Abstract
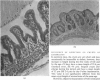
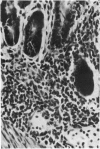
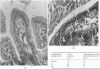
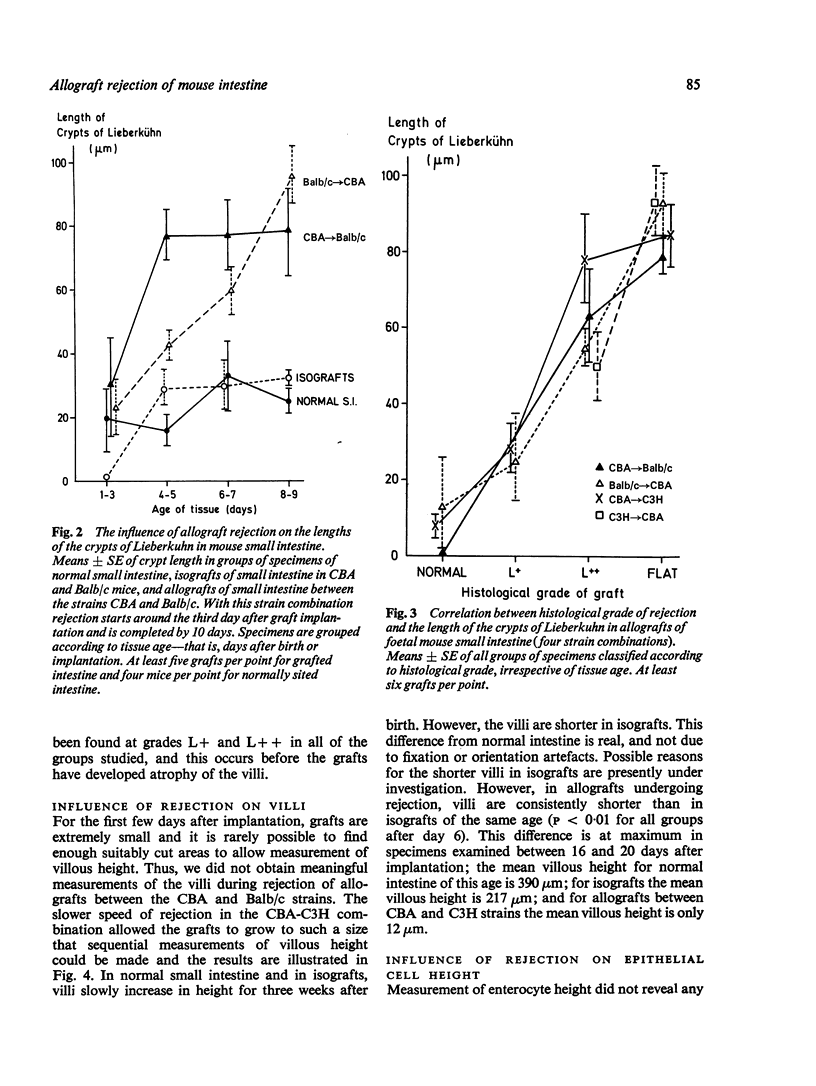
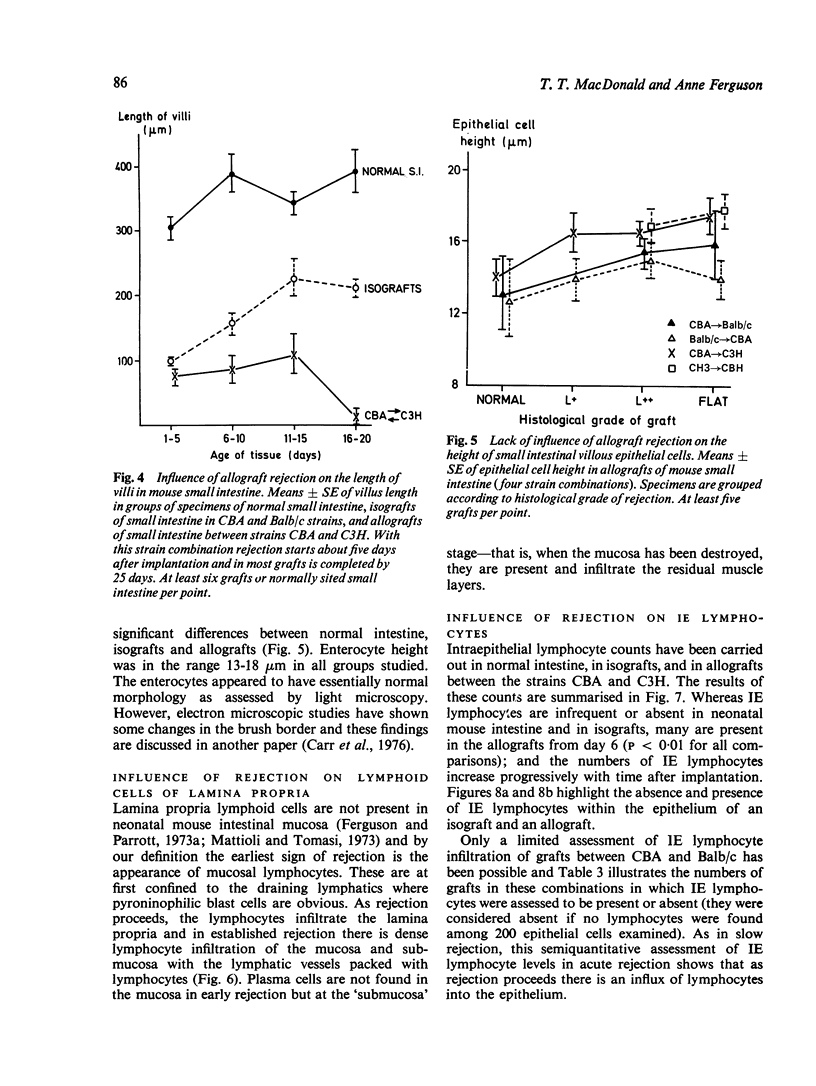
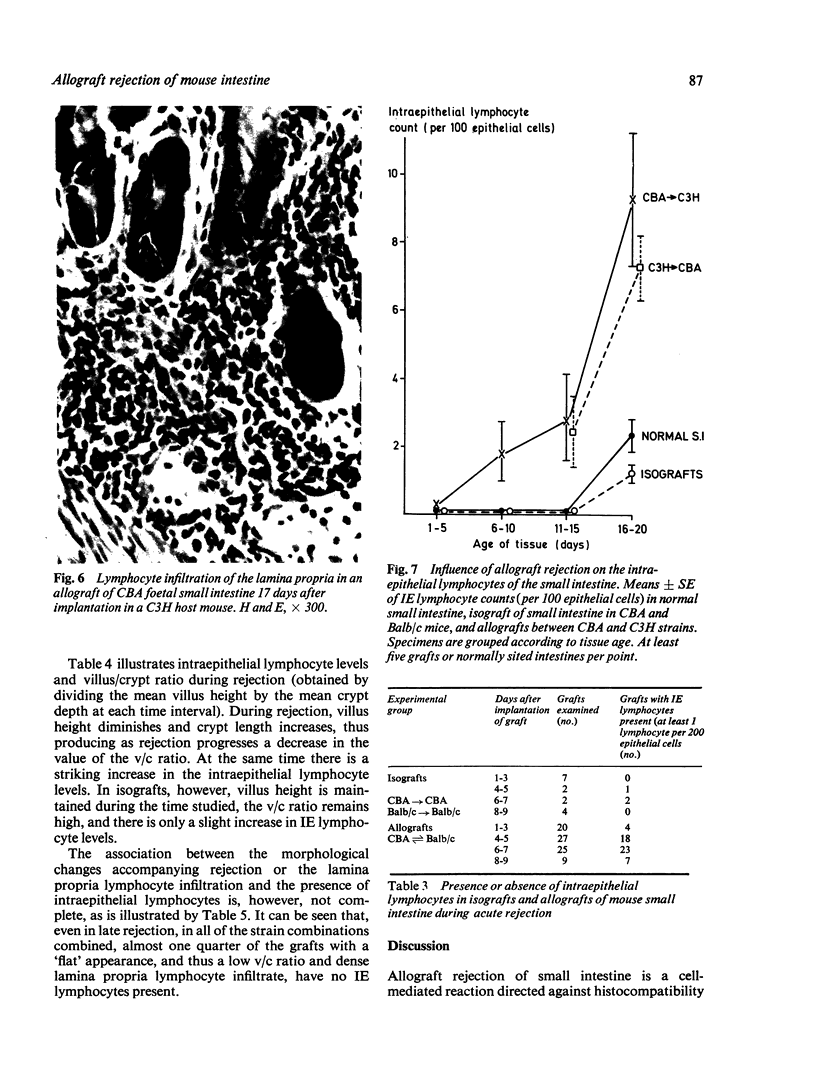
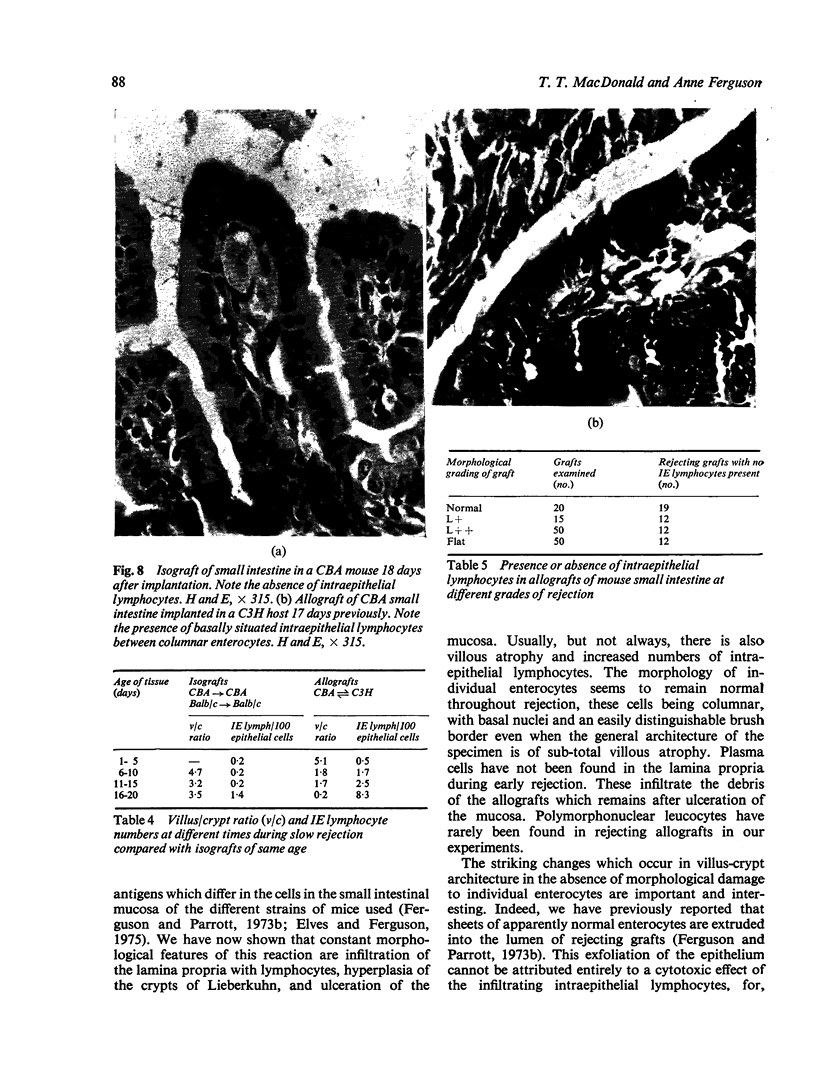
Small intestinal mucosa contains both thymus dependent and thymus independent lymphoid cells and thus has the capacity to act via humoral and cellular mechanisms as a site of local immunity and local hypersensitivity. Allograft rejection of mouse small intestine is a model of a local cell mediated reaction. The effects of this clearly defined, immunologically mediated damage villi, crypts, enterocytes, and lymphoid cell infiltrate have been assessed by comparing the morphology of rejecting allografts with that of isografts and normal small intestine of the same age. In rejection there is infiltration of the lamina propria with lymphocytes, hyperplasia of the crypts of Lieberkuhn, and an eventual sloughing off of the mucosa. Usually, but not always, there is villous atrophy and increased numbers of intraepithelial lymphocytes. However, the morphology of individual enterocytes remains normal throughout rejection and neither plasma cells nor polymorphonuclear leucocytes infiltrate the lamina propria before mucosal ulceration. These results show unequivocally that a local T cell mediated immune response causes villous atrophy and crypt hyperplasia in this animal model, and since there is no evidence of local enterocyte cytotoxicity, a lymphokine may be the link between the activated T cell and the effects on mucosal architecture. We suggest that a local CMI reaction may be the cause of villous atrophy, crypt hyperplasia, and malabsorption in many clinical and experimental conditions, including coeliac disease, food allergy, and intestinal infections.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy J. E., Nielsen N. O. Immune-mediated emigration of neutrophils into the lumen of the small intestine. Infect Immun. 1974 Apr;9(4):615–619. doi: 10.1128/iai.9.4.615-619.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calman K. C. Why are small bowel tumours rare? An experimental model. Gut. 1974 Jul;15(7):552–554. doi: 10.1136/gut.15.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius E. A. Protein-losing enteropathy in the graft-versus-host reaction. Transplantation. 1970 Mar;9(3):247–252. doi: 10.1097/00007890-197003000-00008. [DOI] [PubMed] [Google Scholar]
- Delorme E. J., Hodgett J., Hall J. G., Alexander P. The cellular immune response to primary sarcomata in rats. I. The significance of large basophilic cells in the thoracic duct lymph following antigenic challenge. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):229–236. doi: 10.1098/rspb.1969.0089. [DOI] [PubMed] [Google Scholar]
- Dineen J. K., Ogilvie B. M., Kelly J. D. Expulsion of Nippostrongylus brasiliensis from the intestine of rats. Collaboration between humoral and cellular components of the immune response. Immunology. 1973 Mar;24(3):467–475. [PMC free article] [PubMed] [Google Scholar]
- Elves M. W., Ferguson A. The humoral immune response to allografts of foetal small intestine in mice. Br J Exp Pathol. 1975 Oct;56(5):454–458. [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. Immunological roles of the gastrointestinal tract. Scott Med J. 1972 Mar;17(3):111–118. doi: 10.1177/003693307201700307. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Jarrett E. E. Hypersensitivity reactions in small intestine. I Thymus dependence of experimental 'partial villous atrophy'. Gut. 1975 Feb;16(2):114–117. doi: 10.1136/gut.16.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., MacDonald T. T., McClure J. P., Holden R. J. Cell-mediated immunity to gliadin within the small-intestinal mucosa in coeliac disease. Lancet. 1975 Apr 19;1(7912):895–897. doi: 10.1016/s0140-6736(75)91689-x. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. Growth and development of "antigen-free" grafts of foetal mouse intestine. J Pathol. 1972 Feb;106(2):95–101. doi: 10.1002/path.1711060205. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. Histopathology and time course of rejection of allografts of mouse small intestine. Transplantation. 1973 Jun;15(6):546–554. doi: 10.1097/00007890-197306000-00005. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972 Dec;12(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Fichtelius K. E., Yunis E. J., Good R. A. Occurrence of lymphocytes within the gut epithelium of normal and neonatally thymectomized mice. Proc Soc Exp Biol Med. 1968 May;128(1):185–188. doi: 10.3181/00379727-128-32974. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hedberg C. A., Reiser S., Reilly R. W. Intestinal phase of the runting syndrome in mice. II. Observations on nutrient absorption and certain disaccharidase abnormalities. Transplantation. 1968 Jan;6(1):104–110. doi: 10.1097/00007890-196801000-00010. [DOI] [PubMed] [Google Scholar]
- Holmes J. T., Klein M. S., Winawer S. J., Fortner J. G. Morphological studies of rejection in canine jejunal allographs. Gastroenterology. 1971 Nov;61(5):693–706. [PubMed] [Google Scholar]
- Jarrett E. E., Urguhart G. M. The immune response to nematode infections. Int Rev Trop Med. 1971;4:53–96. [PubMed] [Google Scholar]
- Krejcí J., Pekàrek J., Svejcar J., Johanovskỳ J. The effect of lymphokines on the development of delayed hypersensitivity to an unrelated antigen. Immunology. 1973 Nov;25(5):875–879. [PMC free article] [PubMed] [Google Scholar]
- Mattioli C. A., Tomasi T. B., Jr The life span of IgA plasma cells from the mouse intestine. J Exp Med. 1973 Aug 1;138(2):452–460. doi: 10.1084/jem.138.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. R., Hall J. G. Evidence for a primary association between immunoblasts and small gut. Nature. 1972 Sep 15;239(5368):161–162. doi: 10.1038/239161a0. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Immunity in the parasitic relationship between helminths and hosts. Prog Allergy. 1973;17:93–144. [PubMed] [Google Scholar]
- Ogilvie B. M., Love R. J. Co-operation between antibodies and cells in immunity to a nematode parasite. Transplant Rev. 1974;19(0):147–169. doi: 10.1111/j.1600-065x.1974.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. H., Reilly R. W. Bile salt depletion in the runting syndrome. Transplantation. 1971 Dec;12(6):479–483. doi: 10.1097/00007890-197112000-00011. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- REILLY R. W., KIRSNER J. B. RUNT INTESTINAL DISEASE. Lab Invest. 1965 Jan;14:102–107. [PubMed] [Google Scholar]
- Remold H. G. Purification and characterization of lymphocyte mediators in cellular immunity: comparative studies on migration inhibitory factor (MIF) chemotactic factor for macrophages and lymphotoxin. Transplant Rev. 1972;10:152–176. doi: 10.1111/j.1600-065x.1972.tb01542.x. [DOI] [PubMed] [Google Scholar]
- SHINER M., DONIACH I. Histopathologic studies in steatorrhea. Gastroenterology. 1960 Mar;38:419–440. [PubMed] [Google Scholar]
- SNELL G. D., HOECKER G., AMOS D. B., STIMPFLING J. H. A REVISED NOMENCLATURE FOR THE HISTOCOMPATIBILITY-2 LOCUS OF THE MOUSE. Transplantation. 1964 Nov;2:777–784. doi: 10.1097/00007890-196411000-00010. [DOI] [PubMed] [Google Scholar]
- Shiner M. Ultrastructural changes suggestive of immune reactions in the jejunal mucosa of coeliac children following gluten challenge. Gut. 1973 Jan;14(1):1–12. doi: 10.1136/gut.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. II. Recirculating lymphocytes derived from antigen-activated thymus cells. Cell Immunol. 1972 Mar;3(3):385–404. doi: 10.1016/0008-8749(72)90245-6. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]