Abstract
The Sendai virus (SeV) C proteins are known to interact with Stat1 to prevent interferon (IFN)-induced pY701-Stat1 formation and IFN signaling. Nevertheless, pY701-Stat1 levels paradoxically increase during SeV infection. The C proteins also induce bulk Stat1 instability in some cells, similar to rubulavirus V proteins. We have found that SeV infection increases pY701-Stat1 levels even in cells in which bulk Stat1 levels strongly decrease. Remarkably, both the decrease in bulk Stat1 levels and the increase in pY701-Stat1 levels were found to be independent of the IFN signaling system, i.e., these events occur in mutant cells in which various components of the IFN signaling system have been disabled. Consistent with this, the C-induced decrease in Stat1 levels does not require Y701 of Stat1. We present evidence that C interacts with Stat1 in two different ways, one that prevents IFN-induced pY701-Stat1 formation and IFN signaling that has already been documented, and another that induces pY701-Stat1 formation (while decreasing bulk Stat1 levels) in a manner that does not require IFN signaling. These two types of Stat1 interaction are also distinguishable by C gene mutations. In particular, the IFN signaling-independent Stat1 interactions specifically require the amino-terminal extensions of the longer C proteins. The actions of the SeV C proteins in counteracting the cellular antiviral response are clearly more extensive than previously appreciated.
In response to virus infection, mammalian cells adopt a variety of countermeasures that create an intracellular environment that is nonconducive to virus replication (the interferon [IFN]-induced antiviral state) and simultaneously warn neighboring cells of the presence of the invader (paracrine IFN “priming”) (46). Infected cells also undergo programmed cell death to limit further virus replication if the antiviral state fails to contain the infection (24, 44). The cellular antiviral response is exceedingly complex, as it protects cells against all viruses; the steady-state levels of several hundred mRNAs are altered in response to IFN treatment alone (11, 14) or double-stranded RNA (20).
The antiviral state is composed of multiple components (e.g., a cap on the overall rate of protein synthesis), each of which is due to the action of multiple cellular genes. Moreover, this state for any particular virus is thought to be due to the accretion of multiple functions rather than the function of a single cellular gene (54). All viruses must deal with this antiviral response by first avoiding detection and then counteracting the cell's antiviral response when detection can no longer be avoided. In the face of the multifaceted cellular response, even simple viruses must counteract several aspects of this response as part of their own survival program. This paper details the different ways in which the Sendai virus (SeV) C proteins interact with Stat1 to counteract the cellular antiviral response.
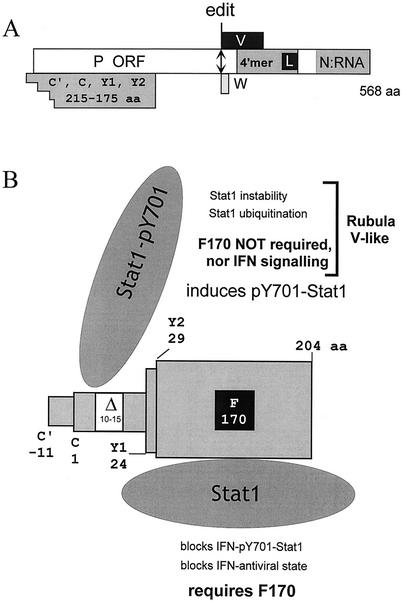
The SeV C gene was an early example of an overlapping gene and has been an enigma since its discovery because of its unusual properties (22, 34). The C gene open reading frame is found overlapping the amino-terminal end of the open reading frame for the P, V, and W proteins (which are also expressed from the P gene mRNA due to mRNA editing) (Fig. 1A). A nested set of four C proteins are initiated from four ribosomal start codons, ACG87/C′, AUG114/C, AUG183/Y1, and AUG201/Y2, due to a combination of leaky ribosomal scanning (C′ and C) and a ribosomal shunt (Y1/Y2) (36). This gene has also been referred to as an “accessory” gene, like the human immunodeficiency virus type 1 nef or influenza virus NS1 gene, as not all viruses of their respective families express these essentially nonstructural proteins (3). Moreover, they do not form part of their virus replication machines in a strict sense, as viruses in which these genes are deleted are viable (33).
FIG. 1.
(A) Open reading frame organization and expression of the SeV P gene. The four open reading frames (ORFs) expressed as proteins P, C, V, and W are shown as horizontal boxes, drawn roughly to scale. Several domains of the P protein, notably its tetramerization domain, including the L protein binding site and that which binds to the N: RNA nucleocapsid, are indicated. The double-headed arrow shows the site (codon 317) where G residues are added cotranscriptionally to access the V and W open reading frames. The crenellated beginning of the C open reading frame box indicates the four independently initiated C proteins, which all terminate at codon 205. aa, amino acid. (B) Sendai virus C proteins and their interactions with Stat1. The nested set of four C proteins (C′, C, Y1, and Y2) that initiate from ACG87, AUG114, AUG183, and AUG201, respectively, are shown as a telescoping set of boxes. Their respective lengths in amino acids are indicated; AUG114/C is set at amino acid position 1. The critical Phe170 present in all four C proteins and the deletion of amino acids 10 to 15 (which are present only in C′ and C) are indicated. The IFN-dependent interactions of C and Stat1 require F170 but not the N-terminal extensions found on C′ and C, whereas the IFN-independent interactions of C and Stat1 require the N-terminal extensions found only on C′ but not necessarily F170. These two different C/Stat1 interactions are shown schematically, along with the consequences of these interactions. The IFN-independent properties shared by the SeV C proteins and the rubulavirus V proteins are also indicated.
The SeV C proteins were first characterized as inhibitors of viral mRNA synthesis (9), then as (genomic) promoter-specific inhibitors of viral RNA synthesis (3), and then as replication fidelity factors (49). All these properties affecting viral RNA synthesis presumably result from the direct interaction of C with the L protein of viral RNA polymerase (P4-L) (26). These effects are relatively severe, as C function must be suppressed for virus to be recovered from DNA (19, 3). The C proteins were then found to inhibit IFN signaling and IFN-induced pY701-Stat1 formation (16, 17, 23), presumably due to their direct interaction with Stat1 (18, 48). As far as we know, the C/L and C/Stat1 interactions represent separate and possibly unrelated functions of C, even though certain mutations (e.g., CF170S) abolish both interactions.
Although C interacts with Stat1 and prevents IFN signaling in all cells examined, other consequences of this complex formation appear to be cell dependent. In HeLa cells, Stat1 levels remain unchanged by SeV infection, and the constitutive expression of any of the four C proteins prevents IFN from inducing an antiviral state (29). In other cells (mouse embryo fibroblasts [MEFs]), however, Stat1 levels are clearly reduced due to SeV infection, and only recombinant SeV that express the longer two of the four C proteins during infection are able to dismantle the IFN-induced antiviral state (15, 18). The possible significance of these different effects of the SeV C gene in different cells remains unclear.
Most recently, our understanding of how C counteracts the cellular antiviral response was further confounded by reports that pY701-Stat1 levels actually increase during SeV infections of HeLa cells, even though SeV prevents IFN-induced pY701-Stat1 formation and IFN signaling (32, 45). This paper reports that SeV infection increases pY701-Stat1 levels even in cells in which bulk Stat1 levels strongly decrease. Remarkably, both the decrease in bulk Stat1 levels and the increase in pY701-Stat1 levels were found to be independent of the IFN signaling system. Moreover, the C-induced decrease in Stat1 levels does not require Y701 of Stat1.
We present evidence that C interacts with Stat1 in two different ways, one that prevents IFN-induced pY701-Stat1 formation and IFN signaling that has already been documented, and another that induces pY701-Stat1 formation while decreasing bulk Stat1 levels in an IFN signaling-independent manner. These two types of Stat1 interaction are also distinguishable by C gene mutations, as the IFN signaling-independent increase in pY701-Stat1 and decrease in bulk Stat1 levels require a longer C protein but not Phe170, whereas the IFN-dependent C/Stat1 interaction requires Phe170 but not a longer C protein. The manner in which the C-induced increase in pY701-Stat1 levels and decrease in bulk Stat1 levels can counteract the cellular antiviral response is discussed.
MATERIALS AND METHODS
Cells and viruses.
MEFs (53) and BSR T7 cells (2) were grown as monolayers in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. 2fTGH cells and their derived cell lines U1A, U1A+TYK2, U3A, U4A, U4A+JAK1, U5A, U5A+IFNAR, and U6A cells were obtained from I. M. Kerr's lab (Imperial Cancer Research Fund, London) and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum in the presence of the relevant maintenance drug (hygromycin [250 μg/ml] or G418 [400 μg/ml]). The generation of recombinant SeV expressing alternative C and P proteins has been described elsewhere (19, 35). All SeV stocks were grown in the allantoic cavity of 10-day-old embryonated chicken eggs. Virus present in the allantoic fluid was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining after virus pelleting. Virus titers were determined by plaquing on LLC-MK2 cells.
Virus infections.
Cells were infected at a multiplicity of infection of 20 in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. After an absorption period of 1 to 2 h, the inoculum was removed and replaced with fresh medium. For proteasome inhibition experiments, the medium contained 0.2% dimethyl sulfoxide supplemented or not with 10 μM MG132 (Sigma). For kinase inhibition experiments, the medium contained 500 nM staurosporine (Sigma).
Coimmunoprecipitation.
Cells were washed once with phosphate-buffered saline and scraped into phosphate-buffered saline, and whole-cell extracts were prepared with lysis buffer containing 0.5% NP-40, 50 mM Tris-Cl (pH 8), 150 mM NaCl, 10 mM EDTA, 1:200 protease inhibitor cocktail (Sigma P8340), and 1:200 phosphatase inhibitor cocktail (Sigma P2850). The mixture was sonicated for 5 s (Branson sonifier B12, position 3) and centrifuged at 13,000 × g for 2 min. The supernatant was used for immunoprecipitation with a mouse monoclonal antibody to ubiquitin (1:100 dilution) (P4D1; Santa Cruz). For the in vitro binding assay, two-thirds of total MEF extracts (corresponding to 4 × 106 cells) were mixed with two-thirds of infected BSR T7 cells (corresponding to 4 × 106 cells) at 20°C for 30 min before immunoprecipitation with a rabbit polyclonal antiserum to SeV C protein (1:300 dilution) (provided by Y. Nagai, Tokyo, Japan) or a mouse monoclonal antibody to Stat1 C terminus (1:300 dilution) (Transduction Laboratories; S21120).
Immunoblotting.
Whole-cell extracts were prepared as described above. Proteins were separated by SDS-PAGE and transferred to Immobilon-P membranes by semidry transfer. The primary antibodies used included a rabbit polyclonal antiserum to SeV P, C, V, and W proteins (to be described elsewhere), a mouse monoclonal antibody to the Stat1 C terminus (Transduction Laboratories; S21120), a rabbit polyclonal antiserum to phospho-Stat (Y701) (Upstate Biotechnology 06-657), a rabbit polyclonal antiserum to actin (provided by G. Gabbiani, Geneva, Switzerland), a mouse monoclonal antibody to ubiquitin (P4D1; Santa Cruz); and a rabbit polyclonal antibody to green fluorescent protein (GFP) (Clontech). The secondary antibodies used were alkaline phosphatase-conjugated goat antibodies specific for either rabbit or mouse immunoglobulin G (Bio-Rad). The immobilized proteins were detected by light-enhanced chemiluminescence (Pierce), and the results were quantified in a Bio-Rad light detector with Quantity One software (Bio-Rad).
Plasmids and transient transfections.
Various SeV C and Stat1 genes were cloned into episomal Epstein-Barr virus-based expression plasmid EBS-PL (1). pEBS expressing simian virus 5 (SV5) protein V and GFP were the generous gift of Olivier Leupin and Michel Strubin (Geneva) (38; unpublished data).
The IFN-α/β-responsive reporter plasmid p(9-27)4tk(239)lucter (30, 31), referred to here as pISRE(f)luc, contains four tandem repeats of the IFN-inducible gene 9-27 internally spaced repeat element (ISRE) fused to the firefly luciferase gene. pTK-r.luc, used as a transfection standard, contains the herpes simplex virus thymidine kinase (TK) promoter region upstream of the Renilla luciferase gene (Promega).
For transfections, 100,000 cells were plated in six-well plates 20 h before transfection with 1 μg of pEBS-C, 0.5 μg of pEBS-GFP, 1 μg of pEBS-Stat1, and 7.5 μl of Fugene (Roche) according to the manufacturer's instructions.
For luciferase assays, 2fTGH cells were transfected with 1 μg of pEBS-C, 1 μg of pISRE(f)luc, 0.3 μg of pTK-r.luc, and 7.5 μl of Fugene (Roche). At 24 posttransfection, the cells were treated or not with 1,000 IU of recombinant IFN-α2/α1 (51) per ml. At 14 h after IFN treatment, cells were harvested and assayed for firefly and Renilla luciferase activity (dual luciferase reporter assay system; Promega). Relative expression levels were calculated by dividing the firefly luciferase values by the Renilla luciferase values.
RESULTS
Reciprocal action of SeV infection on bulk Stat1 and pY701-Stat1 levels in MEFs.
Stat proteins are activated by phosphorylation of a single tyrosine near their carboxyl end (Y701 for Stat1) (10). Stats are long-lived in uninfected cells, and their inactivation involves dephosphorylation by a nuclear phosphatase and recycling to the cytoplasm (25). SeV infection prevents the rapid phosphorylation of Stat1 on Y701 associated with IFN-α signaling (i.e., that measured 45 min after the addition of 1,000 IU of IFN-α to the culture), in all cells examined (12, 15, 29). Nevertheless, pY701-Stat1 levels were paradoxically found to increase during SeV infection of HeLa cells (32, 45). As bulk Stat1 levels remained unchanged during HeLa infections and SeV prevented the normal loss of pY701-Stat1 when its de novo formation was blocked by treatment with staurosporine (a potent nonspecific kinase inhibitor), the increased pY701-Stat1 levels were attributed to SeV inhibition of the unknown phosphatase that dephosphorylates pY701-Stat1.
We have recently described an MEF cell line that is restricted for the growth of highly IFN-sensitive viruses such as vesicular stomatitis virus and SeV-CF170S (18). These cultures secrete high levels of IFN even in the absence of SeV infection, and they contain high constitutive levels of Stat1. These MEFs appear to have already established an IFN-induced antiviral (vesicular stomatitis virus) state or are primed to do so (see Discussion). In contrast to HeLa cells, SeV infection of these MEFs leads to a clear decrease in Stat1 levels; the MEFs become permissive for vesicular stomatitis virus at 18 h post-SeV infection, and this permissiveness increases with time. SeV can thus apparently dismantle this existing anti-vesicular stomatitis virus state, presumably in part by lowering Stat1 levels.
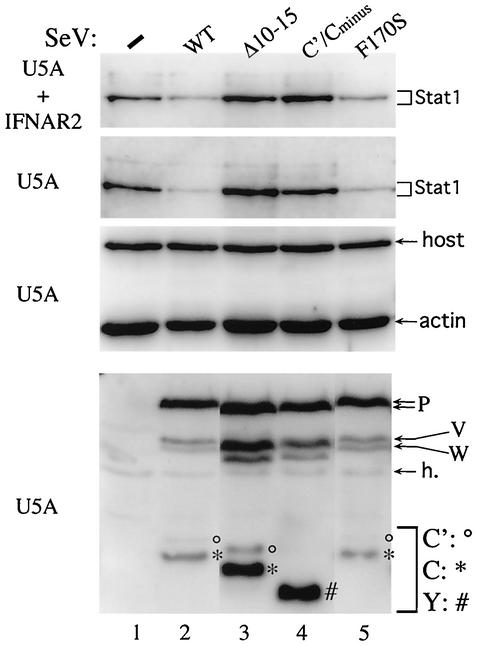
To determine whether SeV infection also increased pY701-Stat1 levels in cells in which bulk Stat1 levels decreased and whether this was due to its C protein, MEFs were infected or not with wild-type SeV (SeV-wt), SeV-CΔ10-15 (Fig. 1B), or SeV-[C′/C-minus] (whose C′/C initiation codons are inactive), and bulk Stat1 and pY701-Stat1 levels were followed (only a very small fraction of Stat1 is phosphorylated on Y701). Even though these three SeV replicate similarly in MEFs when the level of viral structural proteins is examined (e.g., P protein, Fig. 2A, lower panel), only SeV-wt infection concomitantly increased pY701-Stat1 levels while simultaneously decreasing bulk Stat1 levels (Fig. 2A, upper panel). Thus, only SeV that express wild-type C′ and C proteins increase pY701-Stat1 levels.
FIG. 2.
Effect of SeV infection on bulk Stat1 and pY702-Stat1 levels of MEFs. (A) Parallel cultures of MEFs were infected or not with 20 PFU of SeV-wt, SeV-CΔ10-15, or SeV-[C′/C-minus] (or Y1/Y2) per cell. Total cell extracts were prepared at 24 h postinfection, and equal amounts were Western blotted with anti-Stat1 antibody (bulk), anti-pY701-Stat1 plus antiactin antibodies (middle panel), or a polyclonal antibody to the viral P, C, V, and W proteins. Each pair of lanes represent duplicate, independent infections. °, *, and #, C′, C, and Y protein bands, respectively. (B) MEFs were infected or not with 20 PFU of SeV-wt per cell. After 24 h, some of the cultures were treated with 0.5 μM staurosporine for either 1 or 2 h. Total cell extracts were prepared, and equal amounts of extract were Western blotted with either anti-Stat1 (bulk) plus antiactin (the latter as a loading control) or anti-pY701-Stat1 antibodies in the ECL system. The results were quantitated in a Bio-Rad light detector and are presented below in graphic form and as their ratios. h., a host band.
To determine whether the increased pY701-Stat1 levels resulted from the increased stability of this protein, MEFs infected for 24 h with SeV-wt were treated with staurosporine. In both uninfected and SeV-infected cells, staurosporine treatment led to a clear loss of pY701-Stat1, but there was little difference in the rate of this loss over time (Fig. 2B, bottom). Thus, in contrast to the HeLa cell infections, no evidence was found that SeV alters the half-life of pY701-Stat1. Unexpectedly, bulk Stat1 levels increased during staurosporine treatment of both infected and control cultures. In summary, SeV infection decreases bulk Stat1 levels while increasing pY701-Stat1 levels in MEFs. Staurosporine has the opposite effect and partially reverses the effects of SeV infection.
SeV-induced Stat1 instability and pY701-Stat1 formation are independent of IFN signaling in 2fTGH cells.
SeV lowers bulk Stat1 levels in several cell lines, including 2fTGH human fibrosarcoma cells, from which sublines defective in specific components of the IFN signaling system have been generated (43). U5A cells, for example, are defective in the IFN-α/β receptor 2 chain, and these cells are restored to IFN sensitivity by complementation with the IFNAR2 gene (39). As shown in Fig. 3A, SeV-wt infection lowered Stat1 levels relative to the mock-infected control in both U5A and U5A+IFNAR2 cells. SeV CΔ10-15 and SeV-[C′/C-minus] infections, in contrast, slightly increased Stat1 levels, even though these infections accumulated more C proteins than SeV-wt infections (Fig. 3). Thus, similar to MEFs, in which CΔ10-15 was unable to decrease Stat1 levels, this decrease in fibrosarcoma cells also required the longer C proteins.
FIG. 3.
Effect of various SeV infections of U5A cells with and without IFNAR2 on bulk Stat1 levels. Parallel cultures of U5A or U5A+IFNAR2 cells were infected or not with 20 PFU of either SeV-wt, SeV-CΔ10-15, SeV-[C′/C-minus], or SeV-CF170S per cell. After 24 h, total cell extracts were prepared, and equal amounts of extract were Western blotted with anti-Stat1 (bulk) plus antiactin, or polyclonal antibodies that recognize the SeV C, P, V, and W proteins.
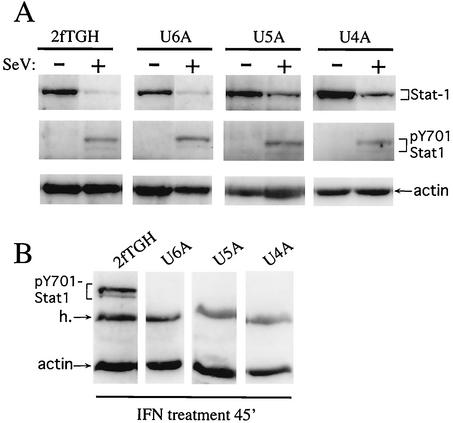
Although SeV-CF170S did not grow in MEFs, it grew as well as SeV-wt in U5A cells (P protein, lane 5, Fig. 3). Unexpectedly, SeV-CF170S infection also appeared to lower Stat1 levels, almost as strongly as SeV-wt (at least during the first 24 h of infection; see below). More importantly, SeV-induced Stat1 instability apparently did not require a functional IFN receptor that is essential for IFN signaling. This point was examined in more detail by infecting U4C (JAK1-minus [40]), U5A (IFNAR2-minus), and U6A (Stat2-minus [37]) cells as well as the parental 2fTGH cells with SeV. As shown in Fig. 4B, cells defective for any of these essential components of the IFN signaling system were unable to respond to IFN treatment by increasing pY701-Stat1 levels. When the changes in bulk Stat1 and pY701-Stat1 levels were followed, SeV infection was found to increase pY701-Stat1 levels even though bulk Stat1 levels decreased in all these cell lines. Thus, neither Stat2, an essential component of IFN-stimulated gene factor 3, nor a functional IFN receptor (IFNAR2), nor a key Stat kinase (JAK1) is required for SeV to increase pY701-Stat1 levels or to decrease bulk Stat1 levels in 2fTGH cells.
FIG. 4.
Effect of SeV infection of various cell lines defective in specific components of the IFN signaling system on bulk Stat1 and pY7021-Stat1 levels. Parallel cultures of U6A (Stat2-minus), U5A (IFNAR2-minus), U4A (JAK1-minus), or the parental (wild type) 2fTGH cells were infected or not with 20 PFU of SeV-wt per cell. Total cell extracts were prepared at 24 h postinfection, and equal amounts of extract were Western blotted with either anti-Stat1 (bulk) plus antiactin or anti-pY701-Stat1 antibodies (panel A). Panel B shows the effect of 45 min of IFN treatment on pY701-Stat1 levels of the various cell lines.
We also investigated whether Y701 itself was critical for SeV-induced Stat1 instability. BSR T7 cells, whose resident Stat1 is not detected with our anti-Stat1, were transfected with plasmids expressing human Stat1 (1 to 750), Stat1-Y701F, and Stat1-Δ694-750 (missing the entire activation domain). After 24 h, the transfected cells were infected or not with SeV for a further 24 h, and their bulk Stat1 levels were determined by immunoprecipitation (Fig. 5). In all cases, SeV infection clearly reduced the Stat1, Stat1-Y701F, and Stat1-Δ694-750 levels. Thus, SeV-induced Stat1 instability requires neither the critical Y701 of Stat1 nor the entire carboxyl activation domain. This is consistent with the finding that Stat1 instability is largely independent of IFN signaling.
FIG. 5.
Effect of SeV infection on transfected Stat1 levels in BSR T7 cells. Parallel cultures of BSR T7 cells were transfected with pEBS plasmids expressing either human wild-type Stat1 (1 to 750), Stat1-Y701F, Stat1-Δ694-750, or empty plasmid. After 24 h of transfection, the cultures were infected with 20 PFU of SeV-wt per cell for a further 24 h. Total cell extracts were prepared, equal amounts of extract were precipitated with anti-Stat1, and the precipitates were Western blotted with anti-Stat1.
Effects of various C genes in cells deficient in IFN signaling components.
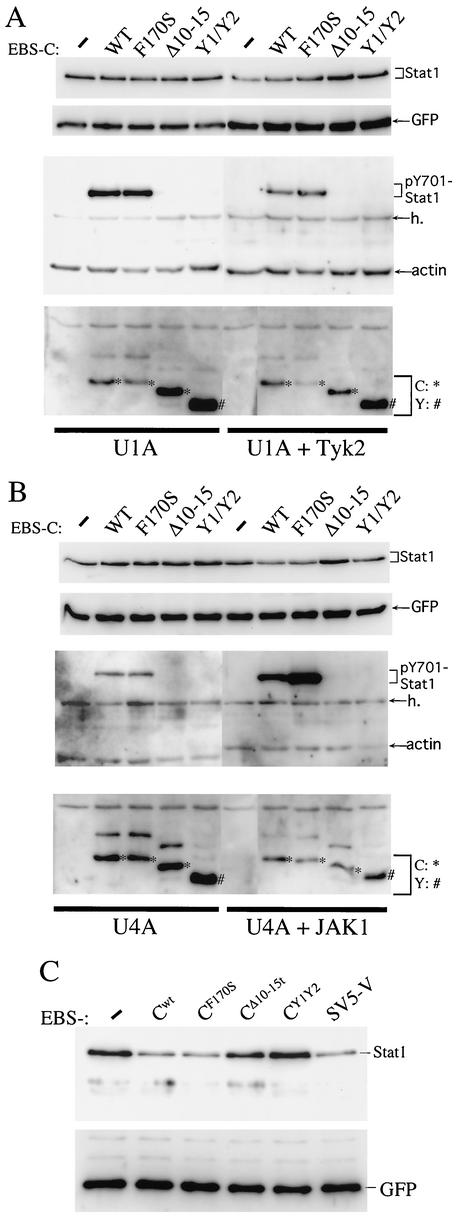
SeV-induced Stat1 instability apparently requires a function specific to the longer C proteins, as infections that do not express C′/C do not induce Stat1 instability (15, 18) (Fig. 2 and 3). The concomitant increase in pY701-Stat1 levels also appears to require this specific function of the longer C proteins (Fig. 2). To further examine this increase in pY701-Stat1 levels, pEBS plasmids expressing various C genes were transfected into U1A cells with and without Tyk2, U4A cells with and without JAK1, U5A cells with and without IFNAR2, and U6A cells with and without Stat2, along with plasmids expressing Stat1 and GFP (the latter to control for transfection efficiency). The C genes included C-wt, CF170S, and CΔ10-15, which all begin at AUG114/C and should not express C′ or P (AUG104).
These plasmids should express essentially only C because the ribosomal shunt operates poorly here, but there was also a more slowly migrating shadow band of variable intensity and unknown origin (Fig. 6A and B). The C′/C-minus (or Y1/Y2) gene begins on AUG183/Y1, and all the C genes end at UAA728. Overexpressing Stat1 by cotransfection raises its intracellular level at least 10-fold and should provide an abundant target for C-induced phosphorylation. As shown in Fig. 6 (and data not shown), the expression of the C-wt and CF170S genes increased pY701-Stat1 levels in all the 2fTGH cell lines tested, whereas the CΔ10-15 and C′/C-minus genes were inactive in this respect. In particular, C-wt and CF170S were also active in Stat2-minus U6A cells (see Discussion).
FIG. 6.
Effect of various transfected C genes on cotransfected Stat1 in cell lines defective in known IFN-dependent signaling components. Parallel cultures of U1A and U1A+Tyk2 cells (A) and U4A and U4A+JAK2 cells (B) were cotransfected with pEBS plasmids expressing Stat1 and GFP along with one of the various C gene plasmids listed or an empty plasmid. Total cell extracts were prepared at 24 h posttransfection, and equal amounts of extract were Western blotted with either anti-Stat1 (bulk) plus anti-GFP (top panel) or anti-pY701-Stat1 plus antiactin (middle panel) or anti-C (bottom panel) antibodies. (C) 2fTGH cells were also transfected with the various C gene constructs (as well as the SV5 V gene) along with Stat1 and GFP for 72 h, and the steady-state level of Stat1 remaining was determined as in panels A and B.
To control for the specificity of our anti-pY701-Stat1 antibody, Stat1-minus U3A cells were transfected with various C genes along with GFP and either Stat1 or Stat1-Y701F. Our anti-pY701-Stat1antibody detected a band only when Stat1 (and not Stat1-Y701F) was present (data not shown). Thus, similar to C-induced Stat1 instability, a function specific to the longer C proteins is required for the C-induced increase in pY701-Stat1 levels. This function of the longer C proteins also acts independently of the other components of SeV in increasing pY701-Stat1 levels, consistent with a direct interaction of C and Stat1. We also note that the cotransfected C genes had little or no effect on the overexpressed Stat1 levels when they were examined at 24 h posttransfection. However, when these transfections are carried out until 72 h, the effect of C-wt and CF170S on reducing bulk Stat1 levels was clearly evident (Fig. 6C). Moreover, the SeV C proteins were as active in this respect as the SV5 V protein, a dedicated Stat1 destabilizer (13).
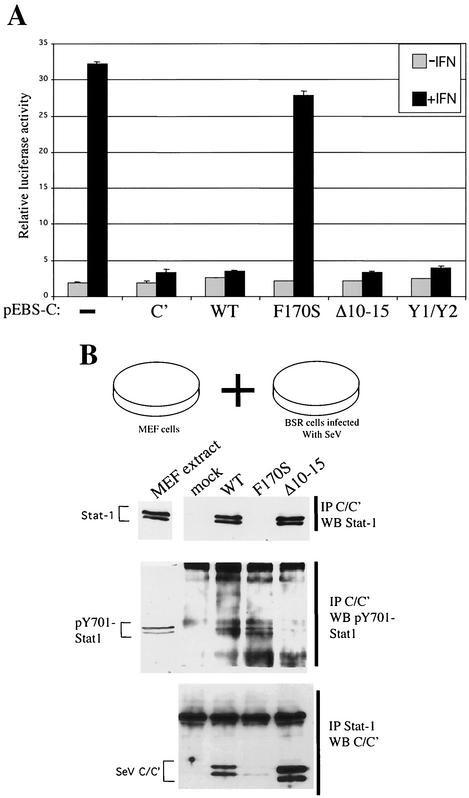
We were surprised to find that CF170S was (at least) as active as C-wt in increasing pY701-Stat1 levels, as this mutation has inactivated various functions of C examined so far. For example, CF170S is unable to form a complex with Stat1 in vitro under conditions in which CΔ10-15 and Y1/Y2 are active (18). Since pEBS plasmids are now being used to express the C genes (Fig. 6), we reexamined their ability to interdict IFN signaling to a cotransfected reporter construct in which luciferase is controlled by an IFN-stimulated response element (pISRE-luc). As expected, C-wt, C-Δ10-15, and Y1/Y2 expression all efficiently prevented IFN-induced luciferase activity, and only CF170S was inactive in this respect (Fig. 7A).
FIG. 7.
IFN-dependent C/Stat1 interaction requires F170 but not the longer C proteins, whereas IFN-independent C/pY701-Stat1 interaction requires the longer C proteins but not F170. (A) Parallel cultures of 2fTGH cells were cotransfected with pISRE-luc (firefly) and pTK-r.luc (Renilla) as well as one of the various C genes listed or an empty plasmid. Some of the cultures were treated with 1,000 IU of IFN-α for 14 h at 24 h posttransfection. Cell extracts were then prepared, and the relative activities of both luciferases were determined. The bars show the results of duplicate determinations. (B) MEF cell extracts were mixed with those of BSR T7 cells that had been infected or not for 24 h with 20 PFU of either SeV-wt, SeV-CF170S, or SeV-CΔ10-15 per cell. The mixed extracts were then either precipitated with anti-C and Western blotted with anti-Stat1 or anti-pY701-Stat1 or precipitated with anti-Stat1 and Western blotted with anti-C.
Taken together with the results in Fig. 6, the critical CF170 is not required for IFN signaling-independent C-induced pY701-Stat1 formation, whereas the longer C proteins are clearly required. This prompted us to examine whether CF170S could form a complex with pY701-Stat1 in vitro. MEF cell extracts (as a source of Stat1 and pY701-Stat1) were mixed with those of BSR T7 cells infected with either SeV-wt, SeV-CF170S, or SeV-CΔ10-15 (Fig. 7B). The mixed extracts were then immunoprecipitated with anti-C and Western blotted with either anti-Stat1 or anti-pY701-Stat1. As found previously (18), Stat1 formed a complex with C-wt and CΔ10-15 but not with CF170S (Fig. 7B). C-wt/pY701-Stat1 complex formation also occurred in this mixed-extract assay, but this complex was just visible. Nevertheless, CF170S was as active as C-wt in forming a complex with pY701-Stat1 under these conditions, whereas CΔ10-15 was relatively inactive. Thus, C/pY701-Stat1 complex formation in vitro requires a longer C protein but does not require the critical Phe170, whereas C/Stat1 complex formation requires the critical Phe170 but not a longer C protein. C is apparently interacting with Stat1 and pY701-Stat1 in different ways. The different interactions of C and Stat1 are summarized in Fig. 1B.
Respirovirus C proteins and rubulavirus V proteins.
Most if not all paramyxoviruses induce Stat instability, at least in some cells. Rubulaviruses SV5, SV41, and mumps virus also induce Stat1 instability, whereas human parainfluenza virus type 2 induces Stat2 instability (reviewed in references 41 and 42). Rubulaviruses do not express C proteins as such, and this activity is due to its V protein, another P gene-derived product (13). As proteasome inhibitors such as MG132 and lactacystin prevent these paramyxovirus-induced decreases in Stat1 levels (13, 18), Stat1 instability is thought to be due to proteasomal degradation. It may be relevant in this respect that only the longer SeV C proteins induced what appears to be a ubiquitinated form of Stat1 (albeit with only a single ubiquitin adduct), a hallmark of protein degradation (18).
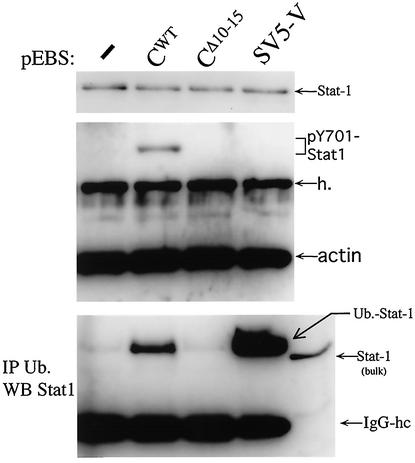
We therefore examined whether the rubulavirus SV5 V protein also induced this modified form of Stat1, as well as the (IFN signaling-independent) formation of pY701-Stat1. 2fTGH cells were transfected with Stat1 along with various C genes or the rubulavirus SV5 V gene. After 24 h, the transfected cultures were treated with MG132, and the levels of pY701-Stat1 were then determined by immunoblotting. The levels of ubiquitinated Stat1 were determined by first precipitating the extracts with antiubiquitin, followed by Western blotting with anti-Stat1. As shown in Fig. 8, the SV5 V protein was unable to induce pY701-Stat1 formation, in contrast to the SeV C protein. However, similar to the SeV C protein, the SV5 V protein did lead to elevated levels of (what appears to be) monoubiquitinated Stat1. Thus, this modified form of Stat1 is associated with both SeV C-induced and SV5 V-induced Stat1 instability.
FIG. 8.
Rubulavirus SV5 V gene induces Stat1 ubiquitination but not pY701-Stat1 formation. 2fTGH cells were cotransfected with plasmids expressing GFP and Stat1 along with the SeV C, SeV CΔ10-15, or SV5 V gene or an empty plasmid. Total cell extracts were prepared at 24 h posttransfection, and equal amounts of extract were Western blotted with either anti-Stat1 (bulk, top panel) or anti-pY701-Stat1 plus antiactin (middle panel). Equal amounts of extract were also precipitated with antiubiquitin (Ub) and then Western blotted with anti-Stat1 (bottom panel). The right-hand lane in the bottom panel has not been immunoprecipitated and shows the electrophoretic position of bulk Stat1. IgG-hc, IgG heavy chain.
DISCUSSION
Viruses not only need to counteract the establishment of the antiviral state in naïve (i.e., non-IFN-primed) cells, they must also overcome the existing antiviral state in neighboring cells “primed” by the paracrine effects of IFN. SeV infection of MEFs may be representative of this situation. As mentioned above, uninfected MEFs contain high constitutive levels of Stat1, and vesicular stomatitis virus growth is restricted. SeV infection decreases these Stat1 levels, and SeV-infected MEFs become permissive for vesicular stomatitis virus. Moreover, MG132 treatment of SeV-infected MEFs reverses the effects of SeV infection and reestablishes the anti-vesicular stomatitis virus state (18). Thus, SeV-induced degradation of Stat1 appears to be important in dismantling the IFN-induced anti-vesicular stomatitis virus state.
The dismantling of an existing antiviral state is presumably a more arduous task than preventing its establishment. That SeV can carry out the latter task in HeLa cells without lowering Stat1 levels may reflect this; it should not be construed as an indication that lowering Stat1 levels is irrelevant to the viral survival strategy (29). The SeV C proteins appear to act at two levels in counteracting the antiviral state. All four C proteins bind Stat1, preventing pY701-Stat1 formation and IFN signaling, and this requires F170. The two longer C proteins also induce Stat1 instability. Whether one or both of these mechanisms is sufficient to counteract the antiviral state presumably depends on the host cell.
Stat1 is a major transcription factor in the IFN signal transduction pathways that lead to cellular antiviral and immunomodulatory responses. IFNs stimulate gene expression by the phosphorylation of Y701-Stat1 by Janus kinases at the cell membrane (e.g., JAK1 and Tyk2). pY701-Stat1 then homo- or heterodimerizes and migrates to the nucleus, where it associates with IRF-9 and binds to the cognate response elements of IFN-stimulated genes (ISGs) (27, 46). Stat proteins generally are long-lived in uninfected cells, and their inactivation involves dephosphorylation by a nuclear phosphatase and recycling to the cytoplasm. It is interesting that both properties of C (Stat1 binding and instability) are seen in MEF and 2fTGH cells that have abundant constitutive levels of Stat1, whereas only Stat1 binding is seen in HeLa cells, which in our experience have very little Stat1. We measured the t1/2 of Stat1 in 2fTGH cells, and this protein is very stable and is synthesized at very low rates. If HeLa cells contain low levels of Stat1 because of higher turnover rates, a virus-induced further increase in these rates might not be visible in these circumstances. Moreover, in MEFs and 2fTGH cells, a rapid reduction in Stat1 levels would require its virus-induced degradation.
Although phosphorylation of Y701-Stat1 is critical for IFN-stimulated gene expression, it has become increasingly clear, most recently from DNA array studies of Stat1-minus U3A cells reconstituted with the mutant Y701F-Stat1 protein, that unphosphorylated Stat1 is also required for the constitutive expression of many genes (6). These include caspases 1, 2, and 3, which are involved in apoptosis, and LMP2, a subunit of the 20S proteasome that processes viral antigens for presentation to CD8+ T cells. As both apoptosis and antigen presentation are important elements of the cellular antiviral response, unphosphorylated Y701-Stat1 is presumably also a suitable target for viral countermeasures. The adenovirus E1A protein is known to downregulate LMP2 mRNA transcription by directly interfering with the binding of unphosphorylated Stat1 to IRF-1 (5). Moreover, Stat1 is a major transcriptional target of human papillomavirus type 31 (4). We presume that the SeV C-induced Stat1 instability also plays a role in viral countermeasures, and this may explain why only infections that express the longer C proteins can dismantle the antiviral (vesicular stomatitis virus) state of MEFs.
As mentioned above, most if not all paramyxoviruses induce STAT instability. The components of the IFN signaling pathway required for rubulavirus-induced Stat instability have recently been investigated (42). Neither the IFN-α/β receptor nor the tyrosine kinases JAK1 and Tyk2 were required. Thus, surprisingly, rubulavirus-induced Stat degradation was found to be largely independent of IFN signal transduction. The SeV (respirovirus) C proteins share many of the properties of the rubulavirus V proteins; they both bind Stat1 and induce its ubiquitin-like modification and proteasomal (MG132-sensitive) degradation in an IFN-independent manner. Moreover, the SeV C and the mumps virus V proteins apparently bind to the same region of Stat1, as prebinding of mumps virus V to Stat1 prevents the subsequent binding of SeV C (41).
The direct interaction of these two paramyxovirus P gene products with apparently the same region of Stat1 presumably accounts for their ability to induce Stat1 instability independent of IFN signaling. However, whereas rubulaviruses require Stat2 to degrade Stat1, SeV does not (Fig. 4). Stat2 is required for rubulavirus-induced Stat1 degradation (and vice versa) because its V protein forms a heterotrimeric complex with Stat1 and Stat2 that is apparently required to target Stat1 (or Stat2) degradation (42). The SeV C and the rubulavirus V proteins differ further in that SV5 V does not induce pY701-Stat1 formation (Fig. 8). The other major difference is that only SeV C also interacts with Stat1 in an IFN-dependent manner, to block IFN signaling even in cells in which Stat1 levels are not decreased. These similarities and differences are summarized in Fig. 1B.
The central finding of this paper is that C interacts with Stat1 itself in two different ways. The first interaction, which can also be carried out by the shorter Y proteins, leads to the inhibition of IFN-induced pY701-Stat1 formation and IFN signaling, and the CF170S mutation abolishes these activities. The second interaction is IFN signaling independent and does not require F170, but can be carried out only by the longer C proteins. This interaction leads to Stat1 modification and instability and paradoxically to elevated pY701-Stat1 levels.
It is unclear how these two apparently opposing properties of C could be linked operationally, if indeed they are linked. The interaction of one protein with multiple partners is not uncommon, but its interaction in two different ways with the same partner is more unusual. A clue as to how C might interact differently with Stat1 has come from the realization that some proteins contain regions of “native disorder,” as described for acidic activation domains of transcription factors that interact with multiple partners (52, 50). According to this view, native disorder allows these domains to bind different surfaces with high specificity (multiple induced fits) and limited stability (i.e., easy “off” rates that are useful in terminating interactions). The NH2-terminal portion of the measles virus P protein (which contains the overlapping C protein open reading frame) is, in fact, a recent example of such natively disordered proteins, in accordance with the prediction of algorithms that detect unstructured regions (28).
With the same algorithms (www.PONDR.com), the SeV C protein is strongly predicted to be natively disordered, and this property is shared with the common NH2-terminal portions of the rubulavirus V, I, and P proteins (25a; J. Curran and D. Kolakofsky, unpublished data). We have previously noted the astonishing property of the SeV C protein on SDS-PAGE, where deletions of 7 to 9 amino acids in the NH2-terminal portion of the protein paradoxically lead to reduced rather than increased electrophoretic mobility (8). These deletions and the Δ10-15 deletion are in the region of C with the highest prediction of disorder, and this aberrant electrophoretic behavior of C may be a property of natively disordered proteins.
The reasons why SeV would decrease Stat1 levels to its advantage are discussed above. The C-induced (IFN signaling-independent) increase in pY701-Stat1 levels, which occur even in cells in which bulk Stat1 levels decrease, however, is more difficult to fathom, as this modification is normally associated with antiviral activity. Only members of the Janus family of tyrosine kinases (JAK1 to JAK3 and Tyc2) are known to phosphorylate the STATs. However, other signaling receptors (e.g., interleukin-6 and platelet-derived growth factor) also phosphorylate Y701-Stat1, presumably by recruiting JAKs (46). Although C induces pY701-Stat1 formation in JAK1-minus U4A cells, we note that reconstitution of these cells with JAK1 strongly enhances C-induced pY701-Stat1 formation (Fig. 6B). JAK1 may therefore regulate the IFN signaling-independent Stat1 kinase or may itself be one of these kinases.
The Src family of tyrosine kinases may even be involved if C acts as an adaptor to connect Stat1 to the Src kinase. Interleukin-6 can also activate Stat1 in an IFN-independent manner in Stat3−/− MEFs (7, 47), and in this case this pY701-Stat1 acts in an IFN-like manner with regard to ISGs and an antiviral state. The SeV C-induced pY701-Stat1, in contrast, does not activate ISGs. We can speculate that the increased levels of pY701-Stat1 during SeV infection fail to activate ISGs because they are unable to dimerize with pY690-Stat2, as this heterodimer is formed only in a concerted fashion at the cytoplasmic face of the IFN-α/β receptor (46).
Our results show that the pY701-Stat1 that accumulates during SeV infection does so independently of an IFN signaling system and is presumably not heterodimerized with pY690-Stat2. Activated Stat1 can also function to repress gene transcription is some cases (21). Given that the SeV C-induced pY701-Stat1 accumulates in the nucleus of infected HeLa cells (32), it is tempting to speculate that it might act there to negatively regulate ISGs. This could be an alternative way in which the SeV C gene blocks IFN action.
Acknowledgments
We thank Ian Kerr, ICRF, London, for providing the 2fTGH cells and their derivatives and Bob Lamb, Northwestern University, for providing the SV5 V gene and for helpful discussions.
This work was supported by a grant from the Swiss National Science Fund.
REFERENCES
- 1.Bontron, S., C. Ucla, B. Mach, and V. Steimle. 1997. Efficient repression of endogenous major histocompatibility complex class II expression through dominant negative CIITA mutants isolated by a functional selection strategy. Mol. Cell. Biol. 17:4249-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadd, T., D. Garcin, C. Tapparel, M. Itoh, M. Homma, L. Roux, J. Curran, and D. Kolakofsky. 1996. The Sendai paramyxovirus accessory C proteins inhibit viral genome amplification in a promoter-specific fashion. J. Virol. 70:5067-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y. E., and L. A. Laimins. 2000. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J. Virol. 74:4174-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee-Kishore, M., A. F. van Den, and G. R. Stark. 2000. Adenovirus E1A down-regulates LMP2 transcription by interfering with the binding of stat1 to IRF1. J. Biol. Chem. 275:20406-20411. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee-Kishore, M., K. L. Wright, J. P. Ting, and G. R. Stark. 2000. How Stat1 mediates constitutive gene expression: a complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J. 19:4111-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa-Pereira, A. P., S. Tininini, B. Strobl, T. Alonzi, J. F. Schlaak, H. Is'harc, I. Gesualdo, S. J. Newman, I. M. Kerr, and V. Poli. 2002. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc. Natl. Acad. Sci. USA 99:8043-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran, J., and D. Kolakofsky. 1988. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 7:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran, J., J.-B. Marq, and D. Kolakofsky. 1992. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology 189:647-656. [DOI] [PubMed] [Google Scholar]
- 10.Darnell, J. E. J. 1997. STATs and gene regulation. Science JID-0404511 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 11.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J. Virol. JID-0113724 73:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. JID-0113724 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fambrough, D., K. McClure, A. Kazlauskas, and E. S. Lander. 1999. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell 97:727-741. [DOI] [PubMed] [Google Scholar]
- 15.Garcin, D., J. Curran, M. Itoh, and D. Kolakofsky. 2001. Longer and shorter forms of Sendai virus C proteins play different roles in modulating the cellular antiviral response. J. Virol. 75:6800-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcin, D., M. Itoh, and D. Kolakofsky. 1997. A point mutation in the Sendai virus accessory C proteins attenuates virulence for mice, but not virus growth in cell culture. Virology 238:424-431. [DOI] [PubMed] [Google Scholar]
- 17.Garcin, D., P. Latorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 73:6559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcin, D., J. B. Marq, L. Strahle, P. Le Mercier, and D. Kolakofsky. 2002. All four Sendai virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology 295:256-265. [DOI] [PubMed] [Google Scholar]
- 19.Garcin, D., T. Pelet, P. Calain, L. Roux, J. Curran, and D. Kolakofsky. 1995. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: generation of a novel copy-back nondefective interfering virus. EMBO J. 14:6087-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. C. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 276:30178-30182. [DOI] [PubMed] [Google Scholar]
- 21.Gil, M. P., E. Bohn, A. K. O'Guin, C. V. Ramana, B. Levine, G. R. Stark, H. W. Virgin, and R. D. Schreiber. 2001. Biologic consequences of Stat1-independent IFN signaling. Proc. Natl. Acad. Sci. USA 98:6680-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorgi, C., B. M. Blumberg, and D. Kolakofsky. 1983. Sendai virus contains overlapping genes expressed from a single mRNA. Cell 35:829-836. [DOI] [PubMed] [Google Scholar]
- 23.Gotoh, B., K. Takeuchi, T. Komatsu, J. Yokoo, Y. Kimura, A. Kurotani, A. Kato, and Y. Nagai. 1999. Knockout of the Sendai virus C gene eliminates the viral ability to prevent the interferon-alpha/beta-mediated responses. FEBS Lett. 459:205-210. [DOI] [PubMed] [Google Scholar]
- 24.Hardwick, J. M. 2001. Apoptosis in viral pathogenesis. Cell Death Differ. 8:109-110. [DOI] [PubMed] [Google Scholar]
- 25.Haspel, R. L., and J. E. Darnell, Jr. 1999. A nuclear protein tyrosine phosphatase is required for the inactivation of Stat1. Proc. Natl. Acad. Sci. USA 96:10188-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-β induction and interferon signalling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 26.Horikami, S. M., R. E. Hector, S. Smallwood, and S. A. Moyer. 1997. The Sendai virus C protein binds the L polymerase protein to inhibit viral RNA synthesis. Virology 235:261-270. [DOI] [PubMed] [Google Scholar]
- 27.Horvath, C. M. 2000. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem. Sci. JID-7610674 25:496-502. [DOI] [PubMed] [Google Scholar]
- 28.Karlin, D., S. Longhi, V. Receveur, and B. Canard. 2002. The N-terminal domain of the phosphoprotein of morbilliviruses belongs to the natively unfolded class of proteins. Virology 296:251-262. [DOI] [PubMed] [Google Scholar]
- 29.Kato, A., Y. Ohnishi, M. Kohase, S. Saito, M. Tashiro, and Y. Nagai. 2001. Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis. J. Virol. JID-0113724 75:3802-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King, P., and S. Goodbourn. 1994. The beta-interferon promoter responds to priming through multiple independent regulatory elements. J. Biol. Chem. 269:30609-30615. [PubMed] [Google Scholar]
- 31.King, P., and S. Goodbourn. 1998. STAT1 is inactivated by a caspase. J. Biol. Chem. 273:8699-8704. [DOI] [PubMed] [Google Scholar]
- 32.Komatsu, T., K. Takeuchi, J. Yokoo, and B. Gotoh. 2002. Sendai virus C protein impairs both phosphorylation and dephosphorylation processes of Stat1. FEBS Lett. 511:139-144. [DOI] [PubMed] [Google Scholar]
- 33.Kurotani, A., K. Kiyotani, A. Kato, T. Shioda, Y. Sakai, K. Mizumoto, T. Yoshida, and Y. Nagai. 1998. Sendai virus C proteins are categorically nonessential gene products but silencing their expression severely impairs viral replication and pathogenesis. Genes Cells 3:111-124. [DOI] [PubMed] [Google Scholar]
- 34.Lamb, R. A., B. W. J. Mahy, and P. W. Choppin. 1976. The synthesis of Sendai virus polypeptides in infected cells. Virology 69:116-131. [DOI] [PubMed] [Google Scholar]
- 35.Latorre, P., T. Cadd, M. Itoh, J. Curran, and D. Kolakofsky. 1998. The various Sendai virus C proteins are not functionally equivalent, and exert both positive and negative effects on viral RNA accumulation during the course of infection. J. Virol. 72:5984-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latorre, P., D. Kolakofsky, and J. Curran. 1998. Sendai virus Y proteins are initiated by a ribosomal shunt. Mol. Cell. Biol. 18:5021-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung, S., S. A. Qureshi, I. M. Kerr, J. E. Darnell, Jr., and G. R. Stark. 1995. Role of STAT2 in the alpha interferon signaling pathway. Mol. Cell. Biol. 15:1312-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin-Marq, N., S. Bontron, O. Leupin, and M. Strubin. 2001. Hepatitis B virus X protein interferes with cell viability through interaction with the p127-kDa UV-damaged DNA-binding protein. Virology 287:266-274. [DOI] [PubMed] [Google Scholar]
- 39.Lutfalla, G., S. J. Holland, E. Cinato, D. Monneron, J. Reboul, N. C. Rogers, J. M. Smith, G. R. Stark, K. Gardiner, and K. E. Mogensen. 1995. Mutant U5A cells are complemented by an interferon-alpha beta receptor subunit generated by alternative processing of a new member of a cytokine receptor gene cluster. EMBO J. 14:5100-5108. [DOI] [PMC free article] [PubMed]
- 40.Muller, M., C. Laxton, J. Briscoe, C. Schindler, T. Improta, J. E. Darnell, Jr., G. R. Stark, and I. M. Kerr. 1993. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and-gamma signal transduction pathways. EMBO J. 12:4221-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishio, M., D. Garcin, V. Simonet, and D. Kolakofsky. 2002. The carboxyl segment of the mumps virus v protein associates with stat proteins in vitro via a tryptophan-rich motif. Virology 300:92.. [DOI] [PubMed] [Google Scholar]
- 42.Parisien, J. P., J. F. Lau, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2002. Selective STAT protein degradation induced by paramyxoviruses requires both STAT1 and STAT2 but is independent of alpha/beta interferon signal transduction. J. Virol. 76:4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellegrini, S., J. John, M. Shearer, I. M. Kerr, and G. R. Stark. 1989. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol. Cell. Biol. 9:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 45.Saito, S., T. Ogino, N. Miyajima, A. Kato, and M. Kohase. 2002. Dephosphorylation failure of tyrosine-phosphorylated STAT1 in IFN-stimulated Sendai virus C protein-expressing cells. Virology 293:205-209. [DOI] [PubMed] [Google Scholar]
- 46.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 47.Strobl, B., V. Arulampalam, H. Is'harc, S. J. Newman, J. F. Schlaak, D. Watling, A. P. Costa-Pereira, F. Schaper, I. Behrmann, K. C. Sheehan, R. D. Schreiber, F. Horn, P. C. Heinrich, and I. M. Kerr. 2001. A completely foreign receptor can mediate an interferon-gamma-like response. EMBO J. 20:5431-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi, K., T. Komatsu, J. Yokoo, A. Kato, T. Shioda, Y. Nagai, and B. Gotoh. 2001. Sendai virus C protein physically associates with Stat1. Genes Cells JID-9607379 6:545-557. [DOI] [PubMed] [Google Scholar]
- 49.Tapparel, C., S. Hausmann, T. Pelet, J. Curran, D. Kolakofsky, and L. Roux. 1997. Inhibition of Sendai virus genome replication due to promoter-increased selectivity: a possible role for the accessory C proteins. J. Virol. 71:9588-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tompa, P. 2002. Intrinsically unstructured proteins. Trends Biochem. Sci. 27:527.. [DOI] [PubMed] [Google Scholar]
- 51.Weber, H., D. Valenzuela, G. Lujber, M. Gubler, and C. Weissmann. 1987. Single amino acid changes that render human IFN-alpha 2 biologically active on mouse cells. EMBO J. JID-8208664 6:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright, P. E., and H. J. Dyson. 1999. Intrinsically unstructured proteins: reassessing the protein structure-function paradigm. J. Mol. Biol. 293:321-331. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou, A., J. M. Paranjape, S. D. Der, B. R. Williams, and R. H. Silverman. 1999. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology 258:435-440. [DOI] [PubMed] [Google Scholar]