Abstract
West Nile virus (WNV) causes severe central nervous system (CNS) infection primarily in humans who are immunocompromised or elderly. In this study, we addressed the mechanism by which the immune system limits dissemination of WNV infection by infecting wild-type and immunodeficient inbred C57BL/6J mice with a low-passage WNV isolate from the recent epidemic in New York state. Wild-type mice replicated virus extraneuronally in the draining lymph nodes and spleen during the first 4 days of infection. Subsequently, virus spread to the spinal cord and the brain at virtually the same time. Congenic mice that were genetically deficient in B cells and antibody (μMT mice) developed increased CNS viral burdens and were vulnerable to lethal infection at low doses of virus. Notably, a ∼500-fold difference in serum viral load was detected in μMT mice as early as 4 days after infection, a point in the infection when low levels of neutralizing immunoglobulin M antibody were detected in wild-type mice. Passive transfer of heat-inactivated serum from infected and immune wild-type mice protected μMT mice against morbidity and mortality. We conclude that antibodies and B cells play a critical early role in the defense against disseminated infection by WNV.
West Nile virus (WNV), a single-stranded positive-polarity RNA virus, is the etiologic agent of West Nile encephalitis. WNV is maintained in a natural cycle between mosquitoes and birds but also infects humans, horses, and other animals. It is endemic in parts of Africa, Europe, the Middle East, and Asia (24), and outbreaks in the United States over the past 3 years indicate that it has established its presence in the Western Hemisphere (28). Humans develop a febrile illness, with a subset of cases progressing to a meningitis or encephalitis syndrome (24). Currently, no specific therapy or vaccine has been approved for human use.
The molecular basis of WNV infection in humans and other animals is not clearly established. Although prior rodent models of WNV infection (5, 15, 16, 22, 49, 50) have shown evidence of viral replication in serum and the central nervous system (CNS), many of the mechanistic questions about pathogenesis and the immune response remain unanswered. For example, a peripheral site of replication has not been defined and the mechanism of spread to the CNS remains unclear. Additionally, a target cell for infection in the CNS has not been definitively identified, and whether tissue injury is related directly to viral infection or to the immune response remains unknown. Finally, although susceptibility to severe WNV infection correlates with an impaired immune system (1, 24, 46), the mechanisms by which the innate and adaptive immune responses limit disease have not been established.
Experiments with related and unrelated RNA viruses have suggested that antibody may have an important protective role against WNV infection. Passive administration of monoclonal antibodies (MAbs) limits the encephalitis caused by some flaviviruses (Saint Louis encephalitis [32, 38], Japanese encephalitis [25], and yellow fever [7, 40, 41] viruses) and nonflaviviruses (Sindbis [19-21, 44, 48], murine hepatitis [6, 33, 37], and lymphocytic choriomeningitis [43] viruses). However, for many of these viruses the mechanism by which antibody attenuates CNS infection has not been clearly demonstrated. The in vitro properties of MAbs, including isotype, avidity, and neutralization, do not necessarily correlate with protection (2, 47), as antibodies may limit viral infection through different mechanisms at several stages of pathogenesis. Antibody may decrease viral load in the CNS by limiting hematogenous spread through direct neutralization, complement activation, or increased viral uptake by phagocytic cells (52). Alternatively, antibodies may act directly in the CNS by preventing replication and spread in neurons (47).
To date, no systematic infection and pathogenesis studies with WNV have been performed with immunodeficient inbred mice. In this study, we established a mouse model of infection with C57BL/6 mice that paralleled human disease: infection via a subcutaneous route resulted in a subset of mice developing encephalitis. Infectious virus appeared in the lymph node and spleen within the first 4 days of infection and then spread concomitantly to the spinal cord and brain. Congenic mice that were genetically deficient in B cells and antibody (strain μMT) were vulnerable to lethal infection at very low doses of virus and developed higher viral loads in serum and the CNS. Because passive transfer of sera from infected and immune mice protected μMT mice against morbidity and mortality after infection, we conclude that antibodies and B cells have a critical early role in the defense against disseminated infection by WNV.
MATERIALS AND METHODS
Cells and viruses.
BHK-21, Vero, and C6/36 Aedes albopictus cells were cultured as previously described (12, 13). The WNV strain (3000.0259) was isolated in New York state in 2000 (14) and obtained from Laura Kramer (Albany, N.Y.). The initial isolate was harvested after inoculation of a mosquito homogenate into Vero cells (passage 0). All cell culture and in vivo studies used a stock (2 × 108 PFU/ml) of this virus that was propagated (passage 1) once in C6/36 cells. Viruses were diluted in Hanks' balanced salt solution and 1% heat-inactivated fetal bovine serum for injection into mice.
Mouse experiments.
All mice used in these experiments were derived from the inbred C57BL/6J strain (H-2b). The wild-type C57BL/6J and congenic μMT mice (strain B6-Igh6-6tm1Cgn) were purchased from Jackson Laboratories (Bar Harbor, Maine). The congenic RAG1 mice (strain B6-RAG1tm1Mom) were a gift from E. Unanue, Washington University School of Medicine). The mice used for all studies were between 8 and 12 weeks of age and were inoculated subcutaneously with WNV by footpad injection after anesthetization with xylazine and ketamine. Mouse experiments were approved by and performed according to the guidelines of the Washington University School of Medicine Animal Safety Committee. Differences in survival times and outcome were analyzed by Kaplan-Meier analysis and the log rank test.
Adoptive transfer and passive sera administration experiments.
Wild-type mice that survived primary WNV infection were maintained for 28 days. At this point, spleens were harvested and cells were released after dissection. Erythrocytes were removed after Ficoll gradient centrifugation, and the remaining cells were quantitated and phenotyped. Based on immunostaining with directly conjugated MAbs, the splenocyte population was found to consist of B cells (45%), CD4+ T cells (30%), and CD8+ T cells (20%). In some experiments, B cells were isolated to greater than 95% purity after negative selection with antibody-coated magnetic beads (Miltenyi Biotech Inc., Auburn, Calif.). Splenocytes (10 × 106) or purified B cells (4 × 106) were resuspended in endotoxin-free phosphate-buffered saline (PBS) and injected into the peritoneum of RAG1-deficient mice 1 day prior to infection with WNV.
Serum samples were isolated from naïve, infected (day 4 postinfection), or immune (day 28 postinfection) mice, heat-inactivated for 30 min at 56°C, and stored at −80°C. An aliquot was reserved for binding and neutralization titers (see below). For passive transfer experiments, mice were administered 0.5 ml of serum intraperitoneally 1 day prior to and after inoculation with 102 PFU of WNV.
Quantitation of viral burden in mice.
For analysis of virus in tissues of infected mice, organs were recovered after cardiac perfusion with PBS and dissection, cooled on ice, weighed, homogenized using a Bead-Beater apparatus, and titrated for virus by performance of a plaque assay on BHK-21 cells as described previously (13). Serum samples were obtained from whole blood by phlebotomy of the axillary vein immediately prior to sacrifice. Viral RNA was harvested from thawed aliquots (10 μl) of serum by using a Qia-Amp viral RNA recovery kit (Qiagen, Palo Alto, Calif.). For analysis of viral RNA in tissues of infected mice, after dissection, tissue samples were snap-frozen in liquid nitrogen, thawed in a guanidinium isothiocyanate denaturing solution, passed over a silica binding resin (RNEasy kit; Qiagen), and eluted in RNase-free water. Viral RNA was quantitated by real-time fluorogenic reverse transcriptase PCR (RT-PCR) using an ABI 7000 sequence detection system (Applied Biosystems, Foster City, Calif.) according to a previously published protocol (27). For each tissue sample, a parallel real-time RT-PCR was performed to quantify 18S rRNA (Applied Biosystems) to control for the amount of tissue in the original sample. Standard WNV and rRNA curves were run on each plate. Samples were run in duplicate, and the data obtained from several different mice for a particular experimental condition were averaged. The levels of WNV RNA in tissues were normalized for rRNA content so that the data were ultimately expressed as the genetic equivalents of WNV RNA per unit of 18S rRNA.
Quantitation of antibodies.
The titer of neutralizing antibodies was determined by a standard plaque reduction neutralization assay (23). Experiments were performed in duplicate, and plaques were scored visually. The results were plotted, and the plaque reduction neutralization titer for 50% inhibition (PRNT50) was determined. To determine the specific immunoglobulin G (IgG) and IgM titers, a WNV antigen enzyme-linked immunosorbent assay (ELISA) was used (45). Briefly, WNV-infected or uninfected (control) BHK21 cell lysates were adsorbed to Maxi-Sorp microtiter plates (Nalge Nunc International, Rochester, N.Y.). Nonspecific binding sites were blocked after incubation with blocking buffer (PBS, 0.05% Tween 20, 3% bovine serum albumin, 3% horse serum) for 1 h at 37°C. Plates were then incubated with serial dilutions of heat-inactivated serum from infected mice for 1 h at 4°C. After extensive washing, plates were incubated serially with biotin-conjugated goat anti-mouse IgM or IgG (Sigma Chemical) and horseradish peroxidase-conjugated streptavidin (Sigma Chemical) at 4°C and developed after addition of tetramethylbenzidine substrate (Sigma Chemical). Optical densities (ODs) at 450 nm were determined with an automatic ELISA plate reader (Molecular Devices). The OD value for the control antigen was subtracted from the viral antigen wells to obtain the adjusted OD value for each sample.
In some experiments, serum samples were depleted of IgM by chemical or immunologic means. Chemical depletion of IgM was performed by treating sera with 0.05 M β-mercaptoethanol in saline for 1 h at 37°C (35, 42). Immunologic depletion was performed as follows: serum samples were incubated twice with an equal volume of anti-IgM-specific agarose for 1 h at 4°C. After centrifugation, the supernatant (1/4 dilution of the original sample) was titrated for the PRNT. Isotype depletion was confirmed by ELISA.
Immunohistochemistry.
Immediately after euthanasia, organs were perfused with 4% paraformaldehyde in PBS. Freshly removed brains were immersed in 4% paraformaldehyde in PBS overnight. Six-micrometer-thick tissue sections were cut with a microtome after paraffin embedding and were dried overnight. Deparaffinization and antigen recovery treatments were performed (31). Slides were incubated with rat anti-WNV or control serum, biotinylated goat anti-rat IgG, streptavidin-conjugated horseradish peroxidase, and diaminobenzidine. Slides were then counterstained with hematoxylin and reviewed by microscopy.
RESULTS
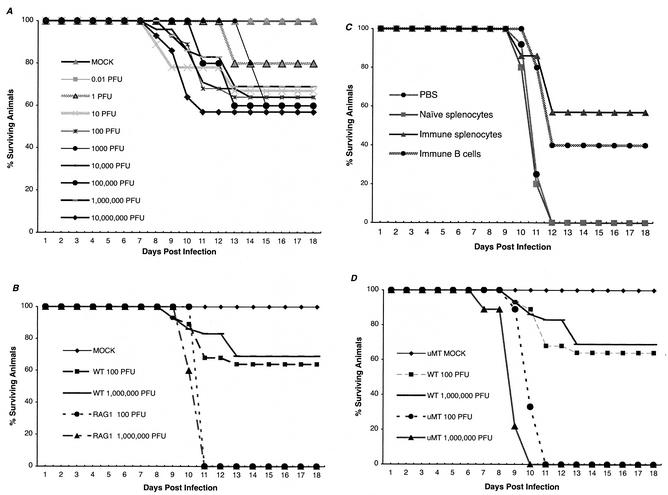
To define how the immune system protects against disseminated WNV infection, a mouse model of infection was developed in an inbred strain (C57BL/6J) that had an array of available genetic deficiencies in individual cells and mediators in the immune system. We first characterized infection in wild-type C57BL/6J mice with a low passage (P = 1) viral isolate that was obtained from the New York state epidemic in 2000 (14). The use of this strain was important because WNV strains isolated from North America are more virulent than those isolated from other regions of the world (4). A peripheral route of inoculation via the footpad was utilized to more closely mimic natural infection and to facilitate an analysis of viral replication and spread. Infection with the New York strain of WNV paralleled human disease. Seven to 10 days after subcutaneous inoculation, mice developed evidence of CNS infection, with a subset progressing to paralysis and death; immunohistochemistry documented WNV infection in CNS neurons (Fig. 1A). During the course of infection, approximately 60% of the wild-type mice developed significant levels of infectious virus in their brains. In contrast, a subset (approximately 20 to 40%) of wild-type mice either never disseminated WNV into the brain or had viral loads that were below the level of detection of our plaque assay. Animals that succumbed to infection showed similar clinical signs 24 to 48 h prior to death, including fur ruffling, weight loss, hunchback posture, and limb paralysis. In adult 8- to 12-week-old mice, a characteristic dose-response curve was not observed (Fig. 2A). Although lower doses (≤1 PFU) were associated with better outcome, there was no significant difference in mortality rates at higher doses (35 to 45% mortality at 102 to 106 PFU). Even at the highest inoculating dose (107 PFU), less than 50% of the wild-type animals succumbed to infection, although all animals developed clinical signs and viral tissue burden consistent with infection (data not shown). This dose-insensitive survival curve is similar to that observed after infection of mice with Murray Valley encephalitis virus (30), a closely related flavivirus.
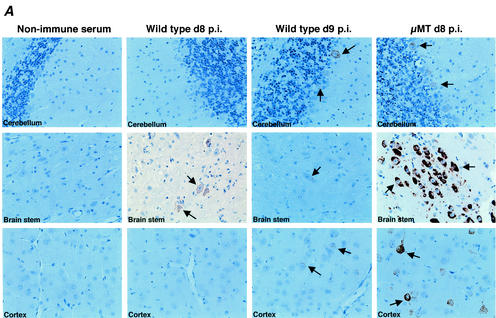
FIG. 1.
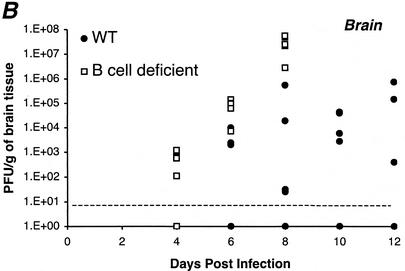
(A) WNV antigen expression in the brains of wild-type (nine left panels) and μMT (three right panels) mice. Brains were harvested 8 or 9 days after infection with WNV, sectioned, and stained with rat anti-WNV polyclonal serum or a control negative polyclonal rat serum. Typical sections from the cerebellum, brain stem, and cerebral cortex are shown and are representative of more than 10 independent brains from either wild-type or μMT mice. At day 8 after infection, approximately 40% of the wild-type mice and 100% of the μMT mice had brains that stained positive for viral antigen by immunohistochemistry. After development with substrate, viral antigen stained dark brown. Arrows indicate examples of heavily infected neurons. In the brain stem and cortex of μMT mice, pyknotic nuclei of heavily infected neurons can be seen. (B) Scatter plot of the levels of infectious WNV in the brains of wild-type and μMT mice. Brains of five wild-type (solid circles) or μMT (open squares) mice at each time point were harvested, homogenized, and subjected to viral plaque assay in BHK21 cells. The limit of sensitivity of the plaque assay is indicated by the dotted line. Viral levels at day 6 (P < 0.03) and day 8 (P < 0.01) were statistically different between wild-type and μMT mice as determined by two-tailed Student's t test. The following percentages of mice had viral burdens below the level of detection (<10 PFU/g): 80% of day 4 wild type, 40% of day 6 wild type, 20% of day 8 and day 10 wild type, 40% of day 12 wild type, 40% of day 4 μMT and 0% of day 6 and day 8 μMT.
FIG. 2.
Survival data for C57BL/6J mice inoculated with WNV. (A) Wild-type mice. Animals were inoculated via footpad with the indicated doses of WNV. The survival curves were constructed using data from three to six separate experiments. The numbers of animals receiving each viral dose ranged from n = 9 (10 PFU) to n = 29 (106 PFU). All animals were inoculated with virus stocks from the same passage (P = 1). (B) RAG1 mice. RAG1 or wild-type C57BL/6J mice were inoculated via the footpad with 102 or 106 PFU of WNV. The survival curves were constructed from data from at least three independent experiments. (C) RAG1 adoptive transfer studies. Splenocytes and purified B cells were harvested from naïve or immune congenic wild-type animals and injected into RAG1 mice 1 day prior to infection with 102 PFU of WNV. Survival data are indicated and reflect results from at least three independent experiments. (D) μMT mice. μMT or wild-type C57BL/6J mice were inoculated via the footpad with 102 or 106 PFU of WNV. The results shown reflect data from at least five independent experiments.
To assess the role of the immune system in limiting WNV infection, preliminary experiments were performed with congenic RAG1 mice that lacked both B and T cells. RAG1 mice were extremely vulnerable to infection. Even at the lowest dose (102 PFU) tested, 100% of the mice rapidly succumbed to infection (P < 0.0001) (Fig. 2B). Virologic analysis revealed extremely high titers of WNV (>108 PFU/g of tissue) in the brains of infected RAG1 mice (data not shown). Because these mice were so susceptible to infection, adoptive transfer studies were performed to define lymphocyte subsets that protected against disseminated WNV infection. Notably, immune splenocytes or B cells were sufficient to confer protection (immune splenocytes, P = 0.006; immune B cells, P = 0.01 [Fig. 2C]) to some RAG1 mice. To confirm the importance of B cells in mediating protection, infection experiments were performed with congenic B-cell-deficient mice. Mice that lack mature B cells (strain μMT) exhibited a similar susceptibility to infection. At either the high (106 PFU) or low (102 PFU) dose tested, all animals succumbed to infection (P < 0.0001) (Fig. 2D). The vulnerability of μMT mice to infection was reflected by the fact that 50% of the animals died after a dose of 1 PFU (50% lethal dose) via footpad inoculation (data not shown).
To understand the mechanism by which a deficiency in B cells made mice vulnerable to lethal infection by WNV, infectious virus was measured in tissues by using plaque assays. Because the analysis was limited by the small amount of sample for some tissues (e.g., lymph nodes), the level of WNV RNA in tissues was assessed independently by real-time fluorogenic RT-PCR using primers that corresponded to a conserved nucleotide sequence in the WNV envelope gene (27). The sensitivity of the RT-PCR assay was greater than that of the plaque assay, as WNV RNA that corresponded to 1 to 5 PFU (or ∼100 to 500 copies of viral RNA) per gram was reliably detected (data not shown).
Distribution of WNV in tissues of wild-type and B-cell-deficient mice.
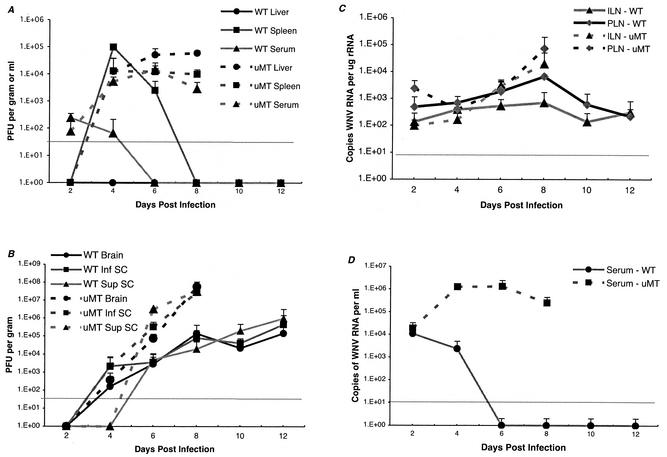
The levels of infectious virus and/or viral RNA were determined from sera, peripheral organs (spleen and liver), and the CNS (brain cortex and inferior and superior spinal cord) of wild-type (days 2, 4, 6, 8, 10, and 12) and μMT (day 2, 4, 6, and 8) mice after infection with 106 PFU per mouse (Fig. 3). Because μMT mice rapidly succumbed to infection, data from the last two time points (days 10 and 12) were not collected. Several observations were noted.
FIG. 3.
WNV burden in peripheral nervous system and CNS tissues in wild-type and μMT mice. (A) Levels of infectious virus in peripheral tissues. Virus levels from serum, spleen, and liver of wild-type and μMT mice were measured using a viral plaque assay in BHK21 cells after tissues were harvested at the indicated days after inoculation. Data shown are the average PFU per gram of tissue or milliliter of serum for five wild-type or μMT mice per time point. The dotted line represents the limit of sensitivity of the assay. (B) Infectious virus levels in the CNS. Virus levels from brain and the inferior and superior spinal cord were determined as described above. (C) Levels of viral RNA in draining lymph nodes. WNV RNA was harvested from draining popliteal or inguinal lymph nodes at the indicated days after inoculation and quantitated using a real-time fluorogenic RT-PCR assay. Data are expressed as genomic equivalents of WNV RNA per microgram of rRNA after normalization for tissue content and represent results for tissues harvested from five wild-type or μMT mice per time point. The dotted line represents the limit of sensitivity of the assay. (D) Levels of viral RNA in serum. Viral RNA levels were determined from serum of wild-type or μMT mice after WNV infection at the indicated days using a real-time fluorogenic RT-PCR assay. Data are expressed as genomic equivalents of WNV RNA per milliliter of serum and represent the averages for five independent mice per time point.
(i) Viremia.
In wild-type mice, viremia was detected at day 2 after subcutaneous infection but rapidly decreased to a level below detection by day 6. In μMT mice, a comparable level of infectious virus (∼102 PFU/ml) was measured in sera at day 2 but this was followed by a sustained increase through day 8 until levels exceeded 104 PFU/ml (Fig. 3A). Parallel results were observed when viral RNA levels were measured, with ∼500-fold higher levels of WNV RNA being detected in μMT mice within 4 days of infection (Fig. 3D). Thus, B cells and antibody appear to be essential for containing the levels of WNV in serum.
(ii) Lymph nodes.
The levels of WNV RNA in the draining popliteal and inguinal lymph nodes were similar between wild-type and μMT mice early in the course of infection. Viral RNA was detected at day 2 after infection and remained elevated throughout the course of infection. However, by day 8 after infection, ∼10-fold higher levels of WNV RNA were measured in the lymph nodes from μMT mice (Fig. 3C).
(iii) Spleen.
In wild-type mice, the levels of infectious WNV in the spleen peaked at day 4 after infection, decreased by day 6, and were absent at day 8. Although comparable levels were observed in μMT mice at days 4 and 6, there was no clearance phase, as levels of virus (104 PFU/g) persisted in the spleen after day 6 (Fig. 3A). A similar trend was observed when viral RNA levels were measured (data not shown).
(iv) CNS.
Similar levels of infectious virus were detected in the brain and spinal cord of wild-type and μMT mice at 4 days after infection. Since infectious virus appeared concurrently at more than one site in the CNS, WNV appears to spread by a hematogenous route. By day 6, markedly increased viral loads were detected in the brain and inferior and superior spinal cord of μMT mice; by day 8, B-cell- and antibody-deficient mice had 100- to 500-fold higher levels of infectious virus and viral RNA in multiple regions of the CNS (Fig. 3B and data not shown). Immunohistochemistry corroborated these results (Fig. 1). Prominent staining for viral antigen was observed in the brain stem, cerebral cortex, hippocampus, and cerebellum of μMT mice. Based on histological appearance, high-grade infection was found to occur primarily in neurons and, in many fields, coincided with evidence of neuronal injury or cell death. The difference in CNS viral loads between wild-type and μMT mice was not explained by the ultimate bias of the survival curves, because no individual wild-type mouse had viral titers in the brain that approached those in the μMT mice (e.g., the maximum titer in the brain of a wild-type mouse was 5.5 × 105 PFU/ml) at any day after infection.
(v) Liver.
The absence of B cells and antibody affected the tropism of WNV infection. In wild-type mice, no infectious virus and very low levels of RNA (<2 × 102 copies of WNV RNA per μg of 18S rRNA) were measured from livers throughout the course of infection. In contrast, after day 4, infectious virus (>104 PFU/g) and viral RNA (>2 × 104 copies of WNV RNA per μg of 18S rRNA) were detected in the livers of all μMT mice. At later time points, the levels of infectious WNV in livers of μMT mice exceeded those found in the serum (Fig. 3A).
Role of B cells and immunoglobulin.
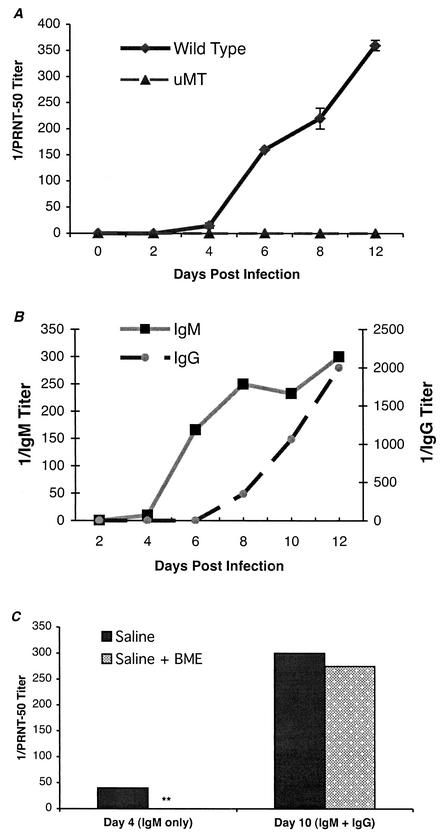
Although the virologic, histologic, and clinical analyses showed that μMT mice had increased CNS viral burdens and mortality rates relative to wild-type mice, the mechanism for this remained unclear. We speculated that specific antibody against WNV directly prevented dissemination of WNV in the CNS. To evaluate this hypothesis, we assessed the kinetics of neutralizing-antibody formation by a viral plaque reduction assay (Fig. 4A). As expected, no neutralizing antibodies were detected in μMT mice at any point during the course of infection. In contrast, low levels (inhibitory titer of 1/10 to 1/20) of neutralizing antibodies were detected in wild-type mice at day 4 after infection. After day 4, inhibitory titers increased. Finally, at the level of sensitivity of our plaque reduction assay, neutralizing antibodies were not detected in serum from naïve animals or from wild-type animals within 2 days of the initial infection.
FIG. 4.
Development of specific antibodies against WNV. Serum samples were collected from wild-type or μMT mice at the indicated days after infection. (A) Neutralizing antibody titers were determined by a PRNT assay. Samples were performed in duplicate, and results represent the average of three independent experiments with at least three mice per group. Data are expressed as the reciprocal PRNT50, the antibody titers that reduced the number of plaques by 50%. (B) Isotype-specific ELISA. The development of the isotype (IgM or IgG) of specific antibodies was determined after incubation of serum with adsorbed control or viral antigen. Data are the averages of three separate experiments performed in duplicate. (C) IgM depletion studies. IgM was depleted from day 4 or day 10 serum samples after treatment with β-mercaptoethanol (see Materials and Methods). Serum was analyzed for remaining neutralizing antibodies by using the PRNT assay as described above. Data represent results from one experiment that is representative of three.
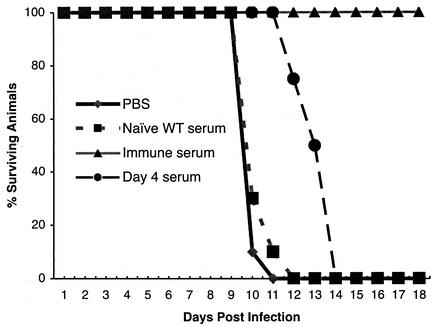
To directly address the protective nature of antibody, independent of B cells, μMT mice were passively administered sera collected from wild-type mice that were naïve or immune to WNV or that were exposed to WNV for 4 days, the earliest time when neutralizing antibodies were detected. μMT mice were inoculated with 0.5 ml of heat-inactivated serum 1 day prior to and after infection with 102 PFU of WNV (Fig. 5). Although similar quantities of naïve serum protected μMT mice against infection with other viruses (34), it had no significant effect on mortality or average survival time (P > 0.7). When μMT mice were given immune serum obtained from wild-type mice, they were completely protected against infection with 102 PFU of WNV (P < 0.0001). Treatment of μMT mice with serum from wild-type mice that were at 4 days postinfection led to an intermediate phenotype; although there was a significant increase in average survival time (14 versus 10 days; P < 0.0001), all animals ultimately succumbed to infection. To determine the role of IgM or IgG in mediating protection, an isotype-specific ELISA against solid-phase WNV antigen was performed. Specific IgM was detected as early as day 4 after infection, whereas specific anti-WNV IgG was not detected until 8 days after infection (Fig. 4B). Chemical and immunologic depletion of IgM confirmed this result. Treatment with 0.05 M β-mercaptoethanol (which destroys IgM but not IgG [42]) or preclearing with anti-IgM agarose completely abolished the neutralizing activity of serum obtained at day 4 after infection but not at day 10 or 28 (Fig. 4C and data not shown). Thus, day 10 and immune sera contained primarily IgG-specific antibodies against WNV but day 4 serum contained exclusively IgM-specific antibodies against WNV.
FIG. 5.
Passive administration of serum to μMT mice. Serum samples were collected from mice that were naïve or immune or at 4 days postinfection with WNV. After heat inactivation, 0.5 ml of serum was administered to μMT mice 1 day prior to and after infection with 102 PFU of WNV. Data represent results from at least three independent experiments with five mice per condition.
DISCUSSION
Although prior WNV infection models in rodents documented the neurotropic character of the virus (15, 16, 49, 50), the mechanism of viral spread and the role of the adaptive immune system in limiting dissemination were not determined. In this paper, by comparing infection in wild-type and congenic B-cell- and antibody-deficient mice, we established the kinetics of the spread of WNV infection from lymphoid tissue to serum to the CNS and determined how the development of antibody impedes this process. In wild-type C57BL/6 mice, WNV infection began peripherally and, in a subset of animals, disseminated to the CNS by a hematogenous route. Congenic mice that lacked B cells and antibody were more viremic, and this corresponded with high-grade dissemination in the CNS and adverse outcome.
Infection model in wild-type C57BL/6 mice.
Wild-type mice replicated virus locally in draining lymph nodes and developed a peak level of viremia within 2 days of infection. By day 4, viral infection was detected in the spleen and multiple sites in the CNS, a pattern most consistent with hematogenous spread. Overall, high-grade viral dissemination to the brain was limited to a subset of wild-type animals: these mice were morbidly ill whereas wild-type mice that had lower viral burdens in the CNS exhibited milder clinical symptoms. Since all wild-type mice showed evidence of peripheral infection, the extent of CNS dissemination may be determined by the kinetics of production of anti-WNV antibody during the early phase of infection. Interestingly, clearance of viral infection in the serum and spleen later in the infection course did not correlate with CNS dissemination or outcome; wild-type moribund mice with evidence of disseminated CNS infection nonetheless retained the ability to clear infectious virus from these peripheral compartments.
WNV replication was observed at several extraneural sites, including lymph nodes, spleen, and kidney (data not shown). Our data are consistent with those of prior studies (26, 50) that documented WNV replication at several extraneural sites, including spleen, kidney, and muscle. At present, the extraneural cellular target of WNV replication remains uncertain; histopathologic and in situ hybridization studies to define permissive cell types in the spleen are under way. Based on cell culture infection studies, cells of myeloid origin (9, 10, 17, 18), including tissue macrophages and dendritic cells, may be targets for WNV infection.
There was little difference in overall survival or average survival times over a broad range of inoculating doses. This striking lack of dose-dependent mortality was not seen in previous studies with WNV in which C3H/HeN or outbred mice were inoculated via the intraperitoneal route (15, 16, 49). It was, however, observed after infection with the related Murray Valley encephalitis flavivirus (30).
Infection in immunodeficient mice.
RAG1 and μMT mice were more vulnerable to lethal WNV infection. Animals developed a rapid-onset paralysis, and high levels of virus and viral RNA were detected peripherally and in the CNS. Similarly, SCID mice developed high-grade viremia and CNS infection and succumbed to infection after inoculation with a candidate WNV vaccine strain (22). B cells and antibody have been proposed to protect against encephalitis caused by other flaviviruses (8, 25, 32, 38) and non-flaviviruses (6, 19, 20, 33, 37, 43, 44, 48). In some of these infection models (e.g., Sindbis, murine hepatitis, and lymphocytic choriomeningitis viruses), antibody and B cells contribute to the eradication of infection by clearing virus from the brain (19, 29) or preventing viral recrudescence (6, 43). Our data suggest that antibody and B cells directly limit the dissemination of WNV in the CNS early during the course of infection. μMT mice had an ∼500-fold increase in serum viral load at day 4 after infection; this led to a markedly increased viral burden in neurons in the CNS at day 6 and provoked a rapidly fatal encephalitis.
Antibody protection.
Specific antibodies against WNV were initially detected 4 days after infection in wild-type animals, which was the same time when high-grade viremia was first detected in μMT mice. An isotype-specific ELISA confirmed that these were exclusively IgM. Nonetheless, induced IgM against WNV, derived from wild-type mice 4 days after infection, prolonged but did not guarantee survival of μMT mice. Although these same IgM antibodies were demonstrated to have neutralizing capacity, they could not eradicate infection in μMT mice. Additional studies must be performed to determine whether higher doses of anti-WNV IgM can confer complete protection in μMT mice and whether equivalent amounts of anti-WNV IgM from day 4 serum can protect wild-type mice. Higher doses of anti-WNV IgM may never eliminate WNV infection in μMT mice because IgM cannot trigger the mature IgG response that is necessary for eradication. Indeed, only immune serum that contained both anti-WNV IgM and IgG prevented morbidity and mortality in μMT mice. Specific IgM may have a dual role early during viral infection: to limit dissemination by temporarily containing viremia and to trigger an adaptive IgG response that eliminates viral infection (35). Recent experiments with complement-deficient mice (M. Diamond, E. Mehlhop, and M. Engle, unpublished observations) suggest that anti-WNV IgM may induce a mature humoral response by activating complement and facilitating T-cell-dependent and -independent antibody production (36).
In contrast to induced antibody, “natural” antibody (primarily IgM generated from CD5+ B-1 cells [3, 11, 34]) obtained from naïve mice did not attenuate WNV infection. Our results contrast with data obtained with vesicular stomatitis virus (34), where passive administration of natural antibody to μMT mice improved survival after infection. Several variables may influence the efficacy of natural antibodies in preventing infection, including the viral inoculum, the kinetics of viral replication, the site of virus inoculation and antibody administration, and the absolute amount of natural antibody transferred. More-detailed experiments are required before a definitive conclusion can be reached regarding the protective role of natural antibodies against WNV.
Overall, our data suggest the importance of the early antibody response in containing viremia and limiting disseminated infection of WNV in the CNS. These results are consistent with those of earlier studies that showed that the absence of B and T cells (SCID mice) but not that of T cells alone (nude mice) in the BALB/c background increased mortality associated with WNV infection (22). Nonetheless, it is likely that other aspects of the innate (e.g., interferon and NK cells) and adaptive (T cells) immune systems also have critical roles in controlling WNV infection. Indeed, preliminary experiments in our laboratory demonstrated that genetic deficiencies of gamma interferon or CD4+ or CD8+ T cells cause increased mortality in C57BL/6 mice (M. Engle, B. Shrestha, and M. Diamond, unpublished observations).
It is intriguing that severe human WNV infection, which is heavily biased toward an elderly population, occurs because of a dysfunctional antibody response against WNV early during infection; many elderly people have decreased antibody production and shortened durations of protective immunity following immunization (39, 51). Pharmacological intervention with antibodies, such as immune immunoglobulin or humanized MAbs, may provide a strategy for mitigating CNS disease in patients with West Nile encephalitis (38; Z. Shimoni, M. J. Niven, S. Pitlick, and S. Bulvik, Letter, Emerg. Infect. Dis. 7:759, 2001).
Acknowledgments
We thank L. Kramer and K. Bernard for WNV isolates and cell lines and R. Beatty, E. Harris, S. Shresta, T. Chambers, and S. Virgin for experimental advice and/or reagents. We thank D. Leib, S. Schlesinger, and S. Virgin for critical reading of the manuscript.
The work was supported by grants from the Centers for Disease Control and Prevention (U50/CCU720545-02), the Pharmacia Biomedical Program, the Edward Mallinckrodt, Jr., Foundation, and the NIH (T32 GM07067).
REFERENCES
- 1.Asnis, D. S., R. Conetta, A. A. Teixeira, G. Waldman, and B. A. Sampson. 2000. The West Nile virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin. Infect. Dis. 30:413-418. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., U. Kalinke, A. Althage, G. Freer, C. Burkhart, H. Roost, M. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024-2027. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarth, N., O. C. Herman, G. C. Jager, L. E. Brown, L. A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beasley, D. W., L. Li, M. T. Suderman, and A. D. Barrett. 2002. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology 296:17-23. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Nathan, D., I. Huitinga, S. Lustig, N. van Rooijen, and D. Kobiler. 1996. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 141:459-469. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 167:1575-1583. [DOI] [PubMed] [Google Scholar]
- 7.Brandriss, M. W., J. J. Schlesinger, E. E. Walsh, and M. Briselli. 1986. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J. Gen. Virol. 67:229-234. [DOI] [PubMed] [Google Scholar]
- 8.Broom, A. K., M. J. Wallace, J. S. Mackenzie, D. W. Smith, and R. A. Hall. 2000. Immunisation with gamma globulin to Murray Valley encephalitis virus and with an inactivated Japanese encephalitis virus vaccine as prophylaxis against Australian encephalitis: evaluation in a mouse model. J. Med. Virol. 61:259-265. [DOI] [PubMed] [Google Scholar]
- 9.Cardosa, M. J., S. Gordon, S. Hirsch, T. A. Springer, and J. S. Porterfield. 1986. Interaction of West Nile virus with primary murine macrophages: role of cell activation and receptors for antibody and complement. J. Virol. 57:952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardosa, M. J., J. S. Porterfield, and S. Gordon. 1983. Complement receptor mediates enhanced flavivirus replication in macrophages. J. Exp. Med. 158:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casali, P., and A. L. Notkins. 1989. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol. Today 10:364-368. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, M., T. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebel, G. D., A. P. Dupuis III, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldadah, A. H., and N. Nathanson. 1967. Pathogenesis of West Nile virus encephalitis in mice and rats. II. Virus multiplication, evolution of immunofluorescence, and development of histological lesions in the brain. Am. J. Epidemiol. 86:776-790. [DOI] [PubMed] [Google Scholar]
- 16.Eldadah, A. H., N. Nathanson, and R. Sarsitis. 1967. Pathogenesis of West Nile virus encephalitis in mice and rats. I. Influence of age and species on mortality and infection. Am. J. Epidemiol. 86:765-775. [DOI] [PubMed] [Google Scholar]
- 17.Gollins, S., and J. Porterfield. 1984. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody and viral fate. J. Gen. Virol. 65:1261-1272. [DOI] [PubMed] [Google Scholar]
- 18.Gollins, S., and J. Porterfield. 1986. The uncoating and infectivity of the flavivirus West Nile on interaction with cells: effects of pH and ammonium chloride. J. Gen. Virol. 67:1941-1950. [DOI] [PubMed] [Google Scholar]
- 19.Griffin, D., B. Levine, W. Tyor, S. Ubol, and P. Despres. 1997. The role of antibody in recovery from alphavirus encephalitis. Immunol. Rev. 159:155-161. [DOI] [PubMed] [Google Scholar]
- 20.Griffin, D. E., B. Levine, W. R. Tyor, and D. N. Irani. 1992. The immune response in viral encephalitis. Semin. Immunol. 4:111-119. [PubMed] [Google Scholar]
- 21.Griffin, D. E., S. Ubol, P. Despres, T. Kimura, and A. Byrnes. 2001. Role of antibodies in controlling alphavirus infection of neurons. Curr. Top. Microbiol. Immunol. 260:191-200. [DOI] [PubMed] [Google Scholar]
- 22.Halevy, M., Y. Akov, D. Ben-Nathan, D. Kobiler, B. Lachmi, and S. Lustig. 1994. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch. Virol. 137:355-370. [DOI] [PubMed] [Google Scholar]
- 23.Halstead, S. B., C. N. Venkateshan, M. K. Gentry, and L. K. Larsen. 1984. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 132:1529-1532. [PubMed] [Google Scholar]
- 24.Hubalek, Z., and J. Halouzka. 1999. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 5:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura-Kuroda, J., and K. Yasui. 1988. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J. Immunol. 141:3606-3610. [PubMed] [Google Scholar]
- 26.Kramer, L. D., and K. A. Bernard. 2001. West Nile virus infection in birds and mammals. Ann. N. Y. Acad. Sci. 951:84-93. [DOI] [PubMed] [Google Scholar]
- 27.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 29.Levine, B., J. M. Hardwick, B. D. Trapp, T. O. Crawford, R. C. Bollinger, and D. E. Griffin. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254:856-860. [DOI] [PubMed] [Google Scholar]
- 30.Licon Luna, R. M., E. Lee, A. Mullbacher, R. V. Blanden, R. Langman, and M. Lobigs. 2002. Lack of both Fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J. Virol. 76:3202-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litwin, J. A., S. Yokota, T. Hashimoto, and H. D. Fahimi. 1984. Light microscopic immunocytochemical demonstration of peroxisomal enzymes in Epon sections. Histochemistry 81:15-22. [DOI] [PubMed] [Google Scholar]
- 32.Mathews, J. H., and J. T. Roehrig. 1984. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J. Immunol. 132:1533-1537. [PubMed] [Google Scholar]
- 33.Matthews, A. E., S. R. Weiss, M. J. Shlomchik, L. G. Hannum, J. L. Gombold, and Y. Paterson. 2001. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J. Immunol. 167:5254-5263. [DOI] [PubMed] [Google Scholar]
- 34.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 35.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190:1165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochsenbein, A. F., and R. M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624-630. [DOI] [PubMed] [Google Scholar]
- 37.Ramakrishna, C., S. A. Stohlman, R. D. Atkinson, M. J. Shlomchik, and C. C. Bergmann. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 168:1204-1211. [DOI] [PubMed] [Google Scholar]
- 38.Roehrig, J. T., L. A. Staudinger, A. R. Hunt, J. H. Mathews, and C. D. Blair. 2001. Antibody prophylaxis and therapy for flaviviral encephalitis infections. Ann. N. Y. Acad. Sci. 951:286-297. [DOI] [PubMed] [Google Scholar]
- 39.Saurwein-Teissl, M., T. L. Lung, F. Marx, C. Gschosser, E. Asch, I. Blasko, W. Parson, G. Bock, D. Schonitzer, E. Trannoy, and B. Grubeck-Loebenstein. 2002. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168:5893-5899. [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger, J. J., M. W. Brandriss, and E. E. Walsh. 1985. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J. Immunol. 135:2805-2809. [PubMed] [Google Scholar]
- 41.Schlesinger, J. J., M. Foltzer, and S. Chapman. 1993. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology 192:132-141. [DOI] [PubMed] [Google Scholar]
- 42.Scott, D. W., and R. K. Gershon. 1970. Determination of total and merecaptoethanol-resistant antibody in the same serum sample. Clin. Exp. Immunol. 6:313-316. [PMC free article] [PubMed] [Google Scholar]
- 43.Seiler, P., U. Kalinke, T. Rulicke, E. M. Bucher, C. Bose, R. M. Zinkernagel, and H. Hengartner. 1998. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin- transgenic mice. J. Virol. 72:2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley, J., S. J. Cooper, and D. E. Griffin. 1986. Monoclonal antibody cure and prophylaxis of lethal Sindbis virus encephalitis in mice. J. Virol. 58:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tardei, G., S. Ruta, V. Chitu, C. Rossi, T. F. Tsai, and C. Cernescu. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J. Clin. Microbiol. 38:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai, T. F., F. Popovici, C. Cernescu, G. L. Campbell, and N. I. Nedelcu. 1998. West Nile encephalitis epidemic in southeastern Romania. Lancet 352:767-771. [DOI] [PubMed] [Google Scholar]
- 47.Tyler, K. L., M. A. Mann, B. N. Fields, and H. W. Virgin IV. 1993. Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J. Virol. 67:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyor, W. R., S. Wesselingh, B. Levine, and D. E. Griffin. 1992. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 149:4016-4020. [PubMed] [Google Scholar]
- 49.Wang, T., J. F. Anderson, L. A. Magnarelli, S. J. Wong, R. A. Koski, and E. Fikrig. 2001. Immunization of mice against West Nile virus with recombinant envelope protein. J. Immunol. 167:5273-5277. [DOI] [PubMed] [Google Scholar]
- 50.Weiner, L. P., G. A. Cole, and N. Nathanson. 1970. Experimental encephalitis following peripheral inoculation of West Nile virus in mice of different ages. J. Hyg. (London) 68:435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weksler, M. E., and P. Szabo. 2000. The effect of age on the B-cell repertoire. J. Clin. Immunol. 20:240-249. [DOI] [PubMed] [Google Scholar]
- 52.Zinkernagel, R. M., A. LaMarre, A. Ciurea, L. Hunziker, A. F. Ochsenbein, K. D. McCoy, T. Fehr, M. F. Bachmann, U. Kalinke, and H. Hengartner. 2001. Neutralizing antiviral antibody responses. Adv. Immunol. 79:1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]