Abstract
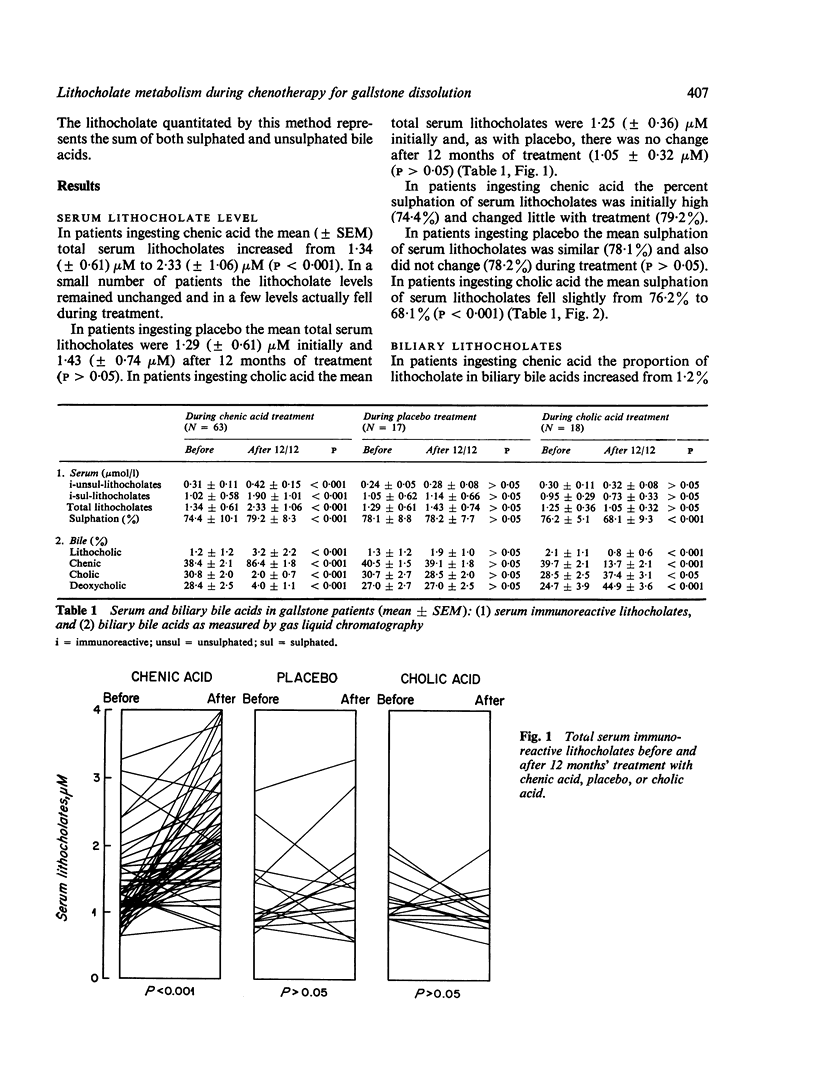
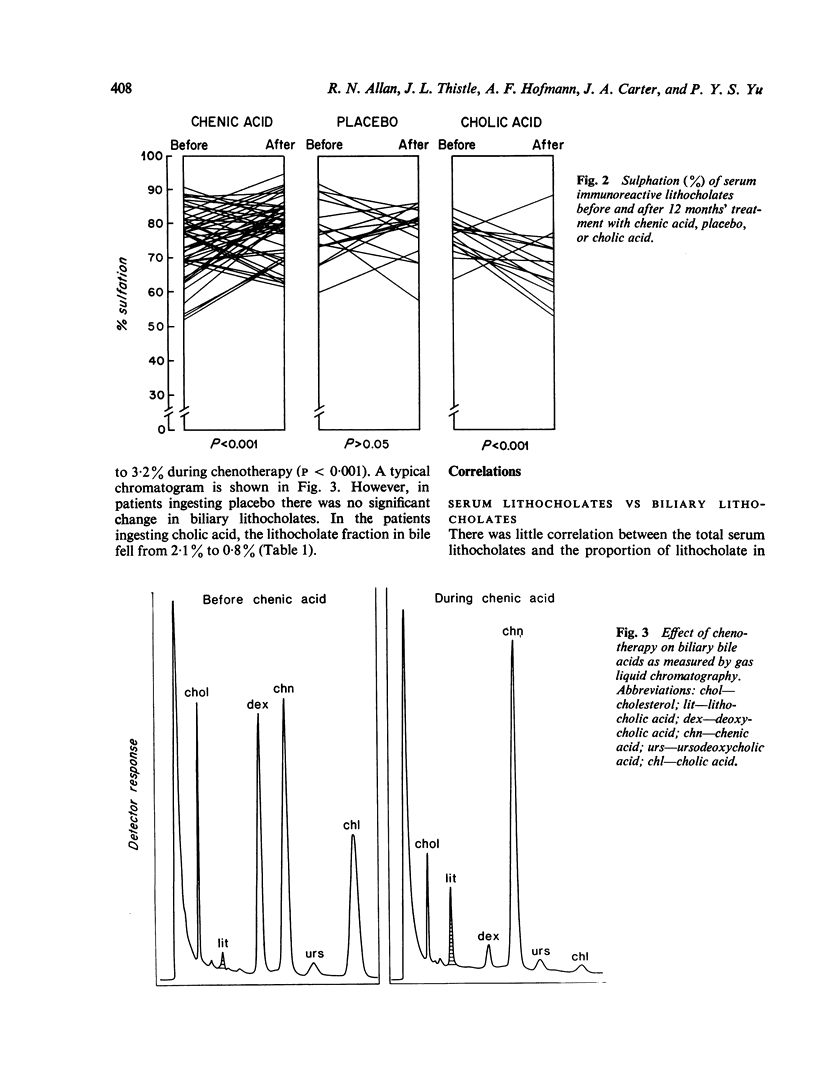
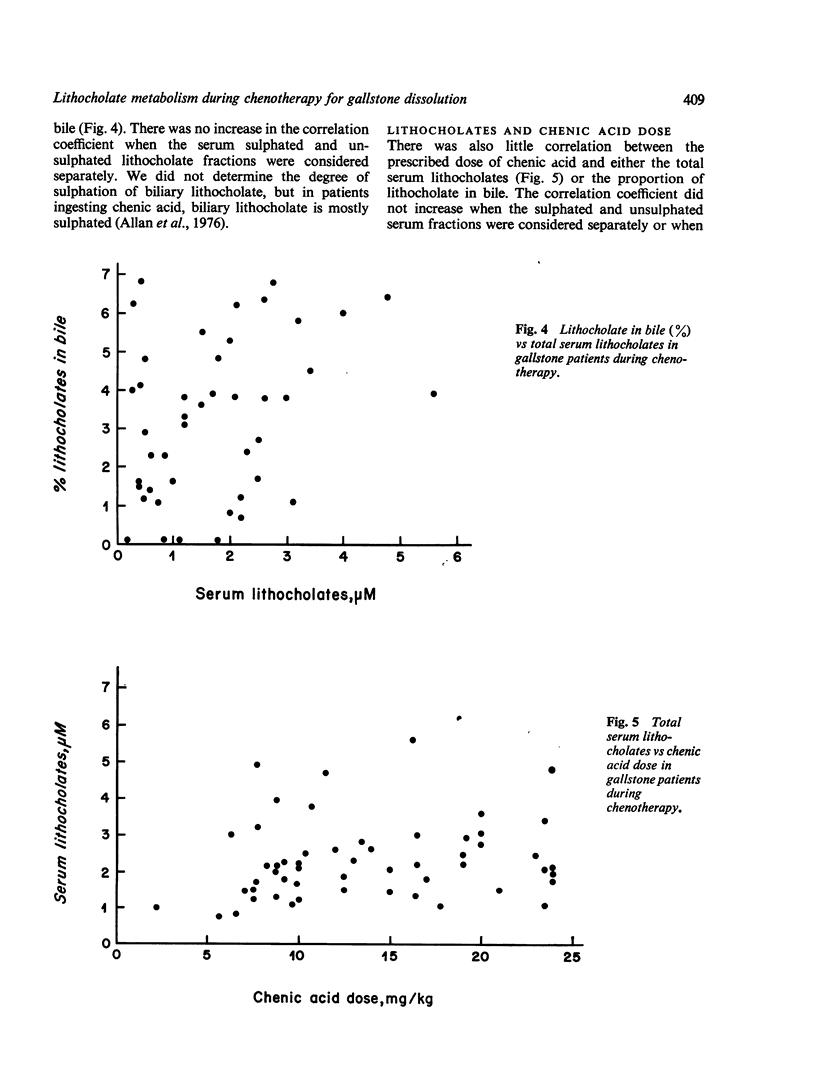
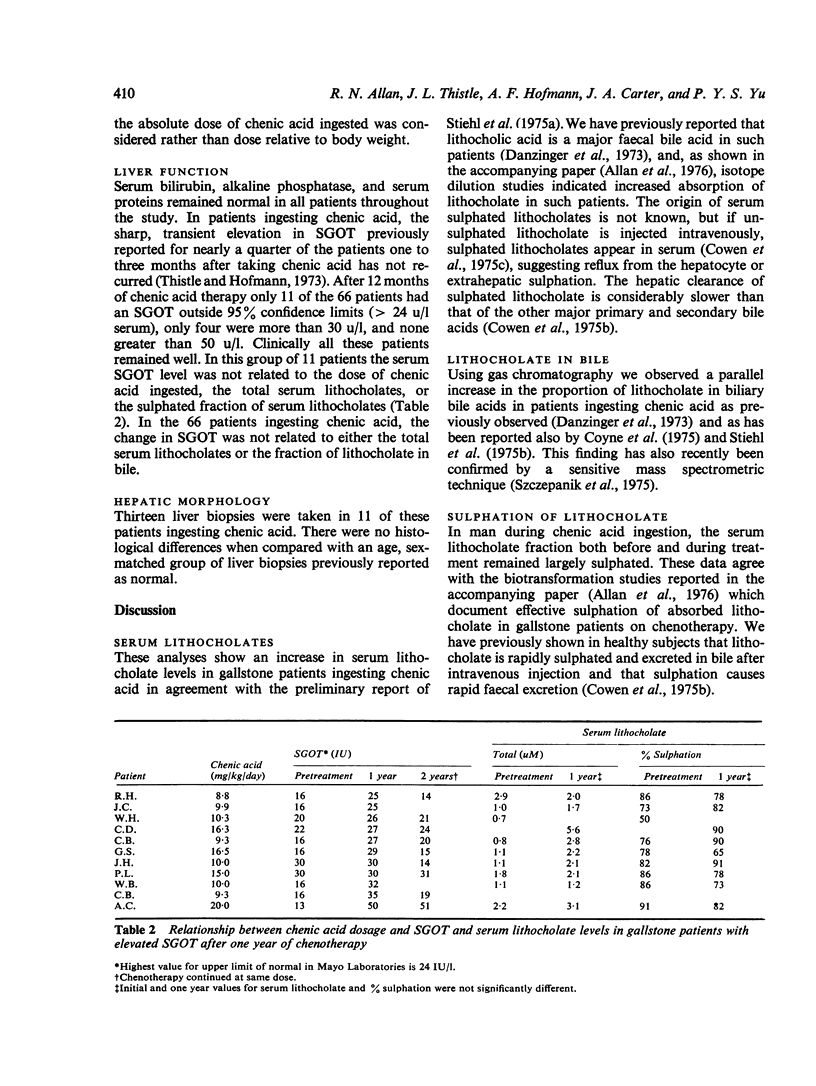
Serum levels of total sulphated and total unsulphated lithocholates were each measured by a specific radioimmunoassay in 66 patients ingesting chenodeoxycholic (chenic) acid for gallstone dissoultion and in 35 gallstone patients ingesting either cholic acid or placebo. No changes occurred in serum lithocholate levels in the control groups. In patients ingesting chenic acid, there was a twofold increase in serum levels of total lithocholate, but the percent sulphation (greater than 75%) remained unchanged during chenotherapy. There was no correlation in the chenic acid treated group between serum lithocholate levels and the proportion of lithocholate in biliary bile acids or changes in serum SGOT. The data suggest that there is effective sulphation of lithocholate in such patients; this may explain the lack of hepatotoxicity observed during ingestion of chenic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. N., Thistle J. L., Hofmann A. F. Lithocholate metabolism during chemotherapy for gallstone dissolution. 2. Absorption and sulphation. Gut. 1976 Jun;17(6):413–419. doi: 10.1136/gut.17.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3 alpha-, 7 alpha- and 12 alpha-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. Biochim Biophys Acta. 1970 May 5;202(3):535–543. doi: 10.1016/0005-2760(70)90124-4. [DOI] [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W., Coffin S. B. Metabolism of lithocholate in healthy man. II. Enterohepatic circulation. Gastroenterology. 1975 Jul;69(1):67–76. [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W. Metabolism of lethocholate in healthy man. I. Biotransformation and biliary excretion of intravenously administered lithocholate, lithocholylglycine, and their sulfates. Gastroenterology. 1975 Jul;69(1):59–66. [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Thomas P. J. Metabolism of lithocholate in healthy man. III. Plasma disappearance of radioactivity after intravenous injection of labeled lithocholate and its derivatives. Gastroenterology. 1975 Jul;69(1):77–82. [PubMed] [Google Scholar]
- Coyne M. J., Bonorris G. G., Chung A., Goldstein L. I., Lahana D., Schoenfield L. J. Treatment of gallstones with chenodeoxycholic acid and phenobarbital. N Engl J Med. 1975 Mar 20;292(12):604–607. doi: 10.1056/NEJM197503202921202. [DOI] [PubMed] [Google Scholar]
- Danzinger R. C., Hofmann A. F., Thistle J. L., Schoenfield L. J. Effect of oral chenodeoxycholic acid on bile acid kinetics and biliary lipid composition in women with cholelithiasis. J Clin Invest. 1973 Nov;52(11):2809–2821. doi: 10.1172/JCI107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrszka H., Salen G., Zaki F. G., Chen T., Mosbach E. H. Hepatic toxicity in the rhesus monkey treated with chenodeoxycholic acid for 6 months: biochemical and ultrastructural studies. Gastroenterology. 1976 Jan;70(1):93–104. [PubMed] [Google Scholar]
- Fischer C. D., Cooper N. S., Rothschild M. A., Mosbach E. H. Effect of dietary chenodeoxycholic acid and lithocholic acid in the rabbit. Am J Dig Dis. 1974 Oct;19(10):877–886. doi: 10.1007/BF01076210. [DOI] [PubMed] [Google Scholar]
- Gadacz T. R., Allan R. N., Mack E., Hofmann A. F. Impaired lithocholate sulfation in the rhesus monkey: a possible mechanism for chenodeoxycholate toxicity. Gastroenterology. 1976 Jun;70(6):1125–1129. [PubMed] [Google Scholar]
- HOFMANN A. F., MOSBACH E. H. IDENTIFICATION OF ALLODEOXYCHOLIC ACID AS THE MAJOR COMPONENT OF GALLSTONES INDUCED IN THE RABBIT BY 5-ALPHA-CHOLESTAN-3-BETA-OL. J Biol Chem. 1964 Sep;239:2813–2821. [PubMed] [Google Scholar]
- Hofmann A. F., Poley J. R. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972 May;62(5):918–934. [PubMed] [Google Scholar]
- Hofmann A. F., Thistle J. L. An algorithm for monitoring and managing drug hepatotoxicity. Gastroenterology. 1974 Aug;67(2):309–313. [PubMed] [Google Scholar]
- Iser J. H., Dowling H., Mok H. Y., Bell G. D. Chenodeoxycholic acid treatment of gallstones. A follow-up report and analysis of factors influencing response to therapy. N Engl J Med. 1975 Aug 21;293(8):378–383. doi: 10.1056/NEJM197508212930804. [DOI] [PubMed] [Google Scholar]
- James O., Cullen J., Bouchier I. A. Chenodeoxycholic acid therapy for gallstones: effectiveness, toxicity and influence on bile acid metabolism. Q J Med. 1975 Apr;44(174):349–367. [PubMed] [Google Scholar]
- Morrissey K. P., McSherry C. K., Swarm R. L., Nieman W. H., Deitrick J. E. Toxicity of chenodeoxycholic acid in the nonhuman primate. Surgery. 1975 Jun;77(6):851–860. [PubMed] [Google Scholar]
- POLEY J. R., DOWER J. C., OWEN C. A., Jr, STICKLER G. B. BILE ACIDS IN INFANTS AND CHILDREN. J Lab Clin Med. 1964 May;63:838–846. [PubMed] [Google Scholar]
- Palmer R. H. Bile acids, liver injury, and liver disease. Arch Intern Med. 1972 Oct;130(4):606–617. [PubMed] [Google Scholar]
- Simmonds W. J., Korman M. G., Go V. L., Hofmann A. F. Radioimmunoassay of conjugated cholyl bile acids in serum. Gastroenterology. 1973 Nov;65(5):705–711. [PubMed] [Google Scholar]
- Thistle J. L., Hofmann A. F. Efficacy and specificity of chenodeoxycholic acid therapy for dissolving gallstones. N Engl J Med. 1973 Sep 27;289(13):655–659. doi: 10.1056/NEJM197309272891303. [DOI] [PubMed] [Google Scholar]
- Thistle J. L., Schoenfield L. J. Induced alterations in composition of bile of persons having cholelithiasis. Gastroenterology. 1971 Oct;61(4):488–496. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Miller J. R., Farrar J. T., Swell L. Kinetics and pool size of primary bile acids in man. Gastroenterology. 1971 Jul;61(1):85–90. [PubMed] [Google Scholar]
- Webster K. H., Lancaster M. C., Hofmann A. F., Wease D. F., Baggenstoss A. H. Influence of primary bile acid feeding on cholesterol metabolism and hepatic function in the rhesus monkey. Mayo Clin Proc. 1975 Mar;50(3):134–138. [PubMed] [Google Scholar]


