Abstract
A series of nonnucleoside, N-α-methylbenzyl-N′-arylthiourea analogs were identified which demonstrated selective activity against varicella-zoster virus (VZV) but were inactive against other human herpesviruses, including herpes simplex virus. Representative compounds had potent activity against VZV early-passage clinical isolates and an acyclovir-resistant isolate. Resistant viruses generated against one inhibitor were also resistant to other compounds in the series, suggesting that this group of related small molecules was acting on the same virus-specific target. Sequencing of the VZV ORF54 gene from two independently derived resistant viruses revealed mutations in ORF54 compared to the parental VZV strain Ellen sequence. Recombinant VZV in which the wild-type ORF54 sequence was replaced with the ORF54 gene from either of the resistant viruses became resistant to the series of inhibitor compounds. Treatment of VZV-infected cells with the inhibitor impaired morphogenesis of capsids. Inhibitor-treated cells lacked DNA-containing dense-core capsids in the nucleus, and only incomplete virions were present on the cell surface. These data suggest that the VZV-specific thiourea inhibitor series block virus replication by interfering with the function of the ORF54 protein and/or other proteins that interact with the ORF54 protein.
Varicella-zoster virus (VZV) causes a disseminated primary infection, chickenpox, and later may reactivate to cause herpes zoster, commonly known as shingles (3, 26). Herpes zoster can result in pain lasting over 1 month from the onset of the rash, termed postherpetic neuralgia (PHN). PHN is often a debilitating condition that is quite difficult to treat (15, 32, 33).
Acyclovir (ACV), valaciclovir, and famciclovir are the primary antivirals used for treating VZV infections in both immunocompetent and immunocompromised individuals (25). Unfortunately, despite these medications, PHN remains a major problem, and the currently available drugs have a limited effect on prevention of this condition. Thus, there is a need for additional, more effective medications to treat herpes zoster in adults.
The development of antiviral resistance remains a concern, particularly when treatment relies on only a small number of approved drugs with common modes of action (13, 14, 25, 26). ACV, valaciclovir (which is metabolized to acyclovir), and famciclovir (the prodrug of penciclovir) are all phosphorylated by the viral thymidine kinase as an initial step for intracellular activation. They are subsequently phosphorylated by cellular kinases, and the activated compounds inhibit the viral DNA polymerase. Sorivudine and brivudine are also potent inhibitors of VZV, and although they represent a different class of nucleoside analogues from ACV, they also require phosphorylation by the viral thymidine kinase for activation (18, 31). While thymidine kinase-deficient VZV can be treated with foscarnet, a direct inhibitor of the viral DNA polymerase (25), this drug requires intravenous administration and has a marked potential for nephrotoxicity. Furthermore, foscarnet-resistant strains of VZV have been reported (23, 30). Since each of the currently available drugs used to treat VZV acts by inhibiting the viral DNA polymerase, additional agents with novel mechanisms of action would be useful treatment options.
Recently, Bloom et al. (4, 5) reported the discovery of a new class of thiourea compounds that inhibit herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) through a novel mechanism of action (29). The HSV UL6 protein, one of the seven HSV proteins shown to be essential for viral DNA cleavage and packaging, was shown to be the likely target of these compounds. Interestingly, these compounds had little activity against other human herpesviruses, including VZV.
In this study, we describe a different class of nonnucleoside inhibitors, N-α-methylbenzyl-N′-arylthiourea analogs, that demonstrate specific and potent activity against VZV. We show that these thiourea compounds appear to target the VZV open reading frame 54 (ORF54) protein, the homolog of the HSV-1 UL6 protein (17, 21, 29).
MATERIALS AND METHODS
Chemical compounds.
All compounds were prepared as follows. 4-N-Boc-phenylendiamine or an appropriately substituted 4-nitroaniline was acylated with the requisite acid chloride. Deprotection with neat trifluoroacetic acid (for the Boc-protected series) or reduction of the nitro groups (cyclohexene, 10% Pd-C, refluxing ethanol) resulted in the desired anilines, which were subsequently acylated with a commercially available isothiocyanate to give the final products. The compounds reported in this paper were tested as racemic mixtures, and later studies indicated that the anti-VZV activity of these compounds resides entirely with the S enantiomer (T. Mansour, J. Upselacis, M. Digrandi, and J. Bloom, Department of Chemical Sciences, Wyeth, Pearl River, N.Y., personal communication). The approximate molecular masses of the compounds are as follows: Comp I, 412 Da; Comp II, 437 Da; and Comp III, 467 Da.
Cells and viruses.
Monolayer cultures of human foreskin fibroblasts (HFF cells) were used for propagation of VZV strain Ellen (American Type Culture Collection, Manassas, Va.) and the resistant isolates EllenrA and EllenrB and for preparation of ROka stocks (ROka, ROka54A3, ROka54A4, and ROka54B) for use in antiviral assays and kinetics of growth analyses.
MeWo (human melanoma) cells were used for transfections and for the initial propagation of VZV ROka and ROka mutant virus stocks (8). Recombinant viruses were derived from cosmids corresponding to the attenuated Oka strain of VZV (7, 27).
Cell-free VZV was derived by scraping infected HFF cells into Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 10% sorbitol followed by brief sonication. The preparation was processed by low-speed centrifugation at 4°C, and the resulting supernatant was checked microscopically for efficient cell disruption. Cell-free supernatants were used on the day of preparation for growth analysis experiments.
Growth curves of VZV parental and mutant viruses were generated by inoculating HFF cells with VZV-infected cell stocks or cell-free virus derived from infected-cell supernatants. Infected monolayers were harvested at the designated times postinfection and subjected to titer determination on uninfected HFF monolayers.
HSV, human cytomegalovirus (HCMV), and respiratory syncytial virus (RSV) strains, their growth, and their respective antiviral assays have been described previously (11, 22, 29).
VZV plaque reduction assay.
HFF cells were infected with approximately 50 to 100 PFU of VZV-infected HFF cell stocks per well. When testing inhibitors, selected compounds were diluted to the desired concentrations in DMEM containing 0.3% dimethyl sulfoxide (DMSO) and applied to uninfected HFF monolayers for a 30 min preincubation before the addition of VZV-infected cells. Positive control wells, which were to receive VZV-infected cells without compound, were also treated with DMEM containing 0.3% DMSO. DMSO at a final concentration of 0.3% was not observed to have any negative or toxic effects on either uninfected or VZV-infected HFF cells (data not shown). Monolayers were incubated for 6 days at 37°C, fixed, and stained. Plaques were counted, and the data are presented as the mean of at least three independent assays.
VZV high-throughput robotic ELISA.
An automated enzyme-linked immunosorbent assay (ELISA) was developed to identify antiviral leads with activity against VZV strain Ellen. The assay was designed to measure the extent to which VZV replication (in HFF cells) was inhibited by various thiourea compounds.
On day 1, VZV-infected HFF cell stocks were thawed and diluted onto uninfected HFF cells. The optimal ratio of VZV-infected cells to uninfected HFF cells was determined by subjecting the VZV-infected cell stock to titer determination on uninfected cells, measuring the VZV-specific ELISA signal, and minimizing the assay background signal while maximizing the VZV-specific signal within a linear range. Tissue culture flasks containing the desired ratio of infected to uninfected cells were incubated overnight at 37°C under 5% CO2.
The assay was run in a 96-well plate format as follows. On day 2, compound stocks (in 100% DMSO) were diluted into DMEM containing 0.3% DMSO to yield 4× working stocks. Then 100 μl of DMEM plus 2% FBS was added to all wells, and 100 μl of 4× drug stock was added to column 3 of the plates. A 100-μl volume was then serially transferred out to column 12. A 100-μl volume was then removed from column 12, leaving 100 μl/well in columns 3 thru 12. VZV-infected cells from day 1 were harvested by trypsinization and resuspended to 3.5 × 105 cells/ml, of which 100 μl was applied to all 96 wells. All plates were incubated at 37°C under 5% CO2 for 3 days.
Column 1 of the 96-well plate was the background signal control, consisting of VZV-infected cells incubated with 100 μg of ganciclovir per ml. Column 2 contained infected cells in DMEM containing 0.3% DMSO without sample compound and served as a positive virus control.
On day 5, the assay mixture was developed as follows. Medium was aspirated from all wells, and 50 μl of fixative solution (50% methanol, 50% acetone) was added per well. Plates were incubated at room temperature for 10 min and then washed with ELISA wash buffer (phosphate-buffered saline [PBS] with 0.05% Triton X-100) three times. A 1:500 dilution of primary monoclonal VZV glycoprotein B (gB) antibody (Advanced Biotechnologies, Inc., Columbia, Md.) in PBS plus 1% bovine serum albumin (BSA) was added to all wells, which were then incubated for 90 min. The plates were washed with ELISA buffer, and then a 1:500 dilution of β-galactosidase-linked mouse IgG (H+L) secondary antibody (American Qualex, San Clemente, Calif.) in PBS plus 1% BSA was added. The plates were incubated for an additional 90 min and washed with ELISA buffer, and 50 μl of the fluorogenic substrate 4-methylumbelliferyl-β-d-galactosidase (Sigma, St. Louis, Mo.) was added per well. The plates were incubated for 60 min at 37°C or 120 min at room temperature, and the intensity of released fluorescent product was determined in a fluorescence plate reader (excitation, 360 nm; emission, 465 nm). The results were analyzed to determine the 50% inhibitory concentrations (IC50) of various compounds by comparison to the maximum possible signal (defined as the signal observed in infected cells without compound [column 2] minus the background noise from infected cells incubated with 100 μg of ganciclovir per ml [column 1]).
MTS cytoxicity assay.
An MTS cytotoxicity assay was performed to determine the 50% cytotoxic concentration (CC50) of various compounds in actively growing (subconfluent) Vero cell monolayers (9). The assay solution contained the tetrazolium compound MTS ([3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium, inner salt) and an electron-coupling reagent, phenazine ethosulfate. The relative in vitro cytotoxic effect of individual compounds was assessed by observing the decrease in the cellular reduction of MTS into its purple formazan product. Mitochondrial enzymes of viable cells convert MTS to the colored product. The relative amount of formazan product generated was determined using a spectrophotometer at an absorbance of 490 nm. The CC50 was defined as the sample compound concentration required to achieve 50% of the reference (cells without compound) MTS cleavage signal, after subtraction of the background (cell-free signal).
Indirect immunofluorescence of VZV-infected cells.
Confluent HFF cell monolayers were either mock infected or infected with cell-free VZV at a multiplicity of infection (MOI) of 0.001 (1 PFU/1,000 cells). Comp I was added at the indicated times at a concentration of approximately 1.0 μg/ml (five times the IC50) in complete medium containing 0.3% DMSO. The cells were fixed in 100% methanol, washed in PBS with 3% BSA, and incubated with monoclonal antibody to either IE62 (immediate-early gene 62) protein or gB (Advanced Biotechnologies). The cells were washed with PBS, incubated with fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody, and examined by fluorescence microscopy.
Isolation of VZV strain Ellen Comp I-resistant isolates: EllenrA and EllenrB.
HFF cells were infected at an MOI of ∼0.01 of parental VZV Ellen-infected cell stock in approximately 0.63 μM Comp I (0.26 μg/ml). After >50% cytopathic effect (CPE) was observed, an infected-cell stock was prepared and a portion was used to infect fresh HFF monolayers in the presence of twofold-increasing concentrations of inhibitor. The process was repeated four times, and the continued observation of CPE on passage indicated that virus was able to grow in the presence of the inhibitor at >10-fold the IC50 of Comp I. Two Comp I-resistant viruses, EllenrA and EllenrB, were plaque purified from separately derived resistant virus pools.
An ACV-resistant VZV strain Ellen mutant (Ellen-ACVr) was also isolated by a method similar to that described above, except that the virus was passaged in increasing concentrations of ACV.
Hirt prep for VZV genomic DNA.
VZV-infected monolayers showing ∼90% CPE were washed twice with ice-cold PBS and solubilized in 0.8 ml of TES with sodium dodecyl sulfate (10 mM Tris [pH 7.5], 0.6% sodium dodecyl sulfate, 50 mM NaCl, 10 mM EDTA). The viscous lysate was incubated with 2 μl of 10-mg/ml RNase A (Sigma) for 45 min at 37°C. After the addition of 8 μl of 20-mg/ml proteinase K (Sigma), the mixture was incubated for an additional 4 h at 37°C. Then 200 μl of 5 M NaCl was added, and the lysate was centrifuged at 12,000 rpm in an Eppendorf 5414C at 4°C. The supernatant was harvested and extracted with phenol-chloroform, and the DNA was precipitated with ethanol. The resulting DNA was used for PCR amplification and cloning.
Transfections and isolation of ROka recombinants.
Cosmids were digested with NotI or Bsu36I to release the VZV insert DNA. Melanoma cells were transfected with plasmid pCMV62 (50 ng) and cosmids VZV NotIA, MstIIB, NotIB, and either MstIIA, MstIIA-54A, or MstIIA-54B (1 μg each) as described previously (8).
Electron microscopy.
Melanoma cells were infected with VZV ROka or ROka54B at an MOI of 0.5 for 1 h. The cells were then washed five times with PBS, and medium was added containing either Comp I (2 μg/ml) in 0.3% DMSO or 0.3% DMSO alone. After 20 h, the medium was removed and the cells were washed in PBS and scraped in 4% paraformaldehyde-1.7% glutaraldehyde in HEPES-PIPES (pH 7.2). After 2 h of fixation, the cells were washed and fixed in 1% osmium tetroxide. The samples were stained with 2% uranyl acetate, dehydrated in graded ethanols, embedded in plastic resin, and sectioned. The sections were mounted on copper grids, stained with lead citrate, and examined by transmission electron microscopy by an observer who was blinded to the treatment of the cells.
RESULTS
New class of thiourea analogs specific for the inhibition of VZV.
van Zeijl et al. (29) described a novel class of thiourea compounds that inhibited the replication of HSV-1 but not VZV (at the highest concentration tested). Screening of new compounds revealed that the addition of a spacer, -HC(CH3)-, between the aryl ring and the thiourea nitrogen yielded activity against VZV but loss of activity against HSV. Subsequently, a novel class of N-α-methylbenzyl-N′-arylthiourea analogs was developed with potent and specific activity for inhibiting VZV replication. Three N-α-methylbenzyl-N′-arylthiourea analogs (Comp I, Comp II, and Comp III) were selected for further study (Fig. 1).
FIG. 1.
Structures of Comp I, Comp II, and Comp III.
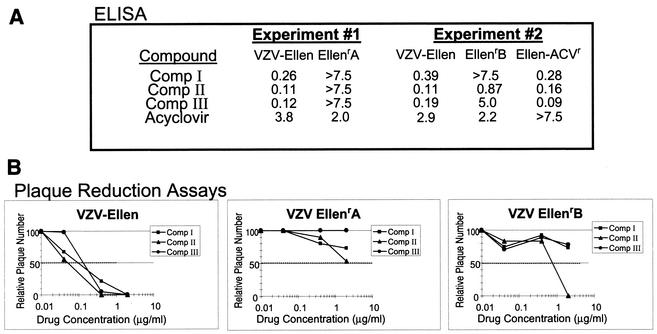
Each of the three thiourea compounds exhibited potent in vitro antiviral activity as measured by VZV-specific ELISA (Table 1) and subsequently confirmed by standard plaque reduction assay. IC50s of all three compounds consistently ranged from 0.1 to 0.6 μM when tested against the VZV laboratory strain Ellen. Similar results were observed for three VZV low-passage clinical isolates, with IC50s ranging from 0.3 to 1.4 μM. Significant activity was not observed against HSV-1, HCMV, or RSV, indicating that the antiviral activity of these compounds is specific for VZV. Minimal cytotoxicity was observed in growing Vero cells, with a CC50 of >36 μM as determined by a tetrazolium dye-based (MTS) assay. HFF monolayers showed no visually apparent cytotoxic effects at compound concentrations of >20 μM when observed during plaque reduction assays.
TABLE 1.
Activity of selected thiourea inhibitors
| Virus | Strain | IC50a of:
|
|||||
|---|---|---|---|---|---|---|---|
| Comp I
|
Comp II
|
Comp III
|
|||||
| μg/ml | μM | μg/ml | μM | μg/ml | μM | ||
| VZV | Ellen | 0.26 | 0.63 | 0.05 | 0.11 | 0.05 | 0.12 |
| VZV | SC44 | 0.32 | 0.78 | 0.14 | 0.32 | NDb | ND |
| VZV | JAB1233 | 0.44 | 1.07 | 0.23 | 0.53 | 0.16 | 0.34 |
| VZV | BKA1244 | 0.59 | 1.43 | 0.18 | 0.41 | ND | ND |
| HSV-1 | Patton | >10 | >25 | >10 | >25 | >10 | >25 |
| HCMV | AD169 | >7 | >17 | >10 | >17 | >10 | >17 |
| RSV | A2 | >10 | >25 | >10 | >25 | >10 | >25 |
| MTS(CC50) | Vero | >15 | >36 | >15 | >36 | >15 | >36 |
IC50s were determined by growing viruses in the presence of various concentrations of compound. Inhibition of viral replication was determined by ELISA as described in Materials and Methods. Values with a > sign represent the highest concentration tested.
ND, not determined.
Comp I does not inhibit VZV immediate-early or late gene expression.
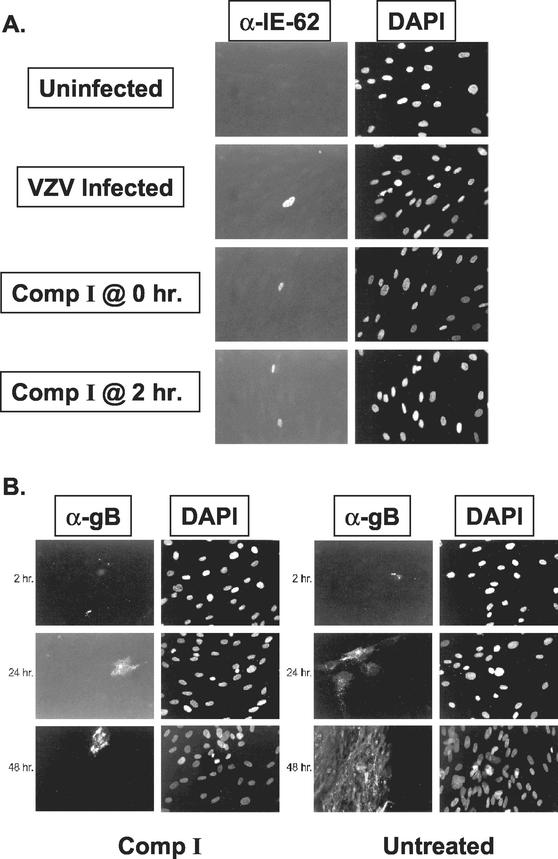
Initial experiments to determine the mechanism of action of one of the thiourea compounds (Comp I) examined the effect of the inhibitor on VZV gene expression. Treatment of HFF with Comp I at 0 or 2 h after infection with cell-free VZV did not affect the expression of the VZV IE62 protein at 8 h postinfection (Fig. 2A). We observed approximately the same number of cells expressing IE62 protein irrespective of the presence or absence of Comp I. Hence, Comp I does not appear to have a significant effect on viral attachment, entry, or immediate-early gene expression.
FIG. 2.
Indirect immunofluorescence analysis of VZV IE62 protein (A) or gB (B) expression in the presence or absence of Comp I. Panels on the left are stained with monoclonal antibodies specific for VZV proteins. Panels on the right show the adjacent field of cells stained with 4′,6-diamidino-2-phenylindole (DAPI).
Cells treated with Comp I at the time of infection and examined at 2, 24, or 48 h postinfection showed viral gB expression in single, isolated cells (Fig. 2B). Individual VZV-infected cells could be observed at 24 and 48 h posttreatment that showed an overaccumulation of gB, both on the surface of and inside the cells, compared with cells that were not treated with Comp I. Infection in the absence of the compound resulted in large foci of gB-expressing cells at 24 and 48 h. Apparently, cells infected in the presence of Comp I can proceed through viral immediate-early gene expression and into the later stages of viral gene expression, but the infection does not spread beyond the initially infected cells. These results suggested that Comp I was targeting a later stage of viral replication such as virion morphogenesis, egress, or, like the previously described HSV thiourea inhibitors, viral DNA cleavage and packaging.
Isolation and characterization of resistant viruses.
In an effort to identify the target of the antiviral compounds, we generated resistant viruses by passaging VZV strain Ellen in the presence of Comp I. Two virus isolates, designated EllenrA and EllenrB, were plaque purified from two separate virus pools obtained from serial passage of Ellen in increasing concentrations of Comp I. The resistant isolates were tested for their ability to replicate in the presence of several structurally diverse compounds from this chemical series. Depending on the compound assayed, IC50s for the resistant viruses ranged from 8- to >50-fold higher than those observed for the parental virus in both the ELISA (Fig. 3A) and the plaque reduction assay (Fig. 3B). The EllenrA and EllenrB isolates were found to be sensitive to ACV at levels similar to parental Ellen, and an ACV-resistant Ellen isolate (Ellen-ACVr) was found to be inhibited by all three of the thiourea compounds (Fig. 3A).
FIG. 3.
Activity of selected compounds against the VZV strain Ellen Comp I-resistant isolates. (A) IC50 (micrograms per milliliter) were derived from automated ELISA results of two separate experiments comparing the parental strain Ellen and the two individually isolated Ellenr mutants. Comp II and Comp III, structurally related compounds within the VZV thiourea inhibitor series, were tested along with Comp I. (B) Plaque reduction assays show the percent reduction in plaque number for various concentrations of the three compounds against parental Ellen and the Ellenr isolates. The data represent the average number of plaques observed in triplicate samples for all viruses at each indicated drug concentration. The dotted line indicates a 50% reduction in the number of plaques. Data were normalized so that the average number of plaques observed in the no-drug control samples for any one virus was 100.
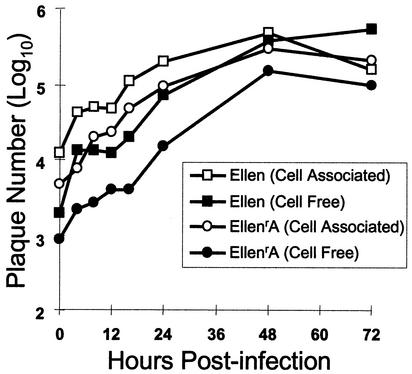
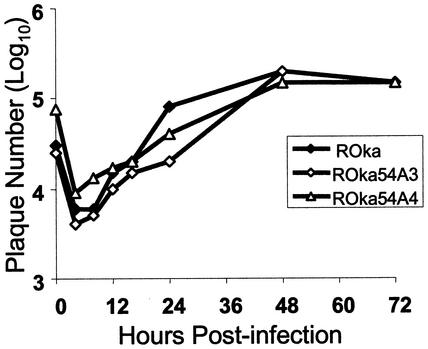
VZV isolates resistant to inhibitor compounds grew to titers similar to those of parental VZV Ellen. Cell-associated and cell-free viruses replicated to peak titers of ∼1.5 and ∼2 logs higher than their inocula, respectively (Fig. 4). Hence, the mutation(s) conferring resistance to the thiourea antiviral compounds had little or no effect on the growth kinetics of the isolates in vitro.
FIG. 4.
In vitro growth kinetics of VZV EllenrA. HFF were inoculated with cell-associated or cell-free virus, and at various times postinfection the cells were harvested and virus titers were determined on fresh HFF monolayers. A comparison of the parental strain Ellen and the EllenrA isolate is shown. Data points represent the average of three independent assays.
Identification of possible sites conferring resistance to the thiourea compounds.
Our results suggested that the VZV inhibitors did not block the initial cycle of viral gene expression but did inhibit the spread of infectious virus to uninfected cells. Since a structurally related series of HSV inhibitors were shown to target the HSV UL6 gene (29), we cloned and sequenced ORF54, the VZV UL6 homolog, from strain Ellen, the two strain Ellen Comp I-resistant isolates (EllenrA and EllenrB), and an ACV-resistant Ellen isolate (Ellen-ACVr). The 2,307-bp sequence of ORF54 from strain Ellen revealed a single nucleotide change of A to G at position 630 compared to the previously sequenced Dumas strain (10). This difference did not result in a change in the predicted amino acid sequence of the Ellen strain ORF54 protein with respect to the predicted amino acid sequence of the ORF54 protein from the Dumas strain. The ORF54 sequence of Ellen-ACVr was identical to the parental Ellen sequence.
Comparison of the nucleotide sequence of ORF54 from EllenrA with that from the parental strain showed two changes, from A to G at nucleotides 971 and 1222, resulting in two amino acid coding changes at residues 324 and 408, respectively (Fig. 5). EllenrB ORF54 showed a single nucleotide change from G to A at nucleotide 1220, resulting in only one amino acid change at residue 407 (Fig. 5). Amino acid 408 changed from asparagine (N) in VZV Ellen to aspartic acid (D) in EllenrA, and amino acid 407 changed from glycine (G) in VZV Ellen to aspartic acid (D) in EllenrB. Interestingly, the two changes resulted in an aspartic acid substitution at adjacent amino acid positions. Amino acid 324 changed from tyrosine (Y) in strain Ellen to cysteine (C) in EllenrA. This change was not observed in ORF54 from EllenrB.
FIG. 5.
ORF54 mutations associated with resistance to Comp I. The amino acid sequences of the ORF54 protein from parental VZV Ellen, recombinant Oka (ROka), and the ACV-resistant isolate (Ellen-ACVr) were identical. The regions corresponding to amino acids 320 to 329 and 400 to 409 of ORF54 are shown. Changes identified for the various resistant isolates are boxed.
Confirmation that changes in ORF54 are sufficient for the resistant phenotypes of EllenrA and EllenrB.
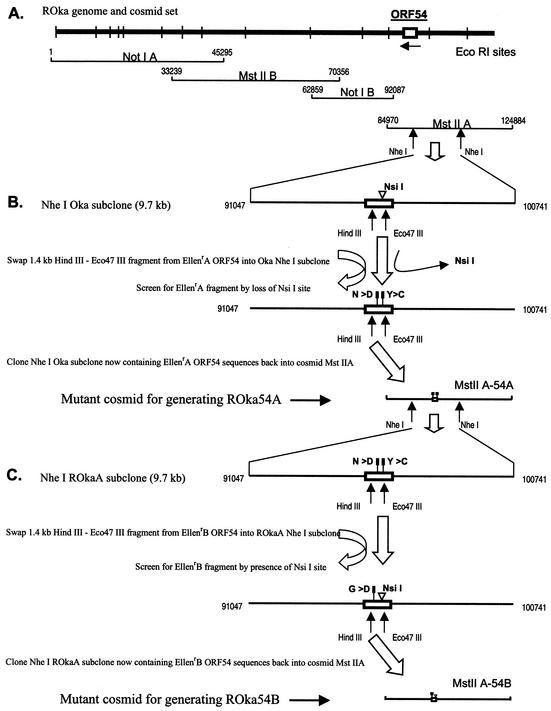
Marker transfer of the mutant ORF54 sequences from EllenrA or EllenrB to VZV strain Oka was performed using the Oka cosmid system. The predicted amino acid sequence of the ORF54 protein for the Oka strain is identical to that for VZV Ellen (2). Recombinant Oka virus (ROka), containing a portion of ORF54 from either EllenrA or EllenrB, was constructed by first replacing the Oka ORF54 sequences from cosmid MstIIA with the mutant Ellen ORF54 sequences and then transfecting the altered cosmid (MstIIA-54A or MstIIA-54B) with three other cosmids into melanoma cells (Fig. 6). Recombinant viruses (ROka54A3, ROka54A4, and ROka54B) were isolated, and the presence of ORF54 mutations was confirmed via sequence analyses (Fig. 5). A single isolate of ROka54B was identified that contained the single amino acid change found in EllenrB. Two independently derived ROka54A isolates, ROka54A3 and ROka54A4, were identified that contained the double amino acid change found in EllenrA.
FIG. 6.
Strategy for construction of recombinant VZV with specific mutations in ORF54. (A) Cosmid clones used for generation of VZV ROka. (B) A 1.4-kb fragment derived from ORF54 of EllenrA was inserted into a 9.7-kb NheI subclone from cosmid MstIIA in place of the wild-type sequence. One of the two nucleotide changes observed for the 1.4-kb EllenrA ORF54 fragment resulted in the elimination of an NsiI restriction site and was used to identify NheI EllenrA clones. The altered NheI fragment was inserted into cosmid MstIIA, in place of the wild-type ROka sequence, to generate cosmid MstIIA-54A. The ORF54 region within MstIIA-54A was sequenced to confirm that the expected changes were present: two nucleotide changes resulting in Y-to-C and N-to-D substitutions at amino acids 324 and 408, respectively. (C) A 1.4-kb fragment derived from ORF54 EllenrB was inserted into a 9.7-kb NheI subclone derived from cosmid MstIIA-54A. The 1.4-kb EllenrB fragment regenerated an NsiI restriction site in ORF54 that was used to identify NheI EllenrB clones. The altered NheI fragment was inserted into MstIIA to generate cosmid MstIIA-54B. The ORF54 region within MstIIA-54B was sequenced to confirm that the expected change was present: a single nucleotide change resulting in a G-to-D substitution at amino acid 407.
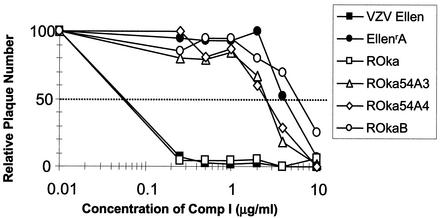
The ROka54A isolates grew to titers similar to that of the parental virus (ROka) in the absence of Comp I, indicating that the presence of the ORF54 mutations did not alter the growth properties of the recombinants in vitro (Fig 7). Each of the three ROkaORF54 mutants was resistant to Comp I at levels similar to those of the original resistant Ellen isolates as determined by the plaque reduction assay (Fig. 8). In contrast, the parental Ellen and ROka viruses were inhibited by Comp I as expected. Since the only changes expected in the recombinant viruses are mutations derived from the ORF54 gene of either EllenrA or EllenrB, we conclude that the likely target of the VZV thiourea compounds is the ORF54 protein.
FIG. 7.
In vitro growth kinetics of ROka54A3 and A4, containing ORF54 mutations derived from EllenrA. HFF were inoculated with VZV-infected cells, and at various times postinfection, cells were harvested and virus titers were determined on fresh HFF monolayers. Data points represent the average of three independent assays.
FIG. 8.
Activity of Comp I against parental and ORF54 mutant viruses. Plaque reduction assay data show the percent reduction in plaque number for VZV strains Ellen, EllenrA, ROka, and the ROka ORF54 mutants (ROka54A3, ROka54A4, and ROka54B) in the presence of increasing concentrations of Comp I. The data represent the average number of plaques observed in triplicate samples of all viruses at each drug concentration. Data were normalized so that the average number of plaques observed in the absence of Comp I was defined as 100.
Effect of inhibitors on capsid morphogenesis.
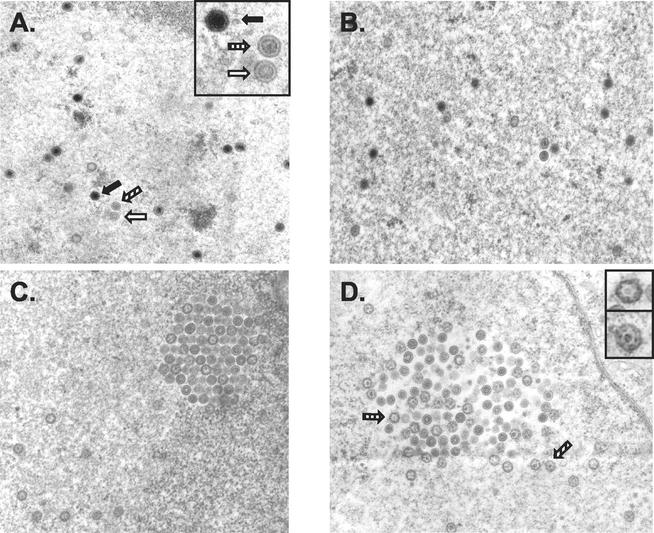
Melanoma cells infected with VZV ROka or ROka54B in either the absence or presence of Comp I were examined by transmission electron microscopy. Multiple capsid forms, similar to those described by Grose et al. (12), were observed in the nucleus of a cell infected with ROka in the absence of Comp I (Fig. 9A). Higher magnification (inset in Fig. 9A) revealed the three capsid types (empty, immature or aberrant, and DNA-containing dense core) observed in untreated control samples. In contrast, examination of >250 capsids in the nuclei of cells infected with ROka in the presence of Comp I showed no DNA-containing dense cores (Fig. 9C and D). Aberrant and immature capsids were readily evident and dominated the Comp I-treated samples. These results suggested that Comp I inhibited the formation of DNA-containing dense-core capsids. Sections prepared from cells infected with ROka54B in either the absence (data not shown) or presence (Fig. 9B) of Comp I were indistinguishable from sections prepared from ROka-infected cells without Comp I.
FIG. 9.
Electron micrographs of nuclei from VZV ROka-infected (A, C, and D) or ROka54B-infected (B) melanoma cells in the absence (A) or presence (B to D) of 2 μg of Comp I per ml. The insets in panels A and D provide a higher-power image of selected capsid particles from their representative panels. Open arrow, empty capsid; striped arrow, aberrant capsid; solid arrow, full DNA-containing, dense-core capsid.
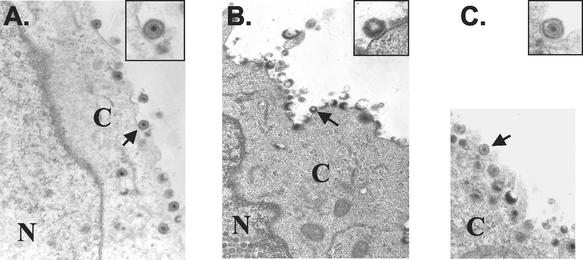
Electron microscopic examination of the cell membrane was performed to confirm that mature, DNA-containing virions were not budding from the surface of ROka-infected cells treated with Comp I. Several mature, fully enveloped capsids were present on the plasma membrane of a melanoma cell that was infected with ROka in the absence of Comp I (Fig. 10A). In contrast, the plasma membranes of melanoma cells infected with ROka in the presence of Comp I showed incomplete and apparently fragile virions that were probably disrupted during sample processing (Fig. 10B). A higher magnification (inset in Fig. 10B) depicts one such particle at the cell surface. As expected, cells infected with ROka54B in the presence of Comp I showed enveloped full capsids at the cell surface (Fig. 10C).
FIG. 10.
Electron micrographs of the cell membrane from VZV ROka-infected (A and B) or ROka54B-infected (C) melanoma cells in the absence (A) or presence (B and C) of 2 μg of Comp I per ml. The insets provide a more detailed view of selected viral particles (arrow) from their representative panels. N, nucleus; C, cytoplasm.
DISCUSSION
We have identified a novel class of thiourea compounds that inhibit the replication of VZV but not HSV-1 or HSV-2. A previous study identified a different series of structurally related thiourea compounds that inhibited HSV-1 and, to a much lesser extent, HSV-2 but had no activity against VZV at the highest concentration tested (29). While the VZV and HSV compounds have similar structures (Fig. 1) (29), minor chemical differences appear to result in a remarkable degree of specificity for inhibition of specific alphaherpesviruses.
Resistance to the N-α-methylbenzyl-N′-arylthiourea analogs was mapped to the VZV ORF54 protein. Based on sequence comparison, VZV encodes homologs for each of the seven essential cleavage and packaging genes of HSV. VZV ORF25, ORF26, ORF30, ORF34, ORF42/45, ORF43, and ORF54 correspond to HSV UL33, UL32, UL28, UL25, UL15, UL17, and UL6, respectively (24, 29). None of the VZV cleavage and packaging homologs has been studied in detail; however, a protein of approximately 83 kDa corresponding to the ORF54 polypeptide was recently identified in VZV-infected cells (M. Kothandaraman, Wyeth, Pearl River, N.Y., personal communication). In addition to our study, recent work with both HSV (1, 29) and HCMV (16, 28) suggests that inhibiting the viral DNA cleavage and packaging process of herpesviruses may be a viable strategy for developing new antiviral agents.
Since the VZV ORF54 protein shows 44% amino acid identity to its HSV-1 UL6 homolog, it is reasonable to speculate that the ORF54 protein may perform a functional role similar to that of UL6 during VZV replication. HSV-1 UL6 is a capsid-associated protein that is essential for cleavage and packaging of viral DNA into capsids (17, 20, 21). The UL6 protein assembles into ring-like structures that form a portal for entry for viral DNA into the nucleocapsid (19). Studies are under way to determine if the ORF54 portein can act as a portal of entry for VZV DNA.
The mutations that conferred resistance in VZV to the thiourea compounds mapped to amino acids 324 and 408 of the ORF54 protein in one resistant isolate and to amino acid 407 in another resistant isolate. These amino acids are located near the middle of the 769-amino-acid ORF54 polypeptide. In contrast, the mutations that conferred HSV-1 resistance to a different class of thiourea compounds mapped to amino acids near the amino and carboxy ends of the UL6 protein. The amino acid changes in ORF54 that conferred resistance were not conservative; two of the changes resulted in the replacement of a nonpolar amino acid (asparagine or glycine) with a charged amino acid (aspartic acid), and the other change replaced a tyrosine with a cysteine. In contrast, the amino acid changes in UL6 that resulted in resistance to the other class of compounds tended to be more conservative. Only one of the three amino acids residues affected in our VZV resistant viruses, glycine 407, is conserved in HSV-1. Furthermore, none of the amino acids changes observed in the resistant VZV isolates were altered to then correspond to an amino acid present in the homologous position of the HSV-1 UL6 sequence. Thus, while the two different classes of structurally related thiourea compounds both appear to target homologous proteins in VZV and HSV-1, resistance appears to map to different regions of the protein for the mutants studied thus far.
While the two different classes of thiourea compounds both appear to inhibit the cleavage and packaging of DNA and act through the ORF54 and UL6 proteins, several other antiherpesvirus inhibitors have been described that have a similar mechanism of action but act through different viral cleavage and packaging proteins. Two benzimidazole ribonucleosides (BDCRB and TCRB) inhibit the replication of HCMV by inhibiting the cleavage of viral DNA (16, 28). This inhibition is mediated through the UL56 and UL89 HCMV proteins. While the UL89 protein has 61% similarity to the VZV ORF42/45 protein, the BDCRB inhibitor has no activity against VZV in vitro. Recently, Buerger et al. (6) described a series of nonnucleoside inhibitors that interfere with HCMV viral DNA cleavage and packaging via the UL89 and UL56 proteins. Interestingly, they reported the observation of additional mutations in the UL104 gene (the HSV UL6 homolog) for both murine CMV and HCMV isolates resistant to the inhibitor series. Their data suggested that the observed UL104 mutations had no direct effect on the resistant phenotypes of the CMV isolates. However, since the UL104 protein and the UL56 and/or UL89 proteins may form a functional complex during the DNA cleavage and packaging process, the authors speculated that UL104 mutations might compensate for potential conformational changes in the altered UL56 and/or UL89 gene products.
Although we have not specifically shown that VZV DNA cleavage and packaging is inhibited by the thiourea compounds, several lines of evidence support this mechanism of action. First, immediate-early and late viral gene expression occurred in the presence of the inhibitor compound. Second, virus isolates resistant to one of the thiourea inhibitors were found to possess mutations in the VZV ORF54 gene, which, by analogy to HSV-1, is likely to be involved in the cleavage and packaging of viral DNA. Third, a similar series of compounds have been shown to inhibit the cleavage and packaging of HSV-1 DNA (29). Last, treatment of wild-type virus with the inhibitor resulted in the absence of DNA-containing capsids. Identification of the function of the ORF54 gene product during VZV replication is currently under investigation. Targeted inhibition of VZV ORF54 protein may provide new agents to complement existing antivirals in the treatment of VZV infections.
Acknowledgments
We thank Tarek Mansour, Janis Upeslacis, and David Shlaes for helpful discussions. The low-passage VZV clinical isolates were kindly provided by Earl Kern (University of Alabama at Birmingham).
REFERENCES
- 1.Akanitapichat, P., and K. F. Bastow. 2002. The antiviral agent 5-chloro-1,3-dihydroxyacridone interferes with assembly and maturation of herpes simplex virus. Antiviral Res. 53:113-126. [DOI] [PubMed] [Google Scholar]
- 2.Argaw, T., J. I. Cohen, M. Klutch, K. Lekstrom, T. Yoshikawa, Y. Asano, and P. R. Krause. 2000. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J. Infect. Dis. 181:1153-1157. [DOI] [PubMed] [Google Scholar]
- 3.Arvin, A. M. 1996. Varicella-zoster virus. Clin. Microbiol. Rev. 9:361-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom, J., K. Curran, M. DiGrandi, R. Dushin, T. Jones, S. Lang, E. Norton, A. Ross, E. A. Terefenko, and B. O'Hara. 6. March 2001. Heterocyclic carboxamide-containing thiourea inhibitors of herpes viruses containing a phenylenediamine group. U.S. patent 6,197,803.
- 5.Bloom, J., K. Curran, M. DiGrandi, R. Dushin, S. Lang, E. Norton, A. Ross, and B. O'Hara. 27. March 2001. Alpha-methylbenzyl-containing thiourea inhibitors of herpes viruses containing a substituted phenylenediamine group. U.S. patent 6,207,715.
- 6.Buerger, I., J. Reefschlaeger, W. Bender, P. Eckenberg, A. Popp, O. Weber, S. Graeper, H. Klenk, H. Ruebsamen-Waigmann, and S. Hallenberger. 2001. A novel nonnucleoside inhibitor specifically targets cytomegalovirus DNA maturation via the UL89 and UL56 gene products. J. Virol. 75:9077-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I., and H. Nguyen. 1998. Varicella-zoster virus ORF61 deletion mutants replicate in cell culture, but a mutant with stop codons in ORF61 reverts to wild-type virus. Virology 246:306-316. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, J. I., and K. E. Seidel. 1993. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc. Natl. Acad. Sci. USA 90:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory, A. H., T. C. Owen, J. A. Barltrop, and J. G. Cory. 1991. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3:207-212. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 11.Gazumyan, A., B. Mitsner, and G. A. Ellestad. 2000. Novel anti-RSV dianionic dendrimer-like compounds: design, synthesis and biological evaluation. Curr. Pharm. Des. 6:525-546. [DOI] [PubMed] [Google Scholar]
- 12.Grose, C., R. Harson, and S. Beck. 1995. Computer modeling of prototypic and aberrant nucleocapsids of varicella-zoster virus. Virology 214:321-329. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa, T., M. Kurokawa, T. A. Yukawa, M. Horii, and K. Shiraki. 1995. Inhibitory action of acyclovir (ACV) and penciclovir (PCV) on plaque formation and partial cross-resistance of ACV-resistant varicella-zoster virus to PCV. Antiviral Res. 27:271-279. [DOI] [PubMed] [Google Scholar]
- 14.Ida, M., S. Kageyama, H. Sato, T. Kamiyama, J. Yamamura, M. Kurokawa, M. Morohashi, and K. Shiraki. 1999. Emergence of resistance to acyclovir and penciclovir in varicella-zoster virus and genetic analysis of acyclovir-resistant variants. Antiviral Res. 40:155-166. [DOI] [PubMed] [Google Scholar]
- 15.Kost, R. G., and S. E. Straus. 1996. Postherpetic neuralgia—pathogenesis, treatment, and prevention. N. Engl. J. Med. 335:32-42. [DOI] [PubMed] [Google Scholar]
- 16.Krosky, P. M., M. R. Underwood, S. R. Turk, K. W. Feng, R. K. Jain, R. G. Ptak, A. C. Westerman, K. K. Biron, L. B. Townsend, and J. C. Drach. 1998. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol. 72:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamberti, C., and S. K. Weller. 1996. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology 226:403-407. [DOI] [PubMed] [Google Scholar]
- 18.Machida, H., M. Nishitani, Y. Watanabe, Y. Yoshimura, F. Kano, and S. Sakata. 1995. Comparison of the selectivity of anti-varicella-zoster virus nucleoside analogues. Microbiol. Immunol. 39:201-206. [DOI] [PubMed] [Google Scholar]
- 19.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 75:10923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel, A. H., and J. B. MacLean. 1995. The product of the UL6 gene of herpes simplex virus type 1 is associated with virus capsids. Virology 206:465-478. [DOI] [PubMed] [Google Scholar]
- 21.Patel, A. H., F. J. Rixon, C. Cunningham, and A. J. Davison. 1996. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology 217:111-123. [DOI] [PubMed] [Google Scholar]
- 22.Razinkov, V., A. Gazumyan, A. Nikitenko, G. Ellestad, and G. Krishnamurthy. 2001. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 8:645-659. [DOI] [PubMed] [Google Scholar]
- 23.Safrin, S., T. G. Berger, I. Gilson, P. R. Wolfe, C. B. Wofsy, J. Mills, and K. K. Biron. 1991. Foscarnet therapy in five patients with AIDS and acyclovir-resistant varicella-zoster virus infection. Ann. Intern. Med. 115:19-21. [DOI] [PubMed] [Google Scholar]
- 24.Sheaffer, A. K., W. W. Newcomb, M. Gao, D. Yu, S. K. Weller, J. C. Brown, and D. J. Tenney. 2001. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J. Virol. 75:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snoeck, R., G. Andrei, and E. D. Clercq. 2000. Novel agents for the therapy of varicella-zoster virus infections. Expert Opin. Investig. Drugs 9:1743-1751. [DOI] [PubMed] [Google Scholar]
- 26.Snoeck, R., G. Andrei, and E. De Clercq. 1999. Current pharmacological approaches to the therapy of varicella zoster virus infections: a guide to treatment. Drugs 57:187-206. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi, M., Y. Okuno, T. Otsuka, J. Osame, and A. Takamizawa. 1975. Development of a live attenuated varicella vaccine. Biken J. 18:25-33. [PubMed] [Google Scholar]
- 28.Underwood, M. R., R. J. Harvey, S. C. Stanat, M. L. Hemphill, T. Miller, J. C. Drach, L. B. Townsend, and K. K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Zeijl, M., J. Fairhurst, T. R. Jones, S. K. Vernon, J. Morin, J. LaRocque, B. Feld, B. O'Hara, J. D. Bloom, and S. V. Johann. 2000. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J. Virol. 74:9054-9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visse, B., B. Dumont, J. M. Huraux, and A. M. Fillet. 1998. Single amino acid change in DNA polymerase is associated with foscarnet resistance in a varicella-zoster virus strain recovered from a patient with AIDS. J. Infect. Dis. 178(Suppl. 1):S55-S57. [DOI] [PubMed] [Google Scholar]
- 31.Whitley, R. J. 1995. Sorivudine: a promising drug for the treatment of varicella-zoster virus infection. Neurology 45:S73-S75. [DOI] [PubMed] [Google Scholar]
- 32.Wood, M. J. 1995. How should we measure pain in herpes zoster? Neurology 45:S61-S62. [DOI] [PubMed] [Google Scholar]
- 33.Wood, M. J. 1996. How to measure and reduce the burden of zoster-associated pain. Scand. J. Infect. Dis. Suppl 100:55-58. [PubMed] [Google Scholar]