Abstract
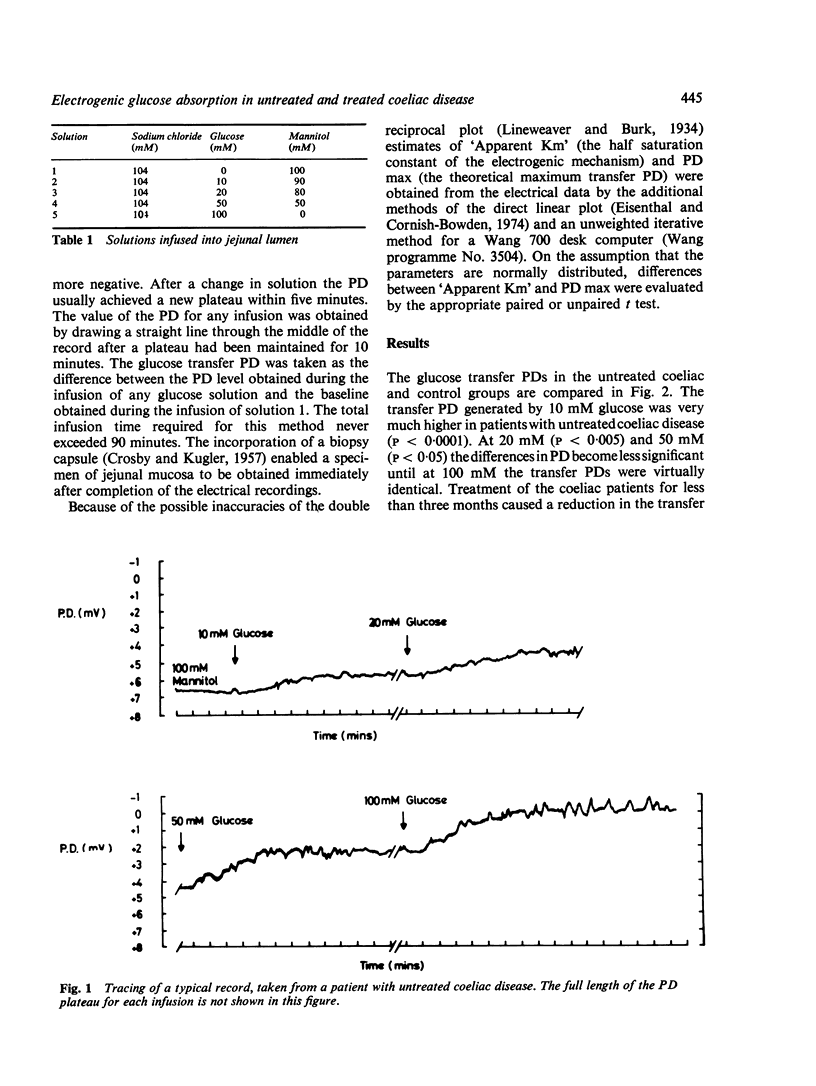
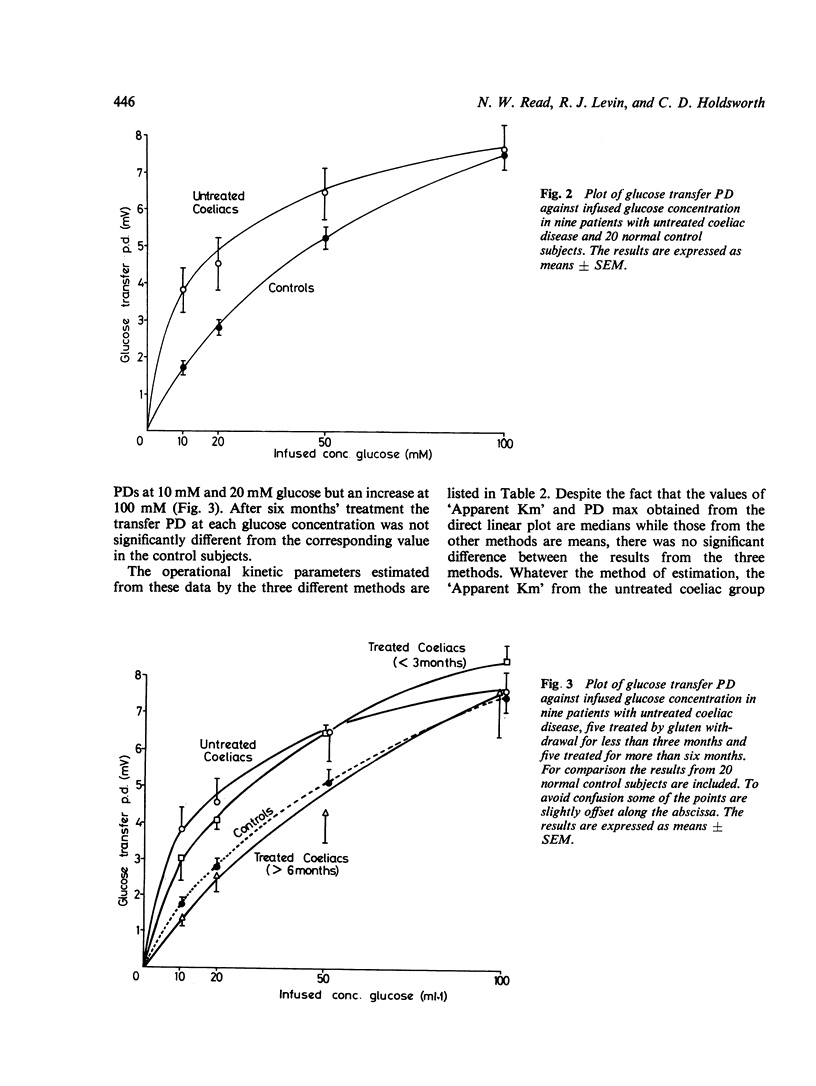
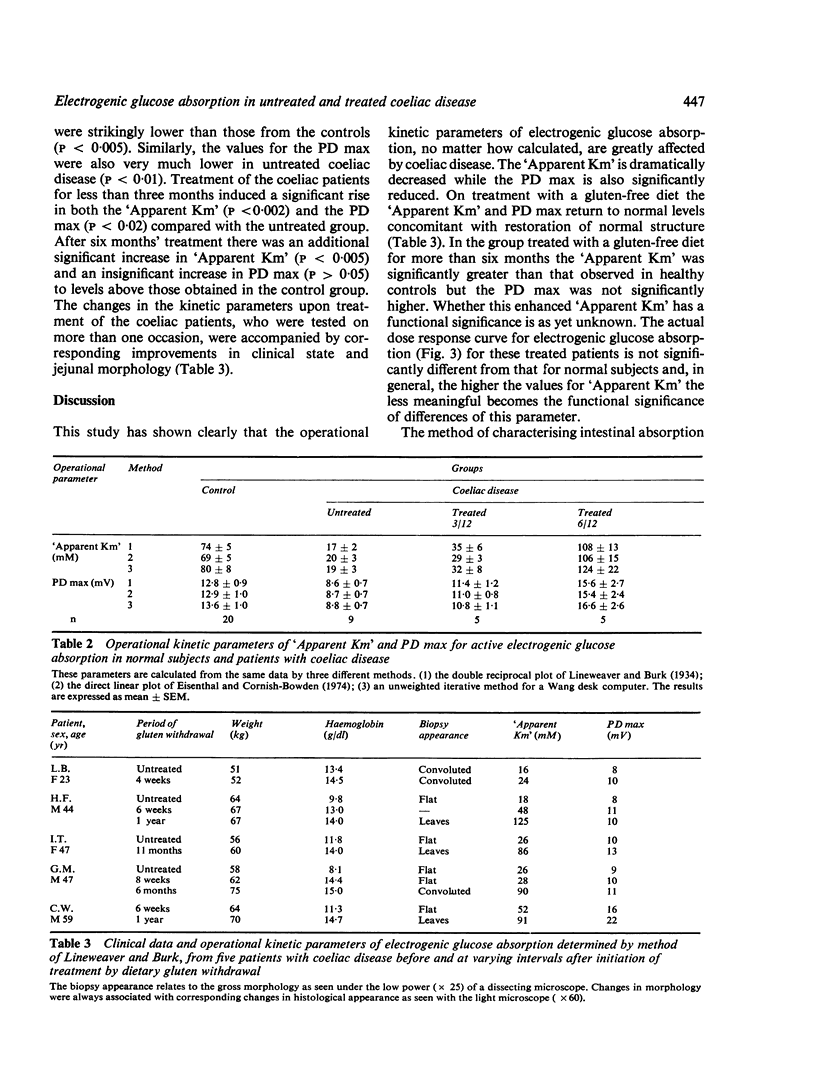
Using a method for measuring changes in transmural potential difference across the human jejunum in vivo, the operational kinetic parameters of 'Apparent Km' and PD max for the active electrogenic component of glucose absorption were estimated in a group of healthy volunteers and in patients with coeliac disease. Both the 'Apparent Km' (17+/2mM; mean +/SEM) and the PD max (8.6+/0.7 mV) in nine patients with untreated coeliac disease were significantly lower (p less than 0.005) than in the control group ('Apparent Km' = 74+/5mM; PD max 12.8+/0.9mV, n=20). Treatment of five coeliac patients by gluten withdrawal for less than three months increased significantly the values of both the "Apparent Km (35+/6mM) and the TPD max (11.4+/1.2mV). Treatment of five patients for more than six months caused a further increase in the values of both kinetic parameters ('Apparent Km' = 108+/13mM; PD max =15.6+/2.7mV) to levels which exceeded those in healthy subjects. The possible interpretations of the differences in the kinetic characteristics of electrogenic glucose transport between coeliac patients and healthy subjects are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO T. METABOLIC DISTURBANCES AND SHORT-CIRCUIT CURRENT ACROSS INTESTINAL WALL OF RAT. Am J Physiol. 1964 Aug;207:415–422. doi: 10.1152/ajplegacy.1964.207.2.415. [DOI] [PubMed] [Google Scholar]
- CROSBY W. H., KUGLER H. W. Intraluminal biopsy of the small intestine; the intestinal biopsy capsule. Am J Dig Dis. 1957 May;2(5):236–241. doi: 10.1007/BF02231100. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J Physiol. 1975 Mar;246(1):181–196. doi: 10.1113/jphysiol.1975.sp010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. Effects of fasting and semistarvation on the kinetics of active and passive sugar absorption across the small intestine in vivo. J Physiol. 1975 Nov;252(3):681–700. doi: 10.1113/jphysiol.1975.sp011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas M. C., Ramaswamy K., Crane R. K. An analysis of the D-glucose influx kinetics of in vitro hamster jejunum, based on considerations of the mass-transfer coefficient. Biochim Biophys Acta. 1975 Apr 8;382(4):576–589. doi: 10.1016/0005-2736(75)90224-2. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. Glucose movements across the wall of the rat small intestine. J Physiol. 1953 Feb 27;119(2-3):210–223. doi: 10.1113/jphysiol.1953.sp004839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth C. D., Dawson A. M. Glucose and fructose absorption in idiopathic steatorrhoea. Gut. 1965 Aug;6(4):387–391. doi: 10.1136/gut.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelfinger F. J., Moss R. E. THE MOTILITY OF THE SMALL INTESTINE IN SPRUE. J Clin Invest. 1943 May;22(3):345–352. doi: 10.1172/JCI101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon I., Crane R. K. Studies on transmural potentials in vitro in relation to intestinal absorption. I. Apparent Michaelis constants for Na+dependent sugar transport. Biochim Biophys Acta. 1966 Feb 7;112(2):278–291. doi: 10.1016/0926-6585(66)90327-x. [DOI] [PubMed] [Google Scholar]
- Read N. W., Holdsworth C. D., Levin R. J. Electrical measurement of intestinal absorption of glucose in man. Lancet. 1974 Sep 14;2(7881):624–627. doi: 10.1016/s0140-6736(74)91946-1. [DOI] [PubMed] [Google Scholar]
- Ritchie J. A., Salem S. N. Upper intestinal motility in ulcerative colitis, idiopathic steatorrhoea, and the irritable colon syndrome. Gut. 1965 Aug;6(4):325–337. doi: 10.1136/gut.6.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. I., Allan J. G., Gerskowitch V. P., Robertson J. W. A study by perfusion techniques of the absorption abnormalities in the jejunum in adult coeliac disease. Clin Sci. 1972 Jun;42(6):735–741. doi: 10.1042/cs0420735. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W. C., Phillips S. F., Summerskill W. H. Jejunal secretion of electrolytes and water in nontropical sprue. J Lab Clin Med. 1969 May;73(5):772–783. [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. The intestinal unstirred layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta. 1974 Aug 21;363(1):112–126. doi: 10.1016/0005-2736(74)90010-8. [DOI] [PubMed] [Google Scholar]
- Winne D. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim Biophys Acta. 1973 Feb 27;298(1):27–31. doi: 10.1016/0005-2736(73)90005-9. [DOI] [PubMed] [Google Scholar]


