells were cultured together in triplicate at ratios of 20:1, 50:1, and 100:1. After 5 h of culture, a 50-μl volume of supernatant from each well was transferred to a fresh 96-well microtiter plate and stored at −80°C. CTL activity was determined by measuring lactate dehydrogenase release from the cytosol of target cells with a lactate dehydrogenase detection kit (CytoTox96 nonradioactive cytotoxicity assay kit; Promega, Madison, Wis.). Supernatants were thawed and subjected to a colorimetric assay according to the manufacturer's instructions, and absorbance was read at 490 nm. Percent cytotoxicity was calculated as [(experimental sample release − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release)] × 100.
Intracellular cytokine staining.
Genital lymph nodes and spleens were collected 4 days (acute) or 4 weeks (memory) following infection of B7KO or BALB/c mice with 186tkΔKpn. Alternatively, mice were reinfected 4 weeks after primary infection, and genital lymph nodes and spleens were collected 4 days later (recall). Single-cell suspensions were prepared as described above, and cells were stimulated in vitro in RPMI containing 2% fetal calf serum and phorbol myristate acetate (50 ng/ml), calcium ionophore A23187 (1 μg/ml), and GolgiStop (1 μl/1.5 ml; PharMingen, San Diego, Calif.) for 5.5 h. Following stimulation, cell surface staining was performed with 0.5 μg of anti-CD4 conjugated to fluorescein isothiocyanate or 0.5 μg anti-CD8 conjugated to fluorescein isothiocyanate (both from Caltag, Burlingame, Calif.), and intracellular staining was performed with anti-IFN-γ conjugated to phycoerythrin (PharMingen) with a Cytostain kit (PharMingen) according to the manufacturer's instructions. Staining was visualized by flow cytofluorometric analysis on a FACSCalibur cytometer (Becton Dickinson, San Diego, Calif.). Ten thousand gated events were collected for each sample, and analysis was conducted with CellQuest software (Becton Dickinson).
Cytokine quantitation.
Spleens and genital lymph nodes were harvested from mice 5 days (acute) or 4 weeks (memory) after primary infection or 5 days after secondary (recall) infection, and single-cell suspensions were prepared as described above. Cells (106 cells/well) were cultured in 96-well plates in medium alone (RPMI with 5% fetal calf serum and 50 μM β-mercaptoethanol) or with UV-inactivated HSV-2 (5BlacZ at a multiplicity of infection equivalent to 0.5) or with 5 × 105 syngeneic splenocytes infected at a multiplicity of infection of 3 to 5 with HSV-2 5BlacZ for 16 h and subjected to 2,000 rads of γ-irradiation. To wells containing medium or UV-HSV, 5 × 104 uninfected, irradiated feeder cells were also added. Supernatants were collected after 48 h of culture at 37°C in 5% CO2. Cytokine concentrations (IFN-γ, IL-4, and IL-10) were determined by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (38).
CD25 and CD40L expression.
Spleen and genital lymph nodes were collected 4 days after infection of mice with 186tkΔKpn. Single-cell suspensions were prepared as described above, and cells (106) were stained with anti-CD4-fluorescein isothiocyanate or anti-CD8-fluorescein isothiocyanate (Caltag) and 0.5 μg of anti-CD25-phycoerythrin (Cedarlane, Ontario, Canada) or 1 μg of biotinylated anti-CD40L (PharMingen) followed by streptavidin-CyChrome in phosphate-buffered saline containing 0.2% bovine serum albumin and 0.1% azide. Staining was visualized by flow cytofluorometric analysis. Ten thousand gated events were collected and analyzed as described above.
Quantitation of serum and secreted antibodies.
To determine the titer and isotype of HSV-specific serum antibodies, blood was collected from the tail vein of mice at various times postinfection with 186tkΔKpn. Serum was prepared by clot retraction and analyzed by ELISA as previously described (35). For the IgG ELISA, anti-mouse IgG-biotin (R&D Systems, Minneapolis, Minn.) was used as the secondary antibody, followed by streptavidin-horseradish peroxidase (Zymed, San Francisco, Calif.). For the IgG2a, IgG1, and IgM ELISAs, anti-mouse IgG2a-horseradish peroxidase, anti-mouse IgG1-horseradish peroxidase, and anti-mouse IgM-horseradish peroxidase (all from Southern Biotechnology Associates, Birmingham, Ala.) were used, respectively. Plates were developed with o-phenylenediamine hydrochloride (Sigma, St. Louis, Mo.) and read at 490 and 630 nm in a Biotek EL340 plate reader. Antibody titers were determined by comparison to standard curves generated with purified antibody of known concentration captured on plates coated with goat anti-mouse kappa light-chain antibody (Caltag).
Statistical analyses.
Significance of differences in antibody titers on individual days was determined by t test.
RESULTS
The influence of B7 costimulation molecules on the development of T-cell effector function in response to HSV was examined. BALB/c and B7KO mice were infected intravaginally with attenuated HSV-2, and splenocytes and genital lymph node cells were analyzed at various times postinfection.
CTL activity.
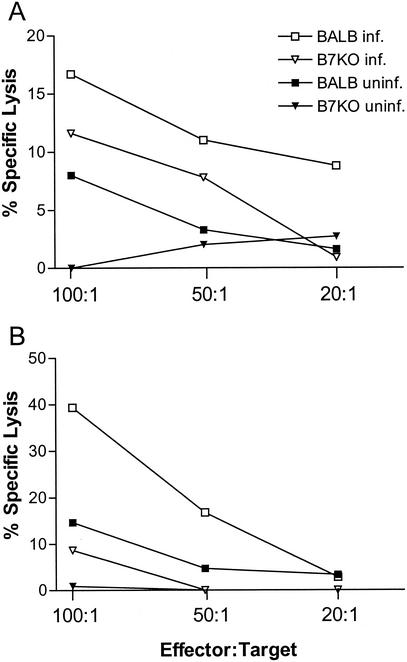
Five days after intravaginal infection, mononuclear cells from the genital lymph nodes and spleens of B7KO and wild-type mice were restimulated in vitro and tested for CTL activity against infected P815 (MHC class I+) and A20 (MHC class I/II+) target cells. Splenocyte cultures from BALB/c mice had a greater capacity to lyse A20 target cells than did cultures from B7KO mice at all effector-to-target cell ratios (Fig. 1A). The deficit in B7KO CTL activity was more pronounced in the genital lymph nodes, where only 9% lysis occurred at an effector-to-target cell ratio of 100:1, compared to 40% lysis by cells from BALB/c mice (Fig. 1B). Lysis of P815 target cells by splenocytes and genital lymph node cells was minimal (data not shown), suggesting that CTL activity in response to infection of the vaginal mucosa was principally mediated by MHC class II-restricted T cells. These results indicate that development of CTL effector function in B7KO mice is impaired in comparison with BALB/c mice and that cells with functional CTL activity are concentrated closer to the site of infection.
FIG. 1.
HSV-specific cytotoxic T-lymphocyte responses. Following intravaginal inoculation, spleens and lymph nodes were harvested from B7KO and BALB/c mice, and mononuclear cells were cultured in vitro for 5 days in the presence of HSV-2-infected, γ-irradiated syngeneic splenocytes. After in vitro stimulation, (A) splenocytes and (B) genital lymph node cells were cocultured for 5 h with uninfected (uninf.) or HSV-infected (inf.) A20 target cells. Supernatants of each culture were then harvested, and lactate dehydrogenase release was assessed as a measure of CTL activity. Data shown are from one of two experiments performed with similar results.
Cytokine-producing cells.
Intracellular cytokine staining and flow cytofluorometric analysis of genital lymph node cells from mice undergoing acute infection revealed that fourfold more CD8+ T cells from BALB/c mice produced IFN-γ than did those from B7KO mice (Table 1, experiment 1). In contrast, no clear difference existed between BALB/c and B7KO mice in either the numbers of IFN-γ-producing CD4+ T cells in the genital lymph nodes or the numbers of IFN-γ-producing CD4+ and CD8+ T cells in the spleen. However, during the memory phase of the response to HSV-2, four- to fivefold more IFN-γ-producing CD8+ and CD4+ T cells were maintained in the spleens of BALB/c mice compared with B7KO mice (Table 1, experiment 2). Upon secondary infection, fivefold more IFN-γ-producing CD8+ T cells were again found in the genital lymph nodes of BALB/c mice than B7KO mice, suggesting that expansion and/or recruitment of cytokine-producing cells in B7KO mice was adversely affected by lack of B7 costimulation.
TABLE 1.
Cytofluorometric quantitation of IFN-γ-producing cells in B7KO and BALB/c micea
| Expt no. and responseb | Mice | Number of lymphocytes staining for IFN-γ (104/mouse)
|
|||
|---|---|---|---|---|---|
| Genital lymph nodes
|
Spleen
|
||||
| CD8+ | CD4+ | CD8+ | CD4+ | ||
| 1 | |||||
| Naïve | BALB/c | 0.1 | 0.1 | 76.2 | 46.2 |
| B7KO | 0.3 | 0.2 | 143.6 | 17.0 | |
| Acute | BALB/c | 19.7 | 9.9 | 129.4 | 51.8 |
| B7KO | 4.8 | 12.4 | 153.0 | 94.6 | |
| 2 | |||||
| Memory | BALB/c | 0.9 | 1.3 | 441.8 | 842.0 |
| B7KO | 0.6 | 0.6 | 79.3 | 66.3 | |
| Recall | BALB/c | 71.5 | 31.2 | 381.8 | 172.7 |
| B7KO | 13.3 | 23.1 | 345.6 | 379.1 | |
Cell surface staining for CD4 and CD8 and intracellular staining for IFN-γ were performed after 6 h of incubation with phorbol myristate acetate, calcium ionophore, and GolgiStop. Each experiment was repeated once with similar results.
Acute, analyzed 4 days after intravaginal infection with 3 × 106 PFU of HSV-2; memory, analyzed 28 days after immunization; recall, analyzed 4 days after intravaginal challenge with HSV-2.
Cytokine production.
Spleen and genital lymph node cells from mice 5 days postinfection were stimulated in vitro with either UV-inactivated virus or infected, irradiated splenocytes, and supernatants were collected for cytokine quantitation by ELISA. UV-inactivated HSV-2 would be expected to preferentially stimulate CD4+, MHC class II-restricted T cells; infected, irradiated splenocytes would preferentially stimulate CD8+, MHC class I-restricted T cells. Splenocytes from BALB/c mice secreted more IFN-γ into the culture medium in response to either stimulus than did those from B7KO mice (Table 2; P = 0.06 to 0.08), even though BALB/c and B7KO splenocytes contained equivalent numbers of IFN-γ-producing cells (Table 1, experiment 1). Considering that baseline levels of IFN-γ were higher in BALB/c mice, it appears that B7KO mice responded well to infection by production of IFN-γ but that the overall amount of IFN-γ produced during infection was inferior to that produced by BALB/c mice.
TABLE 2.
Cytokine production in B7KO and BALB/c mice following primary infectiona
| Cytokine | Stimulusb | Cytokine concn (ng/ml) in culture supernatant
|
|||
|---|---|---|---|---|---|
| Genital lymph nodes
|
Spleen
|
||||
| B7KO | BALB/c | B7KO | BALB/c | ||
| IFN-γ | Medium | 0.33 | 1.58 | <0.02 | 0.66 |
| UV-virus | 14.18 | 8.08 | 11.81 | 25.18 | |
| Infect/irrad | 9.87 | 11.09 | 14.86 | 21.24 | |
| IL-4 | Medium | <0.02 | <0.02 | <0.02 | <0.02 |
| UV-virus | <0.02 | <0.02 | 0.11 | 0.27 | |
| Infect/irrad | <0.02 | 0.05 | 0.28 | 0.24 | |
| IL-10 | Medium | <0.02 | <0.02 | 0.06 | 0.08 |
| UV-virus | 1.00 | 4.02 | 2.40 | 4.60 | |
| Infect/irrad | 1.17 | 6.84 | 2.47 | 8.86 | |
Mononuclear cells were harvested 5 days after challenge and cultured with the indicated stimulus for 48 h before supernatant collection. Standard errors of the mean were ≤12% of th indicated values and were omitted for clarity. Values are from one of two independent experiments with similar results. Limit of detection, 0.02 ng/ml.
UV-virus, UV-inactivated virus; Infect/irrad, HSV-2-infected and γ-irradiated splenocytes.
More IL-4 was produced by BALB/c splenocytes, but only in response to UV-inactivated virus (Table 2; P = 0.01). Genital lymph node cells of B7KO and BALB/c mice secreted equivalent amounts of IFN-γ at 5 days postinfection. Either antigenic stimulus provoked two- to sixfold more IL-10 from both splenocytes and lymph node cells of BALB/c mice compared to B7KO mice (Table 2; P ≤ 0.0001). Cellularity of genital lymph nodes and spleens was comparable between the two mouse strains, suggesting that differences seen in cytokine production in vitro would translate to differences in total amounts of cytokines produced in vivo.
During recall responses to genital infection with HSV-2, BALB/c splenocytes and lymph node cells produced more IFN-γ than cells from B7KO mice (Table 3), even though they had equivalent numbers of IFN-γ-producing cells in the spleen at that time (Table 1, experiment 2). IFN-γ production by genital lymph nodes of BALB/c mice responding to the infected- and irradiated-cell stimulus was twofold greater than that by genital lymph nodes of B7KO mice, but only slightly greater in response to the UV-inactivated virus (Table 3). IL-10 production was again higher in spleen and lymph nodes of BALB/c mice than B7KO mice (Table 3), but IL-4 production was not significantly different between the two mouse strains. Taken together, these results suggest that BALB/c splenocytes were more efficient producers of IFN-γ but that IFN-γ production by lymph node cells from B7KO mice was normal despite lower numbers of IFN-γ-producing CD8+ T cells. The results also suggest that CD4+ T cells may be the principal contributor to IFN-γ production. IL-10 synthesis during both the acute and recall responses was significantly greater when B7-1 and B7-2 could be expressed.
TABLE 3.
IFN-γ production in B7KO and BALB/c mice following secondary infectiona
| Cytokine | Stimulus | Cytokine concn (ng/ml) in culture supernatants
|
|||
|---|---|---|---|---|---|
| Genital lymph nodes
|
Spleen
|
||||
| B7KO | BALB/c | B7KO | BALB/c | ||
| IFN-γ | Medium | <0.02 | 0.06 | <0.02 | <0.02 |
| UV-virus | 8.88 | 11.17 | 5.63 | 13.77 | |
| Infect/irrad | 3.36 | 7.16 | 3.50 | 10.03 | |
| IL-4 | Medium | 0.33 | 0.19 | 0.15 | 0.10 |
| UV-virus | 0.19 | 0.13 | 0.47 | 0.23 | |
| Infect/irrad | 0.13 | 0.15 | 0.32 | 0.19 | |
| IL-10 | Medium | <0.02 | <0.02 | <0.02 | <0.02 |
| UV-virus | <0.02 | 1.85 | 0.28 | 0.52 | |
| Infect/irrad | <0.02 | 1.76 | <0.02 | 0.74 | |
Mononuclear cells were harvested 4 days after infection and cultured with the indicated stimulus for 48 h before supernatant collection. For other details, see Table 2, footnotes a and b.
CD25 expression.
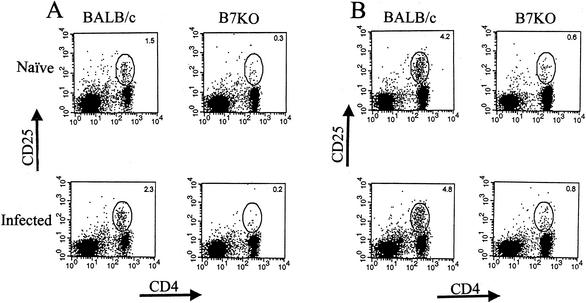
Cells that strongly express the high-affinity IL-2 receptor are the most likely candidates for immediate precursors of effector cells (31). Because B7KO mice have reduced CTL and cytokine responses to HSV-2, we examined the activation state of CD4+ T cells by analyzing CD25 expression following intravaginal infection with HSV-2. Compared with B7KO mice, cells in the genital lymph nodes (Fig. 2A) and spleens (Fig. 2B) of BALB/c mice had a greater percentage of CD4+ T cells also staining for CD25 and a larger increase over background levels in naïve mice. At 7 days postinfection, more CD4+ T cells in both mouse strains expressed CD25, but BALB/c mice still had a greater percentage of CD4+ CD25+ cells. Small numbers of CD8+ T cells expressed CD25 in BALB/c mice by day 7, with fewer in B7KO mice (data not shown). These results indicate that fewer T cells are activated in B7KO mice, which suggests that they are not as capable of expansion and differentiation to effector functions as T cells from wild-type mice.
FIG. 2.
CD25 expression on CD4+ T cells. At 4 days following infection, (A) spleen and (B) genital lymph node cells from infected BALB/c and B7KO mice were analyzed by flow cytometry with antibodies specific for CD4 and CD25. Numbers within each quadrant represent the percentages of the cells within the lymphocyte gate. Cells from two to five mice were pooled. Data shown are from one of two experiments performed with similar results.
Antibody responses.
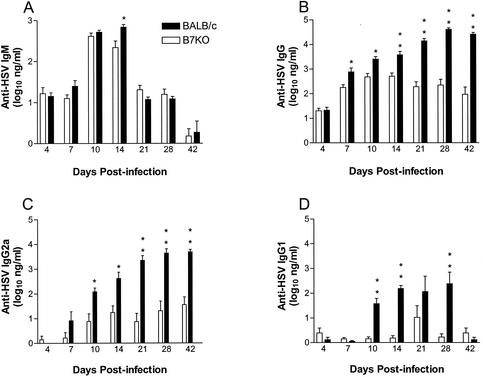
To determine the extent to which lack of B7 costimulation influenced B-cell class switching and antibody production, serum was collected from BALB/c and B7KO mice at intervals of up to 6 weeks after intravaginal infection with HSV-2 and assayed for HSV-specific antibodies by ELISA (Fig. 3). The HSV-specific IgM response rose in both B7KO and BALB/c mice by 10 to 14 days after infection to a peak of 500 ng/ml and then subsided (Fig. 3A). The only statistically significant difference in titer observed between the groups occurred at day 14. In contrast, HSV-specific IgG responses were consistently lower in B7KO mice (Fig. 3B). B7KO IgG responses peaked at day 14 and then reached a nadir on day 42 at a level 310-fold lower than that in BALB/c mice. Titers in BALB/c mice continued to climb through day 28 postinfection and were maintained to 42 days postinfection.
FIG. 3.
Antibody production in mice after intravaginal infection. Serum was collected from groups of six to seven mice at the indicated times after intravaginal inoculation of HSV-2 and analyzed for HSV-specific antibody titers by ELISA. (A) IgM; (B) IgG; (C) IgG2a; (D) IgG1. Geometric mean titers ± standard error of the mean are from one of two experiments performed that yielded similar results. *, P < 0.007; **, P < 0.0006.
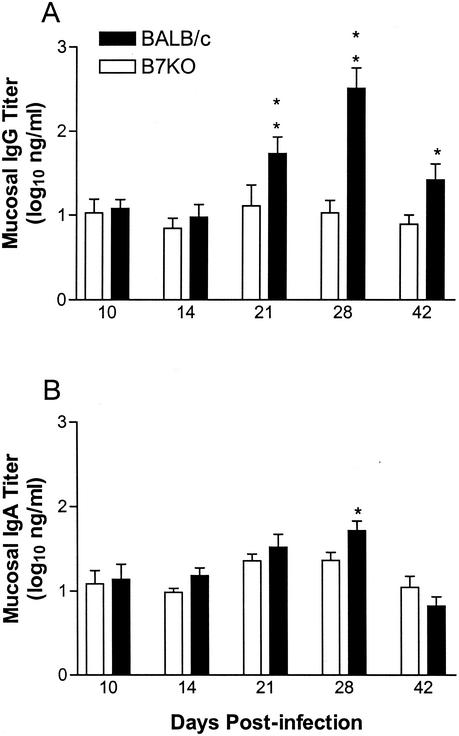
This deficit in B7KO antibody titer was reflected in the HSV-specific IgG2a (Fig. 3C) and IgG1 (Fig. 3D) isotype titers, which both remained steady at low levels in B7KO mice but increased over time in BALB/c mice. At the peak of the response in BALB/c mice, IgG1 and IgG2a titers were approximately 150-fold and 230-fold higher, respectively, than levels in B7KO mice. HSV-specific IgG titers in the vaginal lumens of BALB/c mice mirrored production in the serum, with a peak in IgG at day 28 (Fig. 4A). In comparison, IgG titers in B7KO mice remained constant at a level that was 20-fold lower. Relatively little HSV-specific IgA was produced by either mouse strain (Fig. 4B), and B7KO mice did not show a deficiency compared to BALB/c mice, suggesting that IgA class switching and production are controlled by mechanisms independent of B7 costimulation.
FIG. 4.
HSV-specific antibody in vaginal lumens of infected mice. Vaginal washes were obtained from each mouse in the experiment shown in Fig. 3 28 days after infection and analyzed for HSV-specific (A) IgG and (B) IgA levels by ELISA. Values represent geometric mean titers ± standard error of the mean. *, P < 0.034; **, P < 0.0004.
CD40 ligand expression.
The relative shortage of IFN-γ that we had observed in B7KO mice may have contributed to lower levels of IgG2a produced in B7KO mice because this cytokine helps drive B-cell maturation and class switching to IgG2a (53, 54). A second stimulus to the B-cell differentiation process is CD40L, which contributes to IgG1 isotype switching and antibody production (5, 46, 61). We therefore tested the hypothesis that in B7KO mice with reduced T-cell activation, failure of CD4+ T cells to upregulate CD40L expression leads to diminished CD40L-dependent IgG1 antibody responses.
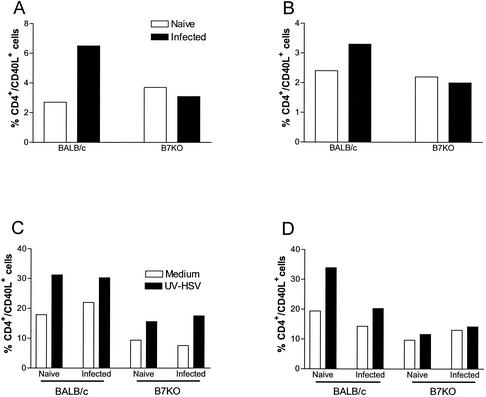
BALB/c and B7KO mice were infected intravaginally with HSV-2, and T cells were analyzed for CD40L expression directly ex vivo 4 days after infection. The percentage of CD4+ CD40L+ T cells more than doubled in the genital lymph nodes of BALB/c mice in response to infection, but no increase was observed in B7KO mice (Fig. 5A). A more modest increase was also seen in the spleen of BALB/c but not B7KO mice (Fig. 5B). These in vivo results indicated that the B7KO mice do not upregulate expression of CD40L as efficiently following virus infection as their wild-type counterparts.
FIG. 5.
Percentage of CD4+ T cells expressing CD40 ligand. Splenocytes and genital lymph node cells from B7KO and BALB/c mice were harvested 4 days after infection with HSV-2 and stained for expression of CD4 and/or CD40L. (A) Genital lymph node cells from mice stained directly ex vivo; (B) splenocytes stained directly ex vivo; (C) genital lymph node cells from mice stimulated for an additional 2 days in vitro with UV-inactivated HSV-2; and (D) splenocytes from mice stimulated in vitro. Data are from one of two experiments performed with similar results.
As shown in Fig. 5C, further in vitro stimulation with UV-inactivated HSV-2 caused CD4+ T cells from B7KO genital lymph nodes to upregulate CD40L expression comparably to those from BALB/c mice. The overall percentage of CD4+ T cells expressing CD40L still lagged, however. In splenocyte cultures, a deficit in induction of CD40L expression on B7KO cells remained (Fig. 5D). These in vitro results indicate that while CD40L is expressed following further stimulation of T cells, significantly fewer CD4+ T cells express CD40L in B7KO mice compared to wild-type mice. Because fewer CD40L+ cells are observed both in vivo and in vitro, we conclude that less CD40L is available to stimulate B cells via CD40 cross-linking and that this likely contributes to the deficit in IgG class switching and/or production.
DISCUSSION
Relationship of decreased T-cell activation to pathology.
Development of antiviral Th1 effector functions involves IL-2-dependent expansion as well as differentiation signals provided by IL-12 and costimulation molecules (31, 39). B7 binding to CD28 transduces a signal for IL-2 production and T-cell proliferation (26). Edelmann and Wilson (13) observed decreased expansion of HSV-specific CD4+ T cells in vitro in the presence of CTLA-4-Ig, which could be partially restored by addition of IL-2. We have demonstrated that activation of T cells to express CD25 for IL-2 responsiveness is impaired in HSV-infected B7KO mice, providing in vivo evidence of decreased proliferative potential. Weaker activation of T cells to expand in B7KO mice, as evidenced by reduced numbers of CD4+ CD25+ T cells, provides one possible mechanism for the reduced CTL activity and cytokine secretion observed. Lack of B7-CD28 interaction may alternatively have direct effects on development of effector functions.
Acute infection of B7KO mice results in more severe genital and neurologic disease and mortality than infection of BALB/c mice (57). The T-cell response to acute infection with HSV-2 mediates virus clearance from the mucosa and limits virus-induced pathology (33, 43). IFN-γ-producing CD4+ T-cell and CD4+ CTL influx into the genital lymph nodes and genital tract occurs 5 to 6 days postinfection and coincides with virus clearance (32, 34). We did not find a difference in the number of CD4+ IFN-γ+ T cells in genital lymph nodes 4 days after intravaginal infection of B7KO and BALB/c mice. However, a transient decrease has been observed at later times after footpad infection of CTLA-4-Ig-treated mice (13), and we did find fewer memory CD4+ IFN-γ+ T cells in the spleens of B7KO mice (Table 1). These observations support the possibility that CD4+ IFN-γ+ T cells do not expand normally in B7KO mice at later times after acute HSV infection or do not efficiently enter a memory state.
CD8+ IFN-γ+ T cells were reduced in number in the genital lymph nodes of B7KO mice during the acute response to HSV-2 infection, consistent with observations in mice treated with CTLA-4-Ig prior to footpad infection with HSV-1 (13). CD8+ IFN-γ+ T cells were also reduced in number during recall responses to HSV infection. IFN-γ production was lower only in the spleens of B7KO mice, however, suggesting that the CD8+ IFN-γ+ T-cell deficit did not have a major impact on the overall amount of IFN-γ produced. These results also suggest that IFN-γ may not be the principal mediator of enhanced protection of wild-type versus B7KO mice after intravaginal infection with HSV-2, but time postinfection and cytokine synthesis by T cells in the genital tract itself remain critical unexplored factors.
CTL activity in the genital tract and draining lymph nodes of HSV-2-infected BALB/c mice is predominantly mediated by CD4+ T cells (34). We observed reduced CTL activity in the genital lymph node cells of B7KO mice, and this CTL activity was manifest only on MHC class II-expressing target cells. Thus, generation of antiviral CD4+ CTL activity, like CD8+ CTL activity (28, 29, 63), may be impaired by B7 deficiency. Compromised CTL activity and, to a lesser extent, IFN-γ production likely contribute to the greater pathological consequences of acute HSV-2 infection in B7KO mice. CD4+ T cells also play a significant role in protecting the host from reinfection with HSV-2 (12, 24; L. A. Morrison, submitted for publication). Because the absence of B7 costimulation resulted in reduced cytokine production and CTL activity in immune mice, we would anticipate less protection of B7KO mice from reinfection, and this was in fact observed (L. G. Thebeau, J. A. Murphy, and L. A. Morrison, unpublished data).
An alternative interpretation of lower CTL activity in the genital lymph nodes of B7KO mice may be a defect in trafficking of suboptimally activated effector T cells into lymph nodes following infection with HSV-2. Hamann et al. (17) demonstrated that activation of T cells rapidly induces significant alterations in their trafficking patterns. In the absence of B7 costimulation, T-cell synthesis and secretion of MIP-1α and RANTES are inhibited (19), and upregulation of CXCR3 on Th1 cells requires B7 signaling (11). In B7KO mice, weaker T-cell activation may cause attenuated responsiveness to chemokines and reduced recruitment of CTL to the site of infection, resulting in lower apparent activity. This possibility is under investigation.
Weaker T-cell activation in the absence of B7 costimulation has multiple consequences for the nascent immune response, but whether the effects are direct or indirect is a matter of conjecture. For example, inhibition of the CD40L-CD40 interaction in T-cell-antigen-presenting cell interactions has been linked to decreased production of IL-12 and IFN-γ for Th1 responses (58, 59). In addition, plasmid DNA-based CD40L expression enhances T-cell proliferation and IFN-γ production in response to coexpressed HSV-2 gD (52). Roy et al. (47) suggested that T-cell receptor-CD4 interactions with antigen-MHC complexes on antigen-presenting cells induce CD40L expression independently of B7 costimulation. On the other hand, CD28-B7 cognate interactions may be important in amplifying CD40L expression for optimal Th1 development (58). Second, strong transcriptional activation of the CD40L promoter in response to T-cell receptor ligation requires stimulation through CD28 (45), presumably via B7 on the initial antigen-presenting cell surface. Our observation that CD40L expression on CD4+ T cells from the genital lymph nodes of infected B7KO mice is reduced suggests that impaired T-cell effector functions in the absence of B7 may be at least in part due to CD40L deficiency.
Role of B7 costimulation in antibody responses to HSV.
We found major deficits in HSV-specific IgG production in B7KO mice compared to BALB/c mice. Production of both HSV-specific IgG2a and IgG1 was decreased, and to equivalent extents. Our results are in concert with those of McAdam with vesicular stomatitis virus infection of B7KO mice (29) but in contrast to those of Gangappa, who found that CTLA-4-Ig treatment of HSV-infected mice suppressed IgG2a but stimulated IgG1 production (15). The basis for this discrepancy is unknown.
Can the specific deficits in class-switched antibody responses in B7KO mice be explained by the defects in T-cell activation that we observed? Contact with specific antigen induces B cells to undergo activation, increasing expression of B7 molecules. In conjunction with antigen receptor engagement, cytokine and CD40L signals provided by antigen-activated T cells stimulate B-cell division and differentiation, including isotype switching (58). Class switching to each isotype of antibody is differentially regulated: IFN-γ induces IgG2a synthesis (53, 54), while IL-4 and IL-10 stimulate production of IgG1 (53). In the absence of B7, diminished cytokine synthesis resulting from attenuated T-cell activation could have a significant impact on class-switched antibody levels, either globally or in an isotype-specific manner.
Strom et al. (55) have shown that CD40 ligation increases germ line Cγ1 but not Cγ2a transcripts and that subsequent Sμ/Sγ1 switch recombination occurs only in the presence of IL-4 or lipopolysaccharide. One implication of these findings is that IFN-γ may be the primary signal determining B-cell competence for IgG2a synthesis, and we did observe less IFN-γ produced by splenocytes of B7KO mice responding to primary or secondary HSV infection, although the difference was only marginally significant. Second, these observations imply that class switching to IgG1 is regulated more by cell-cell interactions than is switching to IgG2a.
CD40L expression on T cells is dependent upon the activation state of the cell (1, 41, 47, 48), and lack of CD28-B7 engagement in some cases depresses IL-4 production (14, 50). Interestingly, IL-4 production was not consistently reduced in B7KO mice, although the low levels of cytokine detected make definitive interpretation difficult. CD40L expression was, however, significantly higher in BALB/c mice, and CD40L transgenic mice have accelerated IgG1 production to T-dependent antigen (46). Thus, CD40L appears to be a limiting factor in class switching to IgG1 and/or IgG1 production. IL-10 synthesis was also significantly greater in BALB/c spleen and lymph node cultures. Whether the IL-10 derives from macrophages, T cells, or B cells themselves, its effect would be to enhance the production of IgG1 by CD40-activated B cells (2, 6). These observations support the concept that the effect of B7 deficiency on HSV-specific IgG1 production occurs chiefly through its effect on T-cell activation to express CD40L and to produce IL-10.
McAdam et al. (29) attributed the differences in vesicular stomatitis virus-specific antibody levels observed between B7KO and BALB/c mice to an inability of B cells from B7KO mice to undergo class switching. Alternatively, class switching may occur normally in B7-deficient mice, but lack of B7 may prohibit production of factors that enhance antibody synthesis. In a system in which antigen-specific resting B cells are stimulated with CD40L and IL-4, ligation of B7-2 on B cells enhances IgG1 production without affecting IgG1 B-cell precursor frequency or the percentage of secretory IgG1-positive B cells (22). In vivo, if B cells undergo class switching but are not successfully stimulated to produce antibody, one would expect the level of IgM to peak and then decline with the switch to IgG. This was indeed what we observed. Further experiments will be necessary to determine whether signal transduction via B7 costimulation molecules on B cells influences B-cell responses and whether it affects IgG2a and IgG1 synthesis rather than isotype switching in vivo.
Interestingly, CD40-deficient, CD40L-deficient, and B7KO mice exhibit remarkably similar deficits in cytokine secretion (20, 61) and class-switched antibody levels in response to virus infection (5, 60, 61). The observations that CD40 ligation results in increased B7-2 expression on B cells (3, 7, 21, 40) and that CD40L−/− mice fail to efficiently upregulate B7 on B cells (5) raise questions about whether the CD40/CD40L deficit influences antibody production directly or indirectly by preventing B7 upregulation for interaction with CD28/CTLA4. One possible explanation for the similarly profound impact on the antibody responses of B7KO and CD40L−/− mice is that ligation of either B7 or CD40 on the B cell sends a competence signal for class switching or Ig production; however, levels of B7 on B cells or CD40L on T cells must be sufficient for signal transduction. Thus, in the absence of B7 costimulation, T cells are suboptimally activated and express diminished levels of CD40L.
With reduced CD40L-CD40 interaction, B cells from B7KO mice are not stimulated to produce class-switched antibody at wild-type levels. Defects in CD40L expression and cytokine production may be the mechanism by which antibody responses are compromised in the context of B7 deficiency. Support for this hypothesis comes from a recent study in which blockade of B7-1, B7-2, and CD40L was required to completely inhibit induction of primary immune responses to an adenovirus vector (62). Experiments to test this hypothesis are in progress.
Acknowledgments
We thank Arlene Sharpe for the generous gift of the B7KO mice and Alex McAdam for helpful advice and discussions. We appreciate the expert technical assistance of Li Zhu, John Patton, and Rob Reass and the sage remarks of Lynn Dustin, who reviewed the manuscript.
This work is supported by PHS grant CA75052.
REFERENCES
- 1.Armitage, R. J., W. C. Fanslow, L. Stockbine, T. A. Sato, K. N. Clifford, B. M. Macduff, D. M. Anderson, S. D. Gimpel, T. Davis-Smith, C. R. Maliszewski, et al. 1992. Molecular and biological characterization of a murine ligand for CD40. Nature 357:80-82. [DOI] [PubMed] [Google Scholar]
- 2.Armitage, R. J., B. M. Macduff, M. K. Spriggs, and W. C. Fanslow. 1993. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J. Immunol. 150:3671-3680. [PubMed] [Google Scholar]
- 3.Azuma, M., D. Ito, H. Yagita, K. Okumura, J. H. Phillips, L. L. Lanier, and C. Somoza. 1993. B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366:76-79. [DOI] [PubMed] [Google Scholar]
- 4.Borriello, F., M. P. Sethna, S. D. Boyd, A. N. Schweitzer, E. A. Tivol, D. Jacoby, T. B. Strom, E. M. Simpson, G. J. Freeman, and A. H. Sharpe. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6:303-313. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P. A., A. Tishon, S. Lee, J. Xu, I. S. Grewal, M. B. Oldstone, and R. A. Flavell. 1996. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 183:2129-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdin, N., C. van Kooten, L. Galibert, J. S. Abrams, J. Wijdenes, J. Banchereau, and F. Rousset. 1995. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 154:2533-2544. [PubMed] [Google Scholar]
- 7.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T-cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle, A. J., and J. C. Gutierrez-Ramos. 2001. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat. Immunol. 2:203-209. [DOI] [PubMed] [Google Scholar]
- 9.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective HSV-2. Virology 232:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Da Costa, X., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. USA 96:6994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Ambrosio, D., A. Iellem, R. Bonecchi, D. Mazzeo, S. Sozzani, A. Mantovani, and F. Sinigaglia. 1998. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 161:5111-5115. [PubMed] [Google Scholar]
- 12.Dudley, K., N. Bourne, and G. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454-463. [DOI] [PubMed] [Google Scholar]
- 13.Edelmann, K. H., and C. B. Wilson. 2001. Role of CD28/CD80-86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foy, T. M., A. Aruffo, J. Bajorath, J. E. Buhlmann, and R. J. Noelle. 1996. Immune regulation by CD40 and its ligand GP39. Annu. Rev. Immunol. 14:591-617. [DOI] [PubMed] [Google Scholar]
- 15.Gangappa, S., E. Manickan, and B. T. Rouse. 1998. Control of herpetic stromal keratitis with CTLA41g fusion protein. Clin. Immunol. Immunopathol. 86:88-94. [DOI] [PubMed] [Google Scholar]
- 16.Gao, M., and D. M. Knipe. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 63:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamann, A., K. Klugewitz, F. Austrup, and D. Jablonski-Westrich. 2000. Activation induces rapid and profound alterations in the trafficking of T cells. Eur. J. Immunol. 30:3207-3218. [DOI] [PubMed] [Google Scholar]
- 18.Harding, F. A., and J. P. Allison. 1993. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J. Exp. Med. 177:1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herold, K. C., J. Lu, I. Rulifson, V. Vezys, D. Taub, M. J. Grusby, and J. A. Bluestone. 1997. Regulation of C-C chemokine production by murine T cells by CD28/B7 costimulation. J. Immunol. 159:4150-4153. [PubMed] [Google Scholar]
- 20.Inagaki-Ohara, K., T. Kawabe, Y. Hasegawa, N. Hashimoto, and Y. Nishiyama. 2002. Critical involvement of CD40 in protection against herpes simplex virus infection in a murine model of genital herpes. Arch. Virol. 147:187-194. [DOI] [PubMed] [Google Scholar]
- 21.Jones, K. W., and C. J. Hackett. 1996. Activated T hybridomas induce upregulation of B7-1 on bystander B lymphoma cells by a contact-dependent interaction utilizing CD40 ligand. Cell. Immunol. 174:42-53. [DOI] [PubMed] [Google Scholar]
- 22.Kasprowicz, D. J., A. P. Kohm, M. T. Berton, A. J. Chruscinski, A. Sharpe, and V. M. Sanders. 2000. Stimulation of the B cell receptor, CD86 (B7-2), and the beta 2-adrenergic receptor modulates the level of IgG1 and IgE produced per B cell. J. Immunol. 165:680-690. [DOI] [PubMed] [Google Scholar]
- 23.Knipe, D. M., M. P. Quinlan, and A. E. Spang. 1982. Characterization of two conformational forms of the major DNA-binding protein encoded by herpes simplex virus 1. J. Virol. 44:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanier, L., S. O'Fallon, C. Somoza, J. Phillips, P. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 26.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T-cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 27.Linsley, P. S., P. M. Wallace, J. Johnson, M. G. Gibson, J. L. Greene, J. A. Ledbetter, C. Singh, and M. A. Tepper. 1992. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257:792-795. [DOI] [PubMed] [Google Scholar]
- 28.Lumsden, J., J. Roberts, N. Harris, R. Peach, and F. Ronchese. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79-85. [DOI] [PubMed] [Google Scholar]
- 29.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8+ cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott, M. R., C. H. Goldsmith, K. L. Rosenthal, and L. J. Brais. 1989. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 159:460-466. [DOI] [PubMed] [Google Scholar]
- 31.McNally, J. M., D. Dempsey, R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1999. Phenotypic identification of antigen-dependent and antigen-independent CD8 CTL precursors in the draining lymph node during acute cutaneous herpes simplex virus type 1 infection. J. Immunol. 163:675-681. [PubMed] [Google Scholar]
- 32.Milligan, G., and D. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 33.Milligan, G., D. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 34.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 35.Morrison, L. A., X. J. Da Costa, and D. M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178-187. [DOI] [PubMed] [Google Scholar]
- 36.Morrison, L. A., and D. M. Knipe. 1994. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J. Virol. 68:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 320:402-413. [DOI] [PubMed] [Google Scholar]
- 38.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobulin contributes to protection from herpes simplex virus type-2 genital infection in the presence of immune T. cells. J. Virol. 75:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 40.Nashar, T. O., T. R. Hirst, and N. A. Williams. 1997. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology 91:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noelle, R. J., M. Roy, D. M. Shepherd, I. Stamenkovic, J. A. Ledbetter, and A. Aruffo. 1992. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc. Natl. Acad. Sci. USA 89:6550-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oxenius, A. K., K. A. Campbell, C. R. Maliszewski, T. Kishimoto, H. Kikutani, H. Hengartner, R. M. Zinkernagel, and M. Bachmann. 1996. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T-cell functions. J. Exp. Med. 183:2209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parr, M. B., and E. L. Parr. 1999. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology 258:282-294. [DOI] [PubMed] [Google Scholar]
- 45.Parra, E., T. Mustelin, M. Dohlsten, and D. Mercola. 2001. Identification of a CD28 response element in the CD40 ligand promoter. J. Immunol. 166:2437-2443. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Melgosa, M., D. Hollenbaugh, and C. B. Wilson. 1999. Cutting edge: CD40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J. Immunol. 163:1123-1127. [PubMed] [Google Scholar]
- 47.Roy, M., A. Aruffo, J. Ledbetter, P. Linsley, M. Kehry, and R. Noelle. 1995. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 25:596-603. [DOI] [PubMed] [Google Scholar]
- 48.Roy, M., T. Waldschmidt, A. Aruffo, J. A. Ledbetter, and R. J. Noelle. 1993. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 151:2497-2510. [PubMed] [Google Scholar]
- 49.Schneweis, K. E., M. Brado, B. Ebers, A. Friedrich, M. Olbrich, and W. Schuler. 1988. Immunological mechanisms giving rise to latency of herpes simplex virus in the spinal ganglia of the mouse. Med. Microbiol. Immunol. 177:1-8. [DOI] [PubMed] [Google Scholar]
- 50.Schweitzer, A. N., and A. H. Sharpe. 1998. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 161:2762-2771. [PubMed] [Google Scholar]
- 51.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 52.Sin, J. I., J. J. Kim, D. Zhang, and D. B. Weiner. 2001. Modulation of cellular responses by plasmid CD40L:CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091-1102. [DOI] [PubMed] [Google Scholar]
- 53.Snapper, C. M., and W. E. Paul. 1987. Interferon gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 54.Snapper, C. M., C. Peschel, and W. E. Paul. 1988. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 140:2121-2127. [PubMed] [Google Scholar]
- 55.Strom, L., J. Laurencikiene, A. Miskiniene, and E. Severinson. 1999. Characterization of CD40-dependent immunoglobulin class switching. Scand. J. Immunol. 49:523-532. [DOI] [PubMed] [Google Scholar]
- 56.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 167:5565-5573. [DOI] [PubMed] [Google Scholar]
- 57.Thebeau, L. G., and L. A. Morrison. 2002. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J. Virol. 76:2563-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Kooten, C., and J. Banchereau. 1996. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv. Immunol. 61:1-77. [DOI] [PubMed] [Google Scholar]
- 59.Whitmire, J. K., R. A. Flavell, I. S. Grewal, C. P. Larsen, T. C. Pearson, and R. Ahmed. 1999. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J. Immunol. 163:3194-3201. [PubMed] [Google Scholar]
- 60.Whitmire, J. K., M. K. Slifka, I. S. Grewal, R. A. Flavell, and R. Ahmed. 1996. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J. Virol. 70:8375-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang, Y., and J. M. Wilson. 1996. CD40 Ligand-dependent T-cell activation: requirement of B7-CD28 signaling through CD40. Science 273:1862-1864. [DOI] [PubMed] [Google Scholar]
- 62.Ziller, C., F. Stoeckel, L. Boon, and H. Haegel-Kronenberger. 2002. Transient blocking of both B7.1 (CD80) and B7.2 (CD86) in addition to CD40-CD40L interaction fully abrogates the immune response following systemic injection of adenovirus vector. Gene Ther. 9:537-546. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann, C., P. Seiler, P. Lane, and R. M. Zinkernagel. 1997. Antiviral immune responses in CTLA4 transgenic mice. J. Virol. 71:1802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]