Abstract
Immunization of macaques with the soluble oligomeric gp140 form of the SF162 envelope (SF162gp140) or with an SF162gp140-derived construct lacking the central region of the V2 loop (ΔV2gp140) results in the generation of high titers of antibodies capable of neutralizing the homologous human immunodeficiency virus type 1 (HIV-1), SF162 virus (Barnett et al. J. Virol. 75:5526-5540, 2001). However, the ΔV2gp140 immunogen is more effective than the SF162gp140 immunogen in eliciting the generation of antibodies capable of neutralizing heterologous HIV-1 isolates. This indicates that deletion of the V2 loop alters the immunogenicity of the SF162gp140 protein. The present studies were aimed at identifying the envelope regions whose immunogenicity is altered following V2 loop deletion. We report that the antibodies elicited by the SF162gp140 immunogen recognize elements of the V1, V2, and V3 loops, the CD4-binding site, and the C1 and C2 regions on the homologous SF162 gp120. With the exception of the V1 and V2 loops, the same regions are recognized on heterologous gp120 proteins. Surprisingly, although a minority of the SF162gp140-elicited antibodies target the V3 loop on the homologous gp120, the majority of the antibodies elicited by this immunogen that are capable of binding to the heterologous gp120s tested recognize their V3 loops. Deletion of the V2 loop has two effects. First, it alters the immunogenicity of the V3 and V1 loops, and second, it renders the C5 region immunogenic. Although deletion of the V2 loop does not result in an increase in the immunogenicity of the CD4-binding site per se, the relative ratio of anti-CD4-binding site to anti-V3 loop antibodies that bind to the heterologous gp120s tested is higher in sera collected from the ΔV2gp140-immunized animals than in the SF162gp140-immunized animals. Overall, our studies indicate that it is possible to alter the immunogenic structure of the HIV envelope by introducing specific modifications.
The elimination of potential N-linked glycosylation sites located in the V1V2 region or the deletion of the V1 and/or V2 loops from the human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) envelope glycoproteins results in an increased susceptibility of these viruses to neutralization by certain monoclonal antibodies (MAbs) or by sera collected from HIV-infected humans or SIV-infected macaques (3, 18, 23, 31, 38). One possible explanation for this change in viral neutralization susceptibility is that such envelope modifications increase the number and/or exposure of available neutralization epitopes within the virion-associated oligomeric viral envelope. The observation that the positioning of the variable loops and sugar molecules within the HIV oligomeric envelope results in the occlusion of neutralization epitopes supports observations made during SIV or simian/HIV infection of macaques, where the emergence of neutralization-resistant viruses coincides with changes in the glycosylation pattern of the envelope protein (2, 4, 6, 24, 29).
It was previously reported that, on the background of the R5-tropic HIV type 1 (HIV-1) SF162 virus, deletion of the central region of the V2 loop or elimination of certain N-linked glycosylation sites from the V1V2 region increases the susceptibility of the virus to neutralization by sera collected from HIV-1-infected patients (38) and by MAbs recognizing the V3 loop and the CD4-binding site (CD4-BS) (23). Based on these observations, studies were initiated to compare the immunogenic properties of the soluble oligomeric gp140 forms of the SF162 envelope (SF162gp140) and of the envelope lacking the V2 loop (termed ΔV2) (ΔV2gp140) in rabbits and rhesus macaques (1). It was reported that although the modified ΔV2gp140 immunogen is as effective as the unmodified SF162gp140 immunogen in eliciting neutralizing antibodies against the homologous SF162 virus, the modified ΔV2gp140 immunogen is more effective in eliciting neutralizing antibodies against heterologous HIV-1 viruses. Although not every heterologous HIV isolate tested so far was susceptible to neutralization by the ΔV2gp140-elicited antibodies and the titers of cross-reactive neutralizing antibodies were lower than those against the homologous SF162 virus, these studies suggested that the deletion of the V2 loop from the SF162 envelope alters the immunogenic properties of this protein.
One possibility for the observed differential ability of the SF162gp140 and the ΔV2gp140 immunogens to elicit cross-reactive neutralizing antibodies is that these two immunogens elicit antibodies that recognize different epitopes on heterologous envelopes and that the epitopes recognized by the ΔV2gp140-elicited antibodies are more frequently present on heterologous envelopes than are those recognized by the SF162gp140-elicited antibodies. A second possibility is that the ΔV2gp140 and SF162gp140 immunogens elicit different relative titers of antibodies that have the same epitope specificity. To distinguish between these two possibilities, we initiated studies to identify the regions on the homologous and heterologous HIV-1 envelopes that are recognized by the antibodies elicited by the SF162gp140 and ΔV2gp140 immunogens.
MATERIALS AND METHODS
Viruses.
The isolation and phenotypic characterization of the SF162, SF2, and SF128A isolates were previously reported (7, 21, 22). The primary HIV-1 isolates used in our present studies (92US657, 92US660, 91US054, and 91US056) were obtained through the AIDS Research and Reference Reagent Program. All virus stocks were propagated and titrated in phytohemagglutinin (PHA)-activated human peripheral blood mononuclear cells (PBMC) in the presence of 40 U of recombinant interleukin 2 (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health [NIH], from Maurice Gately, Hoffmann-La Roche, Inc.) (20) as previously reported (38).
Vaccines and immunization protocol.
Rhesus macaques were immunized with the DNA-prime plus protein-boost vaccination method as previously described in detail (1). Briefly, the animals initially received three monthly intramuscular and intradermal immunizations with a DNA vector (5, 48) expressing the gp140 form of either the SF162 or the ΔV2 envelope. Following a period of 5 to 10 months, they received a fourth DNA immunization and at the same time were immunized intramuscularly with the corresponding recombinant, CHO-produced, soluble oligomeric gp140 envelope proteins. These proteins were purified and characterized as described elsewhere (37) with minor modifications (I. K. Srivastava, L. Stamatatos, E. Kan, L. Vojtech, L. Martin, C. Vita, P. Zhul, K. Roux, Y. Lian, S. Hilt, J. B. Ulmer, and S. W. Barnett, submitted for publication). During the DNA phase of immunization, two animals, H445 and J408, were immunized with the ΔV2gp140 protein containing an intact gp120-gp41 cleavage site (termed ΔV2gp140C for cleavable); two animals, K863 and I708, were immunized with the ΔV2gp140 protein without the gp120-gp41 cleavage site (termed ΔV2gp140F for fused); and two animals, N472 and P655, were immunized with the SF162gp140 protein containing an intact gp120-gp41 cleavage site (termed SF162gp140C). Elimination of the gp120-gp41 cleavage site to generate the fused form of the ΔV2gp140 immunogen was achieved by substituting arginines for the serines in the REKR primary protease cleavage site within the HIV envelope (13, 37). During the protein-boosting phase of immunization, the animals were immunized with recombinant gp140 proteins lacking the gp120-gp41 cleavage site, i.e., the fused gp140 form, mixed with the MF-59C adjuvant. The SF162gp140-immunized animals received the soluble oligomeric SF162gp140 protein and the ΔV2gp140-immunized animals received the soluble oligomeric ΔV2gp140 protein. For simplicity, we identify animals N472 and P655 as being immunized with the cleavable SF162gp140 form (SF162gp140C), we identify animals H445 and J408 as being immunized with the cleavable ΔV2gp140 form (ΔV2gp140C), and we identify animals K863 and I708 as being immunized with the fused ΔV2gp140 form (ΔV2gp140F).
Determination of binding-antibody titers.
The titers of binding antibodies were determined throughout the immunization protocol by using an enzyme-linked immunosorbent assay (ELISA) method as previously described (1, 9, 10). Briefly, similarly purified soluble oligomeric ΔV2gp140 and SF162gp140 proteins or monomeric soluble SF2, US4, and SF162 gp120 proteins were used to coat 96-well ELISA plates (Immulon 2HB; Fisher Scientific Co.) (0.05 μg of protein in 0.1 ml of 100 mM NaHCO3, pH 8.5) by an overnight incubation at room temperature. Nonadsorbed protein molecules were removed by washing with Tris-buffered saline (TBS) (25 mM Tris-144 mM NaCl [pH 7.6]), and the wells were blocked with SuperBlock (SB) (Pierce) according to the manufacturer's instructions. Heat-inactivated (56°C for 35 min) sera collected from the immunized animals were serially diluted in TBS containing 10% SB and 0.03% Tween 20 and added to the wells (0.1 ml per well) for 2 h at room temperature. Preimmunization sera were used as negative controls. Unbound antibodies were removed by washing with TBS, and the HIV envelope-bound antibodies were detected with the use of a goat anti-human immunoglobulin G coupled to alkaline phosphatase (Zymed Immunochemicals) (1:30,000 dilution in TBS containing 10% SB and 0.03% Tween 20) (2-h incubation at room temperature). The substrate for alkaline phosphatase (DAKO Corp.) was added (50 μl per well) for 1 h at room temperature. Upon addition of an equal amount of amplifier (DAKO Corp.) the optical density at 490 nm (OD490) signals were recorded from each well on a VMax microplate reader (Molecular Devices). A plot of the OD490 signals versus serum dilutions was generated, and end-point antibody titers were determined as the highest postimmunization serum dilution that produces an OD490 signal three times that of the OD490 produced by the preimmunization sera at their lowest dilution. Sera collected from the 6 animals at various stages of immunization were tested at the same time.
Neutralization studies.
Neutralization assays were performed by using as target cells human PBMC activated for 3 days with PHA (3 μg/ml; Sigma) (1). Viruses (50 50% tissue culture infective doses in 25 μl of complete RPMI media containing 40 U of recombinant interleukin 2 [Hoffmann-La Roche]/ml) were preincubated for 1 h at 37°C in 96-well U-bottom plates (Corning) with an equal volume of serially diluted heat-inactivated (35 min at 56°C) sera collected either prior to the initiation of the immunizations or following the protein booster immunization. For each serum dilution, triplicate wells were used and 50 μl of complete media containing 0.2 × 106 PHA-activated PBMC was added to each well. Following an overnight incubation at 37°C, 100 μl of media was added to each well, the plates were centrifuged for 10 min at 2,000 × g, and 100 μl of supernatant was removed from each well. This procedure was repeated twice. The p24 antigen concentration in each well was determined at various points following infection (usually 4 and 7 days) by using an in-house ELISA p24 detection assay. The mean percent neutralization from triplicate wells and the standard deviation for each postimmunization serum dilution tested were calculated based on p24 concentrations recorded in wells containing the preimmunization serum at the same dilution.
Identification of the envelope regions recognized by the SF162gp140- and ΔV2gp140-elicited antibodies. (i) Serum antibody-peptide competition studies.
To identify the regions on target HIV envelope proteins, other than the CD4-BS, that are recognized by the antibodies elicited by our soluble gp140 immunogens, we performed antibody-peptide competition studies. During these studies we determined the degree to which the binding of serum antibodies to the homologous SF162 and two heterologous, SF2 and US4, gp120 proteins was affected by the preincubation of sera with peptides derived from various gp120 regions. The SF2 isolate is a laboratory-adapted, X4R5-tropic HIV-1 virus (8) while the US4 isolate is a primary R5-tropic HIV-1 virus (25, 37).
The following gp120-derived peptides were used. CKSITIGPGRAFYATGDC was derived from the central region of the SF162 V3 loop. TTSIRNKMQKEYALF and YKLDVVPIDNDNTSY represent the amino- and carboxy-terminal halves of the crown of the SF162 V2 loop, respectively. CSFKYGAGKLINC represents the V2 loop on the ΔV2 envelope (i.e., following the replacement of the central 30 amino acids from the central portion of the SF162 envelope with the GAG tripeptide) (40). It also represents the amino- and carboxy-terminal halves of the base of the SF162 V2 loop. EKLWVTVYYGVPVWKEATTT and VPVWKEATTTLFCASDAKAY represent the first conserved region of gp120 (C1) minus the first 27 amino acids, which on our immunogens were replaced by the amino acid sequence of the tissue-specific plasminogen activator signal sequence (1). RPGGGDMRDNWRSELYKYKV and VTIEPLGVAPTKAKRRVVQR represent the fifth conserved region of gp120 (C5). CNTSVITQACPKISFEPIPI and HYCAPAGFAILKCNDKKFSG represent the amino-terminal half of the second conserved gp120 region (C2). KGSCKNVSTVQCTHGIRPVV and STQLLLNGSLAEEEVVIRSE represent the carboxy-terminal half of the C2 region. LHCTNLENATNTTSS represents the amino-terminal half of the V1 loop. TSSNWKEMNRGEIKN represents the carboxy-terminal half of the V1 loop. NSTQLFNSTWNNTIG is derived from the central region of the V4 loop, and IRCSSNITGLLLTRD is derived from the V5 loop.
The amino acid sequences of the peptides mapping to the V2, V3, V4, and V5 loops were derived from the HIV-1 SF162 envelope; those of peptides mapping to the C1, C2, and C5 regions were derived from the MN envelope; and those of peptides mapping to the V1 loop were derived from the simian/HIVSF162P3 envelope. The V2 and V3 loop peptides were purchased from Sigma Genosys, and the remaining peptides were obtained from the NIH AIDS Research and Reference Reagent Program. The amino acid composition of the peptides used, the regions of the gp120 they were derived from, and the percentage of amino acid sequence homology between the peptides and the SF162, SF2, and US4 envelope proteins are shown in Table 1. To correct for potential nonspecific inhibition of serum antibody binding to gp120 by the gp120-derived peptides, we included in our studies an unrelated HIV SF2 gag-derived peptide.
TABLE 1.
Amino acid homology between the SF162, SF2, and US4 gp120 envelopes and the peptides used during the antibody-peptide competition studies
| Peptide | Regiona | % Homology withb:
|
||
|---|---|---|---|---|
| SF162 | SF2 | US4 | ||
| LHCTNLENATNTTSS | (V1 Nt) | 87 | 67 | 53 |
| TSSNWKEMNRGEIKN | (V1 Ct)c | 93 | 80 | 60 |
| TTSIRNKMQKEYALF | (V2 Nt) | 100 | 80 | 73 |
| YKLDVVPIDNDNTSY | (V2 Ct)d | 100 | 66 | 93 |
| CSFKVGAGKLINC | (V2 base)e | 77 | 60 | 70 |
| CKSITIGPGRAFYATGDC | V3 loop | 100 | 75 | 94 |
| NSTQLFNSTWNNTIG | V4 loopf | 100 | 60 | 66 |
| IRCSSNITGLLLTRD | V5 loop | 100 | 93 | 93 |
| EKLWVTVYYGVPVWKEATTT | (C1) | 100 | 100 | 90 |
| VPVWKEATTTLFCASDAKAY | (C1) | 100 | 95 | 100 |
| RPGGGDMRDNWRSELYKYKV | (C5) | 100 | 100 | 90 |
| VTIEPLGVAPTKAKRRVVQR | (C5) | 95 | 85 | 85 |
| CNTSVITQACPKISFEPIPI | (C2 Nt) | 95 | 90 | 95 |
| HYCAPAGFAILKCNDKKFSG | (C2 Nt) | 95 | 80 | 90 |
| KGSCKNVSTVQCTHGIRPVV | (C2 Ct) | 85 | 85 | 90 |
| STQLLLNGSLAEEEVVIRSE | (C2 Ct) | 95 | 95 | 90 |
Ct, carboxy terminal; Nt, amino terminal.
The values shown are percentages of homology in the amino acid sequence between the peptide used during our antibody-peptide competition studies and that of the corresponding region on the SF162, SF2, and US4 gp120 proteins. Amino acid insertions or deletions that exist in the SF162, SF2, and US4 amino acid sequence were not taken into consideration when determining the percent amino acid homology.
The carboxy-terminal side of the US4 V1 loop has an insert of 19 amino acids between the second serine and the first asparagine. The 60% homology corresponds to the remaining (shown) 15 amino acids.
The carboxy-terminal side of the SF2 V2 loop has a 5- amino-acid insert between the first aparagine and the second aspartic acid shown. The 66% homology corresponds to the remaining (shown) 15 amino acids.
The first five and last five amino acids forming the V2 loop of SF162 are common between the SF162 and ΔV2 envelopes. The 30 central amino acids of the SF162 V2 loop were replaced with the GAG tripeptide to generate the ΔV2 envelope.
A 2-amino-acid insertion between the third threonine and the isoleucine shown exists in the case of the US4 V4 loop. A single-amino-acid insertion following the tryptophan exists in the case of the SF2 V4 loop.
Ninety-six-well flat-bottom plates (Immulon 2HB) were coated overnight with equal amounts of purified recombinant soluble gp120 derived from the SF162, SF2, or US4 virus as described above. Pooled sera from several HIV-1-positive patients recognized the three adsorbed proteins similarly, indicating that very similar amounts of these proteins became adsorbed onto the ELISA plates (data not shown). Macaque sera collected 2 to 4 weeks following the protein booster immunization were diluted (to their end-point ELISA titers) in TBS containing 10% SB and 0.03% Tween 20 and were either directly added to wells containing the adsorbed proteins or were first incubated with gp120-derived peptides (30 to 100 μg peptide per ml) for 1 h at room temperature and then added to gp120-containing wells. Duplicate or triplicate wells were used for each peptide. The extent of serum antibody binding to the various gp120 proteins tested in the presence and absence of peptide was determined as described above. Upon addition of the amplifier solution, the 96-well plates were placed in the VMax microplate reader and the OD490 signal from each well was recorded over time. The percent reduction in the overall serum antibody binding to gp120 in the presence of peptides was determined for all sera when the OD490 signal associated with the wells not containing peptide was equal to 0.5.
(ii) sCD4 serum antibody competition studies.
To determine whether immunization of macaques with the SF162gp140 and ΔV2gp140 immunogens elicited antibodies against the CD4-BS of gp120, we compared the binding of serum antibodies to gp120 in the presence and absence of soluble CD4 (sCD4). The wells of a 96-well plate were precoated with gp120 proteins as described above. Recombinant sCD4 (Chiron Co.) (37) was added (20 μg/ml in TBS with 0.03% Tween 20) to half of the wells for 5 h at 37°C. Following washing with TBS to remove unbound sCD4, sera diluted to their end-point ELISA titers (in TBS containing 10% SB and 0.03% Tween 20) were added for 1 h at room temperature to all the wells. Detection of serum antibodies bound to gp120 in the presence or absence of sCD4 was performed with the use of goat anti-human immunoglobulin G antibodies coupled to alkaline phosphatase (see above). The percent reduction in serum antibody binding to gp120 in the presence of sCD4 was determined as described above.
Statistical analysis.
Differences in the relative titer of SF162gp140- and ΔV2gp140-elicited antibodies recognizing a particular gp120 region were analyzed with the statistical analysis feature of the Prism software program (GraphPad Software, Inc.) by using unpaired t test analysis and a 99% confidence interval.
RESULTS
The SF162gp140 and ΔV2gp140 envelope immunogens elicit higher titers of binding antibodies against the homologous HIV envelopes than against heterologous HIV envelopes
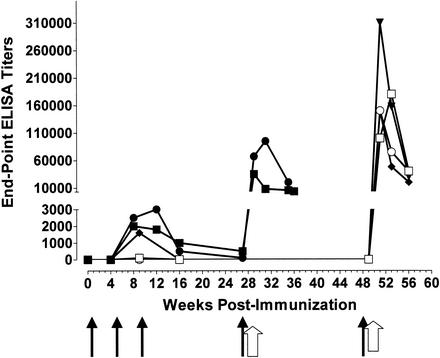
It was previously reported that macaques immunized with the cleavable form of the SF162gp140 and ΔV2gp140 proteins during the DNA-priming phase of immunization and with the corresponding fused form during the protein-boosting phase of immunization generated robust binding antibody responses against the corresponding homologous soluble oligomeric gp140 proteins (1) (Fig. 1). Here we report that animals immunized with the fused form of the ΔV2gp140 immunogen, during both the DNA-priming and the protein-boosting phases of immunization, also elicited potent binding antibody responses (Fig. 1). The presence or absence, therefore, of the gp120-gp41 cleavage site from the ΔV2gp140 immunogen during the DNA-priming phase of immunization does not alter the ability of this immunogen to elicit high titers of binding antibodies during the subsequent protein-boosting phase of vaccination.
FIG. 1.
Development of antibodies (end-point ELISA titers) in animals immunized with the SF162gp140C (animals P655 [□] and N472 [○]) immunogen, the ΔV2gp140C immunogen (animals H445 • and J408 ▪), and the ΔV2gp140F immunogen (animals K863 [⧫] and I708 [▾]) that are capable of binding to the oligomeric gp140 envelope. Sera from the SF162gp140-immunized animals were evaluated against the SF162gp140 protein, whereas those from the ΔV2gp140-immunized animals were evaluated against the ΔV2gp140 protein. Black arrows indicate the time of DNA immunization, and white arrows indicate the time of protein immunization.
The antibodies elicited by our soluble oligomeric gp140 immunogens recognized not only the homologous SF162 gp120 protein (Fig. 2) but also the heterologous SF2 and US4 gp120 proteins. However, the titers of binding antibodies against these two heterologous envelopes were significantly lower than the titers against the homologous envelope. Therefore, of the antibodies elicited by our immunogens that are capable of binding to the homologous SF162 envelope, only a small fraction also recognize the heterologous SF2 and US4 envelopes. Overall, however, the ΔV2gp140 (with or without an intact gp120-gp41 cleavage site) and the SF162gp140 immunogens elicited very similar titers of binding antibodies against the three gp120 proteins tested here.
FIG. 2.
Titration of serum antibodies against the homologous SF162 and heterologous SF2 and US4 gp120 proteins. Sera were collected following the protein administration from animals P655 and N472 (immunized with the SF162gp140C immunogen), animals H445 and J408 (immunized with the ΔV2gp140C immunogen), and animals I708 and K863 (immunized with the ΔV2gp140F immunogen). ∗, nonspecific binding recorded with sera collected prior to the initiation of immunization (pre-bleeds); •, binding of postimmunization sera to the SF162 gp120 protein; □, binding of postimmunization sera to the SF2 gp120 protein; ◊, binding of postimmunization sera to the US4 gp120 protein.
The ΔV2gp140 immunogen elicits higher titers of cross-reactive neutralizing antibodies than the SF162gp140 immunogen.
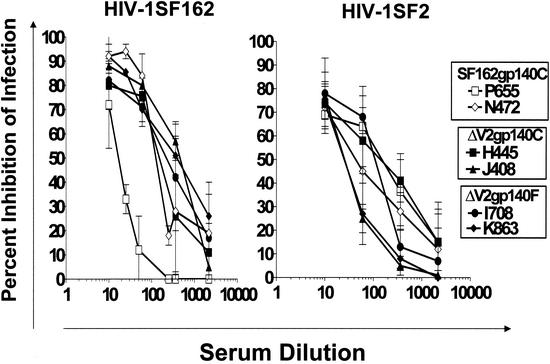
It was previously reported, and we confirmed in our present studies (Fig. 3), that the ΔV2gp140C immunogen is as effective as the SF162gp140C immunogen in eliciting neutralizing antibodies against the homologous SF162 virus (1). Here we report that elimination of the gp120-gp41 cleavage site from the ΔV2gp140 immunogen (ΔV2gp140F) does not affect the ability of this protein to elicit neutralizing antibodies against the SF162 virus (Fig. 3).
FIG. 3.
Neutralization of the homologous SF162 and heterologous SF2 viruses. The neutralizing potential of sera collected from the SF162gp140C-immunized (animals N472 [◊] and P655 [□]), ΔV2gp140C-immunized (animals H445 [▪] and J408 [▴]), and ΔV2gp140F-immunized (animals I708 [•] and K863 [⧫]) macaques against the SF162 and SF2 HIV-1 isolates was evaluated by determining the percent inhibition of PBMC infection at various dilutions. Results have been corrected for nonspecific inhibition of infection (i.e., in the presence of sera collected prior to the initiation of immunization). Results are the averages of three to six independent experiments.
Sera collected from all six immunized animals contained neutralizing antibodies against the laboratory-adapted SF2 isolate. However, the anti-SF2 neutralizing antibody titers present in all six sera were lower than the anti-SF162 neutralizing antibody titers. For example, 80% neutralization of SF162 infection was readily recorded with serum dilutions between 1:10 and 1:100 while it was not recorded in the case of SF2, even at serum dilutions of 1:10. This is not unexpected, since the titers of anti-SF2 binding antibodies were lower than the anti-SF162 binding antibodies (Fig. 2). No difference in the serum neutralizing potency against this laboratory-adapted isolate was recorded between the SF162gp140- and ΔV2gp140-immunized animals. As previously reported, when we compared the neutralizing potential of the sera collected from the SF162gp140- and ΔV2gp140-immunized animals against heterologous primary HIV-1 isolates, we observed that these isolates were more susceptible to neutralization by sera collected from the ΔV2gp140-immunized animals than from the SF162gp140-immunized animals (Table 2). Out of the five heterologous primary HIV-1 isolates tested here, only one (isolate 91US054) was susceptible to neutralization by the sera collected from the SF162gp140C-immunized animals. Four (isolates 92US657, 92US660, 91US054, and 91US056) out of the five isolates were susceptible to neutralization by sera collected from the ΔV2gp140C-immunized animals, and two (isolates 91US054 and 91US056) out the five isolates were susceptible to neutralization by the sera collected from the ΔV2gp140F-immunized animals. Although the number of our immunized animals is small, our present results suggest that elimination of the gp120-gp41 cleavage site may decrease the ability of the ΔV2gp140 immunogen to elicit cross-reactive neutralizing antibodies. In every case, however, where neutralization was recorded, the titer of antibodies capable of neutralizing the heterologous primary HIV-1 isolates was significantly lower than that against the homologous SF162 virus. At serum dilutions of 1:60 or higher, no neutralizing activity against primary HIV-1 isolates tested was recorded (data not shown).
TABLE 2.
Neutralization of heterologous primary HIV-1 isolates
| Isolate | Animals immunized witha:
|
|||||
|---|---|---|---|---|---|---|
| SF162gp140C
|
ΔV2gp140C
|
ΔV2gp140F
|
||||
| P655 | N472 | J408 | H445 | I708 | K863 | |
| 92US657 | ND | ND | 70 ± 10 | 80 ± 9 | ND | ND |
| 92US660 | ND | ND | 76 ± 7 | 88 ± 1 | ND | ND |
| 91US054 | 51 ± 5 | 68 ± 5 | 59 ± 4 | 64 ± 12 | 56 ± 5 | 75 ± 4 |
| 91US056 | ND | ND | 77 ± 13 | 95 ± 2 | 77 ± 5 | 95 ± 5 |
| SF128A | ND | ND | ND | ND | ND | ND |
The values represent the percent inhibition of infection recorded in PBMC at a serum dilution of 1:10. The values shown were determined by taking into account the nonspecific inhibition of infection recorded with preimmunization sera (see Materials and Methods for details). ND, less than 50% inhibition of infection was recorded when the nonspecific inhibition of infection recorded with preimmunization sera was taken into account.
Antibody-epitope mapping studies.
To determine whether the ΔV2gp140 and SF162gp140 immunogens elicit antibodies with the same or different epitope specificity, we performed antibody-peptide competition experiments during which the overall binding of serum antibodies to the SF162 gp120 protein was evaluated in the absence and presence of peptides derived from various gp120 regions. To determine whether the antibodies elicited by our immunogens recognize the same or different regions on homologous and heterologous HIV envelopes, we also performed antibody-binding competition studies with the heterologous SF2 and US4 gp120s as targets. The results from these peptide competition studies are summarized in Tables 3 and 4 and are expressed as percent reductions in overall serum antibody-gp120 binding in the presence of a particular peptide. During immunization, if antibodies were elicited against a specific gp120 region, and if these antibodies were capable of binding to this region on the homologous and heterologous gp120s tested here, then it is anticipated that the presence of a competing peptide derived from the same region would result in an overall reduction in serum antibody binding to gp120. The extent of this reduction would be related to the relative titer of serum antibodies recognizing this particular gp120 region.
TABLE 3.
Peptide-mediated reduction in binding of SF162gp140- and ΔV2 gp140-elicited antibodies to the homologous SF162 gp120 envelopea
| Animal no. | Immunogen | % Decrease in binding in the presence of peptide from region:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | V1 loop (Nt) | V1 loop (Ct) | V2 loop (Nt) | V2 loop (base) | V2 loop (Ct) | C2 (Nt) | C2 (Ct) | V3 loop | V4 loop | V5 loop | C5 | ||
| J408 | ΔV2gp140C | 4 | 3 | 7 | ND | 7 | ND | ND | 17 | 32 | ND | ND | ND |
| H445 | ΔV2gp140C | 15 | ND | 6 | ND | 15 | ND | ND | 16 | 37 | ND | ND | ND |
| I708 | ΔV2gp140F | 5 | 6 | 11 | ND | 19 | ND | ND | 10 | 42 | ND | ND | ND |
| K863 | ΔV2gp140F | 12 | ND | 15 | ND | 16 | ND | ND | 18 | 34 | ND | ND | 3 |
| N472 | SF162gp140C | 26 | ND | 11 | ND | 25 | ND | ND | 21 | 13 | ND | ND | ND |
| P655 | SF162gp140C | 24 | ND | ND | ND | 13 | ND | ND | 23 | 14 | ND | ND | ND |
The values shown represent the percent decrease in serum antibody binding to the indicated gp120 protein in the presence of peptides, at the highest peptide concentration tested (100 μg/ml), derived from the indicated gp120 regions. The amino acid compositions of these peptides are presented in Materials and Methods and Table 1. Values are the averages from 2 to 5 independent experiments. The standard deviations of the means were less than 40% of the values shown. The nonspecific inhibition recorded with an irrelevant peptide derived from the HIV p24 has been subtracted. Bold numbers indicate statistically significant differences in the percent reduction of serum antibody-gp120 binding in the presence of a specific peptide between the SF162gp140- and ΔV2gp140-immunized animals. ND, no reduction in serum antibody-gp120 binding was recorded in the presence of the indicated region-derived peptide; Nt, amino terminal; Ct, carboxy terminal.
TABLE 4.
Peptide-mediated reduction in binding of SF162gp140- and ΔV2gp140-elicited antibodies to heterologous gp120 envelopesa
| gp120 protein | Animal no. | Immunogen | % Decrease in binding in the presence of peptide from region:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | V1 loop (Nt) | V1 loop (Ct) | V2 loop (Nt) | V2 loop (base) | V2 loop (Ct) | C2 (Nt) | C2 (Ct) | V3 loop | V4 loop | V5 loop | C5 | |||
| SF2 | J408 | ΔV2gp140C | 9 | 4 | ND | ND | ND | ND | ND | 11 | 13 | ND | 4 | |
| H445 | ΔV2gp140C | 13 | ND | ND | ND | ND | ND | ND | 20 | 23 | ND | ND | 8 | |
| I708 | ΔV2gp140F | 21 | 15 | 15 | ND | ND | ND | ND | ND | 32 | ND | ND | 8 | |
| K863 | ΔV2gp140F | 3 | ND | 5 | ND | ND | ND | ND | 2 | ND | ND | ND | 46 | |
| N472 | SF162gp140 | 23 | ND | ND | ND | ND | ND | ND | 11 | 55 | ND | ND | ND | |
| P655 | SF162gp140 | 21 | ND | ND | ND | ND | ND | ND | 7 | 65 | ND | ND | ND | |
| US4 | J408 | ΔV2gp140C | 12 | 4 | ND | ND | ND | ND | ND | 10 | 33 | ND | ND | 4 |
| H445 | ΔV2gp140C | ND | ND | ND | ND | ND | ND | ND | 6 | 28 | ND | ND | 3 | |
| I708 | ΔV2gp140F | 25 | 4 | ND | ND | ND | ND | ND | 11 | 38 | ND | ND | 12 | |
| K863 | ΔV2gp140F | ND | ND | ND | ND | ND | ND | ND | 7 | ND | ND | ND | 49 | |
| N472 | SF162gp140C | 19 | ND | ND | ND | ND | ND | ND | 8 | 70 | ND | ND | ND | |
| P655 | SF162gp140C | 18 | ND | ND | ND | ND | ND | ND | 9 | 58 | ND | ND | ND | |
The values shown represent the percent decrease in serum antibody binding to the indicated gp120 protein in the presence of peptides, at the highest peptide concentration tested (100 μg/ml), derived from the indicated gp120 regions. The amino acid compositions of these peptides are presented in Materials and Methods and Table 1. Values are the averages from 2 to 5 independent experiments. The standard deviations of the means were less than 40% of the values shown. The nonspecific inhibition recorded with an irrelevant peptide derived from the HIV p24 has been subtracted. Bold numbers indicate statistically significant differences in the percent reduction of serum antibody-gp120 binding in the presence of a specific peptide between the SF162gp140- and ΔV2gp140-immunized animals. ND, no reduction in serum antibody-gp120 binding was recorded in the presence of the indicated region-derived peptide; Nt, amino terminal; Ct, carboxy terminal.
(i) Reduction in serum antibody binding to the homologous SF162 gp120 envelope in the presence of gp120-derived peptides (Table 3).
The SF162gp140 immunogen elicited antibodies capable of binding to several conserved and variable gp120 regions, in accordance with previous studies conducted with gp140 immunogens derived from the laboratory-adapted HXB2 (12, 32, 42) and SIVmacBK28 (14) isolates. Specifically, the antibodies elicited by the SF162gp140 immunogen recognized epitopes located in the V1, V2, and V3 loops and the C1 and C2 regions on the homologous SF162 gp120 protein. Unexpectedly, however, this immunogen did not elicit antibodies that bind to the crown of the V2 loop (either to its amino- or carboxy-terminal side), but it did elicit binding antibodies against the base of this loop. Only one out of the two SF162gp140-immunized animals (animal N472) elicited anti-V1-loop-binding antibodies, and these antibodies bound to the carboxy-terminal, but not to the amino-terminal, side of this loop. Both SF162gp140-immunized animals elicited anti-C1- and anti-C2-binding antibodies. The later antibodies exclusively recognized the carboxy-terminal half of the C2 region.
All of the ΔV2gp140-immunized animals generated antibodies against the base of the V2 loop, the V3 loop, the C1 region, and the carboxy-terminal half of the C2 region. One of the four animals immunized with the ΔV2gp140 immunogen (animal K863, immunized with the ΔV2gp140F form) elicited low titers of antibodies that bound to the C5 region on the SF162 gp120 protein. In addition, all four animals immunized with the ΔV2gp140 immunogen elicited binding antibodies against the carboxy-terminal half of the V1 loop, and two of these animals (animal J408, immunized with the ΔV2gp140C form, and animal I708, immunized with the ΔV2gp140F form) elicited antibodies that recognize the amino-terminal side of the V1 loop.
The overall binding of ΔV2gp140-elicited serum antibodies to the SF162 gp120 protein was reduced between 32 and 42% in the presence of a peptide derived from the V3 loop. Thus, approximately one-third of the antibodies elicited by the ΔV2gp140 immunogen that are capable of binding to the SF162 gp120 protein recognize the V3 loop. In contrast, the overall binding of SF162gp140-elicited antibodies to the SF162 gp120 protein was reduced by only 13% in the presence of the same V3-loop-derived peptide. Since the SF162gp140 and ΔV2gp140 immunogens elicited the generation of similar overall titers of binding antibodies against the SF162 gp120 protein (Fig. 2), the above results suggest that the sera collected from the animals immunized with the ΔV2gp140 immunogen contain higher relative titers of anti-SF162 V3 loop antibodies than the sera collected from the SF162gp140-immunized animals. The difference in the relative anti-SF162 V3 loop antibody titers present in sera collected from the SF162gp140-immunized and the ΔV2gp140-immunized animals is statistically significant (P, 0.0023; R2, 0.9234).
No significant differences in the relative titer of SF162gp140- and ΔV2gp140-elicited antibodies that recognize the remaining regions on the homologous gp120 envelope were evident from our studies.
(ii) Reduction in serum antibody-binding to the heterologous SF2 and US4 gp120 envelopes in the presence of gp120-derived peptides (Table 4).
As mentioned above, although both the ΔV2gp140 and SF162gp140 immunogens elicited the generation of high titers of antibodies that bound to the homologous SF162 gp120, only a fraction of these antibodies bound to the heterologous SF2 and US4 gp120s (Fig. 2). In part, this is due to the fact that the antibodies elicited by our immunogens recognized fewer regions on the heterologous envelopes tested than on the homologous envelope (compare Tables 3 and 4). The base of the V2 loop was not recognized by the antibodies elicited by the SF162gp140 and ΔV2gp140 immunogens on these two heterologous gp120 proteins, whereas it was on the homologous SF162 envelope. In addition, while five out of the six immunized animals elicited antibodies that bound to the carboxy-terminal side of the V1 loop on the homologous SF162 gp120, none of the immunized animals elicited antibodies that recognize this region on the US4 gp120 and only two animals (animals I708 and K863, immunized with ΔV2gp140F) generated antibodies capable of binding to the carboxy-terminal side of the V1 loop on the SF2 gp120. Also, four out of the six immunized animals generated antibodies recognizing the C1 region of the US4 gp120, whereas all of the animals elicited antibodies that recognize the C1 region of the SF162 (and SF2) envelope. Finally, one out of the four animals immunized with ΔV2gp140 (animal K863, immunized with ΔV2gp140F) did not generate binding antibodies against the V3 loop of the US4 and SF2 gp120s, although it generated such antibodies against the V3 loop of SF162 gp120.
The preincubation of SF162gp140-elicited serum antibodies with a peptide derived from the SF162 V3 loop resulted in a 55 to 65% reduction in overall serum antibody binding to the SF2 gp120 and in up to a 70% reduction in binding to the US4 gp120. In contrast, incubation of serum antibodies elicited by the ΔV2gp140 immunogen with the same peptide (with the exception of sera collected from animal K863) (see above) resulted in 10 to 38% reduction in the overall antibody binding to the SF2 and US4 gp120s. The difference in the relative titer of anti-SF2 or anti-US4 V3 loop antibodies present in the sera collected from the SF162gp140- and ΔV2gp140-immunized animals is statistically significant (P = 0.0165 and R2 = 0.7978 in the SF2 gp120 case and P = 0.0416 and R2 = 0.6863 in the US4 gp120 case).
The antibodies elicited by the SF162gp140 immunogen did not bind to the C5 region on the SF2 and US4 gp120s, similar to what we observed in the case of the SF162 gp120. In contrast, all four ΔV2gp140-immunized animals elicited antibodies capable of binding to the C5 region on the SF2 and US4 gp120s. In fact, half of the total antibodies elicited by animal K863 that are capable of binding to the heterologous gp120s tested are targeted to the C5 region.
(iii) Reduction in the binding of serum antibodies to gp120 in the presence of sCD4 (Table 5).
TABLE 5.
Reduction in serum antibody gp120 binding in the presence of sCD4
| Animal no. | Immunogen | % Decrease in binding to gp120 proteina:
|
||
|---|---|---|---|---|
| SF162 | SF2 | US4 | ||
| J408 | ΔV2gp140C | 6 ± 2 | 7 ± 3 | 5 ± 3 |
| H445 | ΔV2gp140C | 15 ± 4 | 13 ± 7 | 5 ± 5 |
| I708 | ΔV2gp140F | 7 ± 2.5 | 14 ± 9 | 7 ± 3.5 |
| K863 | ΔV2gp140F | 19 ± 2 | 13 ± 4 | 5 ± 5 |
| N472 | SF162gp140C | 12.5 ± 8 | 5 ± 8 | 3 ± 5 |
| P655 | SF162gp140C | 5 ± 3 | 7 ± 10 | 1 ± 3 |
Values represent the percentages of decrease in serum antibody binding to the SF162, SF2, or US4 gp120 proteins in the presence of sCD4. Values are the averages ± standard deviations from two to six independent experiments.
To determine whether our immunogens elicited antibodies against the CD4-BS we examined whether occlusion of the CD4-BS on gp120 by sCD4 resulted in a decrease in the overall binding of serum antibodies to gp120. Our results indicate that both the SF162gp140 and ΔV2gp140 immunogens elicited antibodies against the CD4-BS of the homologous SF162 envelope. The binding of sCD4 to SF162 gp120 reduced the overall serum antibody binding to this viral envelope by between 5 and 19%. Binding of sCD4 to the SF2 gp120 resulted in a similar reduction in serum antibody binding. However, a smaller decrease in the overall serum antibody binding (between 1 and 7%) was recorded in the case of US4 gp120. No difference in the ability of the SF162gp140 and ΔV2gp140 immunogens to elicit anti-CD4-BS antibodies was recorded.
DISCUSSION
The studies presented here were aimed at obtaining information that would assist us in understanding how the deletion of the V2 loop from the SF162gp140 immunogen alters the immunogenic properties of this protein. Specifically, we wanted to identify the regions on the homologous and heterologous HIV-1 envelopes that are recognized by the antibodies elicited by the SF162gp140 and ΔV2gp140 immunogens (1, 39). To this end, we monitored the binding to the homologous SF162 and heterologous SF2 and US4 gp120 proteins of serum antibodies collected from macaques immunized with these two immunogens. These binding experiments were conducted in the presence or absence of sCD4 and peptides derived from various regions of the gp120 subunit. Because we used peptides to compete the binding of serum antibodies to gp120, we primarily evaluated antibodies recognizing linear epitopes. We believe that the sera from the SF162gp140- and ΔV2gp140-immunized animals also contain antibodies that recognize conformation-dependent epitopes and possibly antibodies that preferentially recognize the oligomeric HIV envelope structure. Future studies will examine in detail the existence and epitope-specificity of such antibodies. Additionally, some of the peptides used here may adopt such a conformation in solution so that the epitopes recognized by the vaccine-elicited antibodies are unavailable for binding.
A direct correlation between the greater ability of ΔV2gp140 to elicit cross-reactive neutralizing antibodies and a specific epitope-binding specificity was not established by our present studies. Several reasons may account for this. Our epitope-mapping studies were performed with monomeric gp120s as antibody targets, and the ability of an antibody to neutralize HIV is best correlated with its ability to bind to the oligomeric envelope (15, 34), with a few exceptions (16, 41). Also, as mentioned above, our present studies primarily report on antibodies recognizing linear epitopes. It is possible that cross-reactive neutralizing antibodies recognize conformational epitopes, as suggested by the conformational nature of the epitopes recognized by the currently available broadly reactive anti-HIV MAbs (11, 28, 33, 35). Finally, the broader neutralizing potential of sera collected from the ΔV2gp140-immunzied animals may not be due to the presence of specific antibodies that are absent from sera collected from the SF162gp140-immunized animals. It could be associated with differences in the relative ratios of the same antibodies elicited by these two immunogens (see below).
Our studies, however, indicate that it is possible to alter the way that the immune system recognizes and reacts to the various regions of the HIV envelope once certain modifications are introduced in this protein. We observed that both immunogens elicited higher titers of serum antibodies against the homologous gp120 than against the two heterologous gp120s tested (Fig. 2). This difference may partially explain the greater ability of these immunogens to elicit neutralizing antibodies against the homologous SF162 than against heterologous HIV-1 isolates (1) (Table 2). The fact that similar total binding antibody titers were present in sera collected from the SF162gp140- and ΔV2gp140-immunized animals is an indication that deletion of the V2 loop from the SF162gp140 immunogen does not alter (increase or decrease) the overall ability of this immunogen to elicit binding antibodies against the three gp120s tested here. Therefore, the improved ability of the ΔV2gp140 immunogen, compared to the SF162gp140 immunogen, to elicit cross-reactive neutralizing antibodies is not due to an improved ability to elicit higher binding antibody titers to heterologous envelopes.
Both immunogens elicited binding antibodies against multiple regions on both the homologous and the heterologous gp120 proteins tested (Tables 3 and 4, respectively). Surprisingly, however, the SF162gp140 protein did not elicit measurable titers of binding antibodies against the crown of the SF162-derived V2 loop, but it did elicit antibodies against the base of this loop. Therefore, the crown of the V2 loop does not appear to be immunogenic in the context of the soluble oligomeric SF162gp140 protein. This is consistent with our observation that the SF162gp140 and ΔV2gp140 immunogens elicited similar titers of total binding antibodies.
Deletion of the crown of the V2 loop (ΔV2gp140 immunogen) does not appear to increase the immunogenicity of the base of the V2 loop, since similar relative titers of anti-V2 loop-base binding antibodies were present in sera collected from the SF162gp140- and ΔV2gp140-immunized animals. The V2 loop-base peptide used in our studies represents the amino acid sequence of the V2 loop on the infectious SF162ΔV2 virus (38) and on the ΔV2gp140 immunogen. It comprises the first four and last four amino acids of the SF162 V2 loop linked by the GAG tripeptide. The SF162gp140- and ΔV2gp140-elicited antibodies could recognize the amino acid sequences on either side of the peptide. Alternatively, these two amino acid sequences form an epitope that is present on the native SF162 gp120 envelope, and it is preserved when the crown of the V2 loop is deleted. The fact that the V2 loop-base-derived peptide blocked the binding of serum antibodies to the SF162 gp120, but not to the SF2 and US4 gp120s, even though the amino acid sequence homology between this peptide and the base of the SF162, SF2, and US4 V2 loops (Table 1) is similar, suggests that although our immunogens are capable of eliciting antibodies against the base of the V2 loop, these antibodies do not have access to their epitopes on the heterologous envelopes tested here.
Only one of the two SF162gp140-immunized animals generated antibodies against the carboxy-terminal, but not the amino-terminal, side of the SF162-derived V1 loop, and neither animal elicited anti-V1 loop antibodies capable of binding to the heterologous envelopes examined. In contrast, all four ΔV2gp140-immunized animals elicited antibodies capable of binding to the carboxy-terminal side of the homologous V1 loop, two animals (animals I708 and K863) elicited antibodies capable of binding to the carboxy-terminal side of the SF2 V1 loop, and two animals (animals J408 and I708) elicited antibodies capable of binding to the amino-terminal side of both the homologous and heterologous V1 loops tested. Therefore, deletion of the V2 loop from the SF162gp140 immunogen alters the immunogenicity of the V1 loop.
A second region affected by the deletion of the V2 loop is the V3 loop. The relative ratio of anti-V3-loop to non-anti-V3-loop antibodies that are capable of binding to the SF162 gp120 protein is significantly higher in sera collected from the ΔV2gp140-immunized animals than from the SF162gp140-immunized animals (Table 3). This observation indicates that deletion of the crown of the SF162-derived V2 loop unmasks elements of the V3 loop and alters their immunogenicity. Our present results are in support of previous observations that modifications introduced within and around the V2 loop of the SF162 envelope alter the antigenic structure of the virion-associated envelope and render this virus more susceptible to neutralization by certain anti-V3-loop antibodies (23).
The anti-V3-loop antibodies elicited by the SF162gp140 immunogen are capable of binding to the V3 loop on the heterologous gp120s tested here (Table 4). In fact, up to 70% of the total antibodies capable of binding to these gp120 proteins recognized elements of the V3 loop. Thus, not only does a smaller fraction of the total antibodies elicited by SF162gp140 recognize the heterologous rather than the homologous gp120s tested here but, in addition, the antibodies that bind to the heterologous gp120s recognize primarily one region, the V3 loop. In contrast, the relative ratio of anti-V3-loop binding antibodies in the sera collected from the ΔV2gp140-immunized animals was the same (approximately 30%) regardless of whether the target envelope was the homologous SF162 or the two heterologous gp120s tested. Interestingly, one of the ΔV2gp140-immunized animals, animal K863, which received the fused version of the ΔV2gp140 immunogen, elicited antibodies capable of binding to the homologous but not the heterologous V3 loops examined. It is unclear why the SF162gp140 immunogen elicits higher relative titers of antibodies recognizing the V3 loop on heterologous gp120s. It is possible that epitopes that are common among the gp120s tested here exist on the SF162 gp120 protein and that deletion of the V2 loop alters their structure or exposure.
Although both the SF162gp140 and ΔV2gp140 immunogens elicited antibodies against the V1, V2, and V3 loops, they did not elicit antibodies against the V4 and V5 loops examined. This is not unexpected since the V4 and V5 loops are part of the immunologically silent phase of the HIV envelope (19, 44, 45).
Very similar antibody titers against the C2 region were elicited by our two immunogens. Thus, deletion of the V2 loop does not alter the overall immunogenicity of the C2 region. The fact that the anti-C2 antibodies elicited by the two immunogens bind to the carboxy-terminal, but not the amino-terminal, side of this region most likely suggests that the amino-terminal half of C2 is not immunogenic on the SF162gp140 immunogen and that deletion of the V2 loop does not increase its immunogenicity.
It is believed that the C1 and C5 regions are in close proximity (27) and elements of both regions interact with the extracellular portion of gp41 (43). We expect, therefore, the C1 and C5 regions to be occluded within the oligomeric envelope structure (19, 44, 45) and that antibodies recognizing these regions would preferentially bind to the monomeric rather than to the oligomeric envelope (17), although binding of anti-C5 antibodies to the virion-associated envelope form has been demonstrated (30). However, the SF162gp140 immunogen elicited anti-C1, but not anti-C5, antibodies. This is an indication that on that protein, the C1, but not the C5, region is exposed. It is possible that the replacement of the first 27 amino acids of gp120 by those of the tissue-specific plasminogen activator signal sequence (see Materials and Methods) alters the exposure of the remaining portion of C1. Deletion of the V2 loop does not affect the immunogenicity of the C1 region. However, the fact that animals H445 and K863 (immunized with ΔV2gp140) elicited antibodies capable of binding to the C1 region of SF162 gp120 and SF2 gp120, but not of US4 gp120, suggests that the anti-C1 antibodies generated by these two animals recognize different epitopes than those recognized by the antibodies generated in the remaining animals (which generated anti-C1 antibodies against all three gp120s tested).
In contrast to the SF162gp140-immunized animals, all four animals immunized with the ΔV2gp140 immunogen elicited binding antibodies to C5 on the heterologous gp120s tested and one of these animals (animal K863) elicited antibodies capable of binding to the C5 region on the homologous SF162 gp120. Thus, the C5 region is better exposed on the SF2 and US4 gp120 than on the SF162 gp120. Our results also suggest that the C5 region is poorly immunogenic on the SF162gp140 protein and that deletion of the V2 loop increases its immunogenicity. Based on structural predictions made upon examination of the gp120 protein core (19, 45, 46) and by antibody cross-competition studies (26), we do not believe that the V2 loop is positioned in a way that directly masks the C5 region on the SF162gp140 envelope. Most likely, deletion of the V2 loop results in global structural changes that indirectly affect the structure and/or exposure of C5. Wyatt et al. previously reported that HXBc2-derived gp160 constructs lacking portions of the V1V2 region are not as efficiently processed as the parental envelope, suggesting that deletion of the V1V2 region affects the structure and/or exposure of the C5 region (46).
In addition to the V2 loop, the gp120-gp41 cleavage site also appears to affect the immunogenic structure of the C5 region. Almost half of the total antibodies elicited by animal K863 (one of the two animals immunized with the fused form of the ΔV2gp140 immunogen) that are capable of binding to the heterologous SF2 and US4 gp120s target the C5 region. Previous studies reported that elimination of the gp120-gp41 cleavage site from soluble gp140 constructs can alter the structure and/or positioning of certain C1 and C5 epitopes (36, 47). Our results also suggest that the form (cleavable or fused) of the envelope immunogen used during the DNA-priming phase of our immunization methodology could affect the type of antibodies elicited during the subsequent protein-boosting phase of immunization. Further studies with additional animals need to be performed to verify this possibility.
Our present immunogenicity studies indicate that the partial deletion of the V2 loop from the SF162gp140 immunogen does not increase the immunogenicity of the CD4-BS and are in general agreement with previous envelope antigenicity studies, which indicated that this modification does not result in an increase in the exposure of the CD4-BS on the SF162gp140 protein (39), even though the V1V2 region masks elements of the CD4-BS (26, 44, 45). It is, however, possible that the antibodies elicited by the ΔV2gp140 immunogen recognize different epitopes within the CD4-BS than those recognized by the antibodies elicited by the SF162gp140 immunogen. Interestingly, although deletion of the V2 loop does not directly result in an increase in the immunogenicity of the CD4-BS, the relative ratio of anti-CD4-BS to non-CD4-BS antibodies is higher in sera collected from the ΔV2gp140-immunized animals than in sera collected from the SF162gp140-immunized animals (in the case of antibody binding to the heterologous envelopes tested). For example, the relative ratio of anti-CD4-BS to anti-V3-loop antibodies binding to the SF2 or US4 gp120s, is 4- to 10-fold higher in sera collected from the ΔV2gp140-immunized animals than in sera collected from the SF162gp140-immunized animals.
Overall, our studies indicate that deletion of the V2 loop from the SF162gp140 immunogen differentially alters the immunogenic structure of certain envelope regions that are already immunogenic and renders certain additional regions immunogenic. As a result, sera collected from the SF162gp140- and ΔV2gp140-immunized animals not only contain antibodies that recognize different regions on target HIV envelopes but also contain different relative titers of antibodies that recognize the same regions. We believe that this difference in antibody composition is responsible for the recorded differential ability of these sera to mediate neutralization of diverse HIV-1 isolates.
Acknowledgments
These studies were supported by NIH grant RO1 AI47708 (to L.S.). L.S. also acknowledges the Seattle Biomedical Research Institute's private donors for financial support. Protein purification and characterization studies at Chiron were supported by the NIH (grants N01-A195367-TO#1 and N01-A1-95367-TO#3).
We thank Elaine Kan, Ying Lian, Susan Hilt, and Karen Matsuoka for expert technical assistance and Cheryl Saunders, Nina Derby, and Ruth McCaffrey for critical reading of the manuscript.
REFERENCES
- 1.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns, D. P., C. Collignon, and R. C. Desrosiers. 1993. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J. Virol. 67:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng-Mayer, C., J. Homsy, L. A. Evans, and J. A. Levy. 1988. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc. Natl. Acad. Sci. USA 85:2815-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng-Mayer, C., R. Liu, N. R. Landau, and L. Stamatatos. 1997. Macrophage tropism of HIV-1 and utilization of the CC-CKR5 coreceptor. J. Virol. 71:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherpelis, S., X. Jin, A. Gettie, D. D. Ho, S. W. Barnett, I. Shrivastava, and L. Stamatatos. 2001. DNA-immunization with a V2 deleted HIV-1 envelope elicits protective antibodies in macaques. Immunol. Lett. 79:47-55. [DOI] [PubMed] [Google Scholar]
- 10.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162ΔV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J. Virol. 75:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ditzel, H. J., J. M. Binley, J. P. Moore, J. Sodroski, N. Sullivan, L. S. W. Sawyer, R. M. Hendry, W.-P. Yang, C. F. Barbas III, and D. R. Burton. 1995. Neutralizing recombinant human antibodies to a conformational V2- and CD4-binding site-sensitive epitope of HIV-1 gp120 isolated by using an epitope-masking procedure. J. Immunol. 154:893-906. [PubMed] [Google Scholar]
- 12.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earl, P. L., R. W. Doms, and B. Moss. 1990. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 87:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edinger, A. L., M. Ahuja, T. Sung, K. C. Baxter, B. Haggarty, R. W. Doms, and J. A. Hoxie. 2000. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J. Virol. 74:7922-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouts, T. R., A. Trkola, M. S. Fung, and J. P. Moore. 1998. Interactions of polyclonal and monoclonal anti-glycoprotein 120 antibodies with oligomeric glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res. Hum. Retrovir. 14:591-597. [DOI] [PubMed] [Google Scholar]
- 17.Gorny, M. K., T. C. VanCott, C. Williams, K. Revesz, and S. Zolla-Pazner. 2000. Effects of oligomerization on the epitopes of the human immunodeficiency virus type 1 envelope glycoproteins. Virology 267:220-228. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, W. E., J. Morgan, J. Reitter, B. A. Puffer, S. Czajak, R. W. Doms, and R. C. Desrosiers. 2002. A replication-competent, neutralization-sensitive variant of simian immunodeficiency virus lacking 100 amino acids of envelope. J. Virol. 76:2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature (London) 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahm, H. W., and S. Stein. 1985. Characterization of recombinant human interleukin-2 with micromethods. J. Chromatogr. 326:357-361. [DOI] [PubMed] [Google Scholar]
- 21.Levy, J. A., A. D. Hoffman, S. M. Kramer, J. A. Landis, J. M. Shimabukuro, and L. S. Oshiro. 1984. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science 225:840-842. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Z., C. Wood, J. A. Levy, and C. Cheng-Mayer. 1990. The viral envelope is involved in macrophage tropism of a human immunodeficiency virus type 1 strain isolated from brain tissue. J. Virol. 64:6148-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malenbaum, S. E., D. Yang, L. Cavacini, M. Posner, J. Robinson, and C. Cheng-Mayer. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 74:11008-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, and the NIAID AIDS Vaccine Evaluation Group. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 26.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore, J. P., R. L. Willey, G. K. Lewis, J. Robinson, and J. Sodroski. 1994. Immunological evidence for interactions between the first, second, and fifth conserved domains of the gp120 surface glycoprotein of human immunodeficiency virus type 1. J. Virol. 68:6836-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Y. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 99:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan, S. V., S. Mukherjee, F. Jia, Z. Li, C. Wang, L. Foresman, C. McCormick-Davis, E. B. Stephens, S. V. Joag, and O. Narayan. 1999. Characterization of a neutralization-escape variant of SHIVKU-1, a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology 256:54-63. [DOI] [PubMed] [Google Scholar]
- 30.Nyambi, P. N., H. A. Mbah, S. Burda, C. Williams, M. K. Gorny, A. Nadas, and S. Zolla-Pazner. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol. 74:7096-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 32.Richardson, J. T. M., B. L. Stryjewski, C. C. Broder, J. A. Hoxie, J. R. Mascola, P. L. Earl, and R. W. Doms. 1996. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J. Virol. 70:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomers. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulke, N., M. S. Vesanen, R. W. Sanders, P. Zhu, M. Lu, D. J. Anselma, A. R. Villa, P. W. Parren, J. M. Binley, K. H. Roux, P. J. Maddon, J. P. Moore, and W. C. Olson. 2002. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J. Virol. 76:7760-7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary r5 subtype B human immunodeficiency virus. J. Virol. 76:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 72:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatatos, L., M. Lim, and C. Cheng-Mayer. 2000. Generation and structural analysis of soluble oligomeric envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 16:981-994. [DOI] [PubMed] [Google Scholar]
- 40.Stamatatos, L., M. Wiskerchen, and C. Cheng-Mayer. 1998. Effect of major deletions in the V1 and V2 loops of a macrophage-tropic HIV-1 isolate on viral envelope structure, cell-entry and replication. AIDS Res. Hum. Retrovir. 14:1129-1139. [DOI] [PubMed] [Google Scholar]
- 41.Stamatatos, L., S. Zolla-Pazner, M. Gorny, and C. Cheng-Mayer. 1997. Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells. Virology 229:360-369. [DOI] [PubMed] [Google Scholar]
- 42.Sugiura, W., C. C. Broder, B. Moss, and P. L. Earl. 1999. Characterization of conformation-dependent anti-gp120 murine monoclonal antibodies produced by immunization with monomeric and oligomeric human immunodeficiency virus type 1 envelope proteins. Virology 254:257-267. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt, R., E. Desjardin, U. Olshevsky, C. Nixon, J. Binley, V. Olshevsky, and J. Sodroski. 1997. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J. Virol. 71:9722-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature (London) 393:705-711. [DOI] [PubMed] [Google Scholar]
- 45.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 46.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, X., L. Florin, M. Farzan, P. Kolchinsky, P. D. Kwong, J. Sodroski, and R. Wyatt. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74:4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.zur Megede, J., M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 74:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]