Abstract
The herpes simplex virus (HSV) glycoprotein heterodimer gE/gI plays an important role in virus cell-to-cell spread in epithelial and neuronal tissues. In an analogous fashion, gE/gI promotes virus spread between certain cell types in culture, e.g., keratinocytes and epithelial cells, cells that are polarized or that form extensive cell junctions. One mechanism by which gE/gI facilitates cell-to-cell spread involves selective sorting of nascent virions to cell junctions, a process that requires the cytoplasmic domain of gE. However, the large extracellular domains of gE/gI also appear to be involved in cell-to-cell spread. Here, we show that coexpression of a truncated form of gE and gI in a human keratinocyte line, HaCaT cells, decreased the spread of HSV between cells. This truncated gE/gI was found extensively at cell junctions. Expression of wild-type gE/gI that accumulates at intracellular sites, in the trans-Golgi network, did not reduce cell-to-cell spread. There was no obvious reduction in production of infectious HSV in cells expressing gE/gI, and virus particles accumulated at cell junctions, not at intracellular sites. Expression of HSV gD, which is known to bind virus receptors, also blocked cell-to-cell spread. Therefore, like gD, gE/gI appears to be able to interact with cellular components of cell junctions, gE/gI receptors which can promote HSV cell-to-cell spread.
The alphaherpesviruses herpes simplex virus (HSV) types 1 (HSV-1) and 2, varicella-zoster virus (VZV), and pseudorabies virus (PRV) replicate primarily in epithelial tissues before spreading into neurons where they establish latency. These viruses are extraordinarily adept at spreading rapidly from cell to cell in both these tissues. Given that alphaherpesviruses establish lifelong infections, cell-to-cell spread appears to be especially important in order for these viruses to outrun the immune system, especially after reactivation in hosts that have fully primed immunity. Consistent with this, HSV, PRV, and VZV all remain largely cell associated; large numbers of progeny virions accumulate on cell surfaces, especially at cell junctions in polarized epithelial cells (23). Moreover, virus mutants with specific defects in cell-to-cell spread that do not affect entry at apical surfaces are extremely attenuated in vivo (reviewed in references 15 and 21). It has been proposed elsewhere that alphaherpesviruses have evolved specialized mechanisms to allow specific sorting of virions to epithelial and neuronal cell junctions and the efficient transfer across junctions to promote infection of adjacent cells (14, 21).
HSV, PRV, and VZV all express a glycoprotein heterodimer, gE/gI, that promotes cell-to-cell spread (19, 20, 25, 49, 50). In the case of HSV and PRV, gE/gI was originally thought to be nonessential based on observations that gE− or gI− mutants enter cultured laboratory cells and replicate normally in the cells. However, PRV and HSV gE− or gI− mutants are severely compromised in epithelial and neuronal tissues (1, 6, 7, 12, 13, 33, 37, 43, 44, 46). Moreover, gE/gI can be important for HSV cell-to-cell spread in certain cultured epithelial cells, especially human endometrial HEC-1A cells and keratinocytes, cells that form extensive cell junctions (14, 48), as well as in cultured neurons (13). One mechanism by which gE/gI apparently facilitates cell-to-cell spread involves the sorting of newly assembled HSV virions to lateral cell surfaces and cell junctions (23). Mutant HSV lacking gE, or just the cytoplasmic (CT) domain of gE, accumulated more extensively in the cytoplasm, at apical cell surfaces, and in cell culture supernatants than did wild-type HSV particles, which were found predominantly at cell junctions. The CT domain of gE is essential for gE/gI to mediate cell-to-cell spread (44, 48) and plays an important role, along with other viral glycoproteins, in the envelopment of HSV and PRV particles in the cytoplasm. PRV mutants lacking gE or the gE CT domain and also PRV gM (2) and an HSV lacking gD and gE (A. Farnsworth, K. Goldsmith, and D. C. Johnson, unpublished results) do not form cytoplasmic enveloped particles. Together these results suggest that gE/gI acts to promote envelopment into cytoplasmic vesicles that are sorted to cell epithelial junctions, a process which would obviously facilitate cell-to-cell spread (21).
There is also evidence that the large extracellular (ET) domains of gE/gI can participate in cell-to-cell spread. We observed accumulation of gE/gI at cell junctions at intermediate to late stages of HSV replication in epithelial cells, while other HSV glycoproteins were found more extensively on apical surfaces (14, 31). This appeared to involve anchoring of gE/gI to components of cell junctions that occurred after gE/gI was sorted to lateral cell surfaces. Recently, there were constructed mutant gE molecules with small insertions in the ET domain that fold relatively normally, bind gI, and traffic to the cell surface but do not promote cell-to-cell spread (K. Goldsmith, K. Polcicova, and D. C. Johnson, unpublished data). We hypothesized that gE/gI, and specifically the ET domain of gE/gI, promotes cell-to-cell spread by a receptor mechanism, binding components of cell junctions to promote entry into adjacent cells (14, 21, 23, 48). The genes encoding gE and gI are located adjacent to that of gD and may have evolved by duplication of a common ancestor (29). gD is now a well-characterized receptor-binding glycoprotein (reviewed in 40). Three types of early experiments initially demonstrated that HSV gD binds cellular receptors important for virus entry: (i) comparisons of HSV particles with or without gD demonstrated differential blocking of virus receptors (22), (ii) soluble forms of gD blocked entry and bound to saturable cell surface sites (18), and (iii) cells stably transfected with gD were resistant to HSV infection (4, 24, 36). In the interference experiments, it is believed that gD expressed in trans apparently bound gD receptors either in cytoplasmic membranes or on the cell surface and sequestered the cellular molecules, rendering them unavailable to incoming virus.
In order to examine gE/gI as a potential receptor-binding glycoprotein, we have coexpressed a truncated form of gE lacking the CT domain (gEΔCT) and gI in human keratinocytes by using replication-defective (E1−) adenovirus (Ad) vectors. HSV cell-to-cell spread was markedly reduced in keratinocytes coexpressing gEΔCT and gI. There was no inhibition of HSV replication, and HSV particles accumulated at cell junctions. These results are consistent with the hypothesis that gE/gI acts to bind cellular receptors concentrated at cell junctions.
MATERIALS AND METHODS
Cells and herpesviruses.
All cell culture media were purchased from BioWhittaker Inc., Walkersville, Md. HaCaT cells (48) were grown in Dulbecco's minimum essential medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (HyClone). 293 cells (Microbix, Toronto, Ontario, Canada) and 293-cre4 cells (34) were grown in DMEM with 10% fetal bovine serum and passaged as described by Microbix. Wild-type HSV strain F; a gE-null mutant derived from F, F-gEβ; and a gI-null mutant, F-gIGFP, in which green fluorescent protein sequences replace gI coding sequences, were propagated, and their titers were determined, on Vero cells.
Ads.
AdgD, also designated elsewhere as AdgD(E1−), was described previously (3). Ad vectors expressing wild-type gE and gI from the human cytomegalovirus (HCMV) immediate-early promoter have also been described previously (14). Two additional Ad vectors expressing gE and gI under the control of a tetracycline-transactivated promoter were constructed: AdtetgEΔCT (also designated AdgEΔCT here) and AdtetgI. To construct AdtetgEΔCT, gE structural sequences were PCR amplified with Vent polymerase (Promega) from pUCUS7/8, a plasmid containing a 3.1-kb fragment of HSV-1 F encompassing all of the gE coding sequences and the 3′ end of the gI gene described previously (48). The primers used were 12930 gE-tail-F (5′GACGCAAGCTTATCCCCGGGACATGGATCGC; underlined area is HindIII site) and 12929 gE-tail-R (5′TATCAAATCGCCGGCGCCCTTACCCGAGGCCCTGC; underlined area is NotI site). The PCR product contained all of the 5′ coding sequences of gE but was truncated after residue 462, so that 16 residues of the gE CT domain remained. The PCR was purified, subjected to digestion with HindIII and NotI, and ligated into HindIII/NotI-digested pAdtet7. The entire gE coding region was sequenced. Since no stop codon was inserted at the 3′ end of the gE coding sequences, 13 additional residues were added to the gE CT domain: AGPPRWSSSFCSL. The truncated gE gene was inserted into a shuttle vector, pAdtet7, which contains loxP sites; a basal HCMV promoter that is regulated by a tetracycline transactivator protein; and flanking Ad sequences (9, 45). AdtetgI was constructed by removing gI coding sequences from plasmid pCA3gI (14) by using EcoRI and BamHI and inserting the sequences into pAdtet7, which was digested with EcoRI and BamHI and then sequenced. The gE and gI sequences present in plasmids pAdtetgEΔCT and pAdtetgI were used to construct recombinant E1− Ad vectors as described previously (9, 45). Briefly, 293-cre4 cells (34) were transfected with Ad type 5 (Ad5) psi5 DNA and plasmid pAdtetgEΔCT or pAdtetgI. After 3 to 5 days, infectious virus was harvested and passaged three sequential times on Cre4 cells. Larger virus stocks were prepared, and their titers were determined, on 293 cells.
Radiolabeling of cells and immunoprecipitation.
HaCaT cells were infected with wild-type HSV-1 F, F-gEβ, or F-gIGFP with 10 to 25 PFU/cell; with Ad vectors AdgE(E1−) and AdgI(E1−) with 150 PFU of each virus per cell; or with AdtetgEΔCT (150 PFU/cell), AdtetgI (150 PFU/cell), and AdTet-trans (80 PFU/cell). After 5 h of infection with HSV-1 F or 22 h of infection with Ad vectors, the cells were washed three times with DMEM lacking cysteine and methionine and labeled for 3 h in this medium supplemented with [35S]methionine-cysteine (75 mCi/ml; New England Nuclear, Boston, Mass.). Cell extracts were prepared with 50 mM Tris HCl (pH 7.5)-100 mM NaCl-1% NP-40-0.5% sodium deoxycholate containing 2 mg of bovine serum albumin per ml and 1 mM phenylmethylsulfonyl fluoride. gE/gI complexes were immunoprecipitated with anti-gE monoclonal antibody (MAb) 3063 or gI, and proteins were analyzed on 10% polyacrylamide gels as previously described (48).
Confocal immunofluorescence microscopy.
HaCaT cells were grown on Nunc Permanox eight-well slides until 70% confluent and then coinfected with AdtetgEΔCT (150 PFU/cell), AdtetgI (150 PFU/cell), and AdTet-trans (80 PFU/cell) or with AdgE(E1−) and AdgI(E1−) with 150 PFU of each per cell for 24 h. The cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min and then permeabilized with 0.2% Triton X-100 in PBS for 5 min, washed three times in PBS containing 0.02% Tween 20 (PBS-T), and then incubated in blocking buffer: PBS-T containing 2% goat serum, 1% fish gelatin, and 2% bovine serum albumin. The cells were stained with rat anti-gE/gI and simultaneously with sheep anti-TGN46 or rabbit anti-β-catenin as described previously (31).
Plaque assays measuring cell-to-cell spread.
HaCaT cells growing in 35-mm-diameter plastic dishes were not infected with Ad vectors or were infected with AdTet-trans (300 PFU/cell), AdtetgI (300 PFU/cell), AdgE(E1−) and AdgI(E1−) (150 PFU of each per cell), AdgEΔCT and AdtetgI (150 PFU of each per cell), AdgD (300 PFU/cell) or AdgEΔCT (100 PFU/cell), AdtetgI (100 PFU/cell), and AdgD (200 PFU/cell). After 12 h the cells were infected with wild-type HSV-1 F or F-gEβ (approximately 100 PFU/dish), and then the monolayers were incubated for 48 h in medium containing 0.2% pooled human gamma globulin (a source of anti-HSV neutralizing antibodies). The cells were then fixed in PBS containing 4% paraformaldehyde for 20 min, washed with PBS containing 0.1% Tween 20 for 10 min, and then stained with rabbit polyclonal anti-HSV antibodies and donkey anti-rabbit immunoglobulin G antibodies conjugated with horseradish peroxidase as described previously (48).
Electron microscopy.
HaCaT cells were infected as described for plaque assays with Ad vectors for 12 h and then infected with HSV-1 (F) with 0.3 or 3.0 PFU/cell for 17 to 19 h. The cells were fixed with 2.5% glutaraldehyde in PBS for 20 min, washed with 100 mM sodium cacodylate buffer (pH 7.5), scraped from the plates, pelleted, prestained, and stained as described previously (23). The cell pellets were then embedded in epoxy resin, and thin sections were cut and viewed in a Philips Morgagni 268 transmission electron microscope. Images were captured with an Amount 2K digital camera.
RESULTS
Construction and characterization of an Ad vector expressing HSV gE lacking the CT domain.
Previous results showed that gE/gI accumulates extensively in the trans-Golgi network (TGN) when expressed with Ad vectors and also early after HSV infection (31). The CT domain of gE plays an important part in this TGN localization, as gE/gI was more extensively found on lateral and apical cell surfaces in cells infected with an HSV lacking the gE CT domain (F-gEΔCT) (48). Moreover, transfer of the gE CT domain, but not the gI CT domain, onto gD caused gD to be TGN localized (31). In order to determine if gE/gI could be expressed on the cell surface and potentially inhibit cell-to-cell spread, we constructed a recombinant Ad vector expressing a gE polypeptide lacking the CT domain. All but 16 residues of the 106-amino-acid CT domain were removed, so that this truncated gE lacks all of the recognized sorting motifs. This truncated gE was inserted into a shuttle plasmid and used to produce a recombinant Ad vector, designated AdgEΔCT, by using 293-cre4 cells (34). A second Ad vector, AdtetgI, expressing wild-type (full-length) HSV-1 gI under the control of the tetracycline-regulated HCMV promoter was constructed by similar methods. Expression of both gEΔCT and gI was dependent upon coinfection of cells with another Ad vector, AdTet-trans, that expresses the tetracycline transactivator protein that activates the tetracycline-regulated promoter (9, 45).
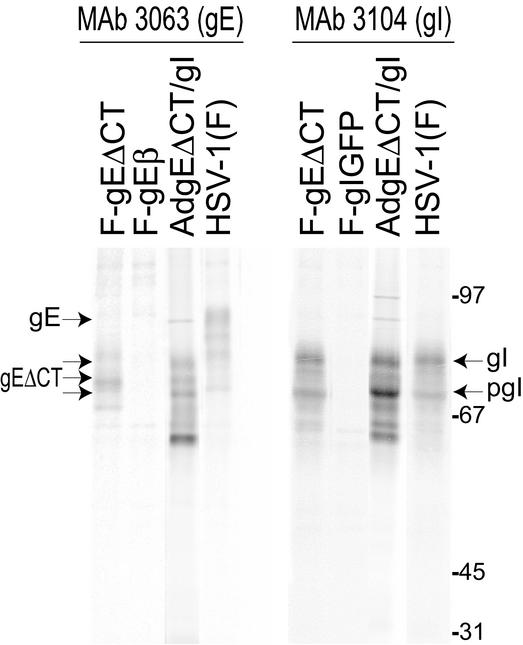
Expression of gEΔCT and gI was assessed by coinfecting HaCaT cells with AdtetgEΔCT and AdtetgI, radiolabeling the cells, and immunoprecipitating the gE/gI complex. MAb 3063 recognizes gE in the context of the gE/gI complex but does not recognize gE when expressed without gI (T. Wisner, unpublished results). In cells coinfected with AdgEΔCT and AdtetgI, MAb 3063 precipitated a series of protein species ranging in apparent molecular mass from 82 to 65 kDa, including several species that comigrated with those observed in F-gEΔCT-infected cells, as well as smaller, immature forms of gE and gI (Fig. 1, left panel). Note that gE and gI are both glycosylated with N- and O-linked oligosaccharides and phosphorylated and that gE is modified with fatty acid and sulfated (22a). However, the important observations were that (i) the largest of the protein bands in AdgEΔCT/gI-infected cells was ∼10 kDa smaller than mature gE in HSV-infected cells (designated gE in Fig. 1, ∼92 kDa), consistent with loss of the gE CT domain, and (ii) a complex of gEΔCT and gI was clearly formed. MAb 3104 recognizes gI without gE but prefers the gI in complex with gE and precipitated complexes of gEΔCT/gI from AdgEΔCT/gI-infected cells (Fig. 1, right panel). We concluded that these Ad vectors could be used to express a complex of gEΔCT/gI in a robust and sustained manner.
FIG. 1.
Expression of gE/gI in cells infected with recombinant Ad vectors and HSV. HaCaT cells were infected with AdgEΔCT and AdtetgI with 100 PFU/cell for 22 h or were infected with wild-type HSV-1 strain F, HSV gE-null mutant F-gEβ, HSV gE mutant F-gEΔCT (lacking the gE CT domain), or HSV gI-null mutant F-gIGFP for 5 h. The cells were radiolabeled with [35S]methionine-cysteine for 3 h, cell extracts were produced, and gE/gI complexes were immunoprecipitated with MAb 3063, which recognizes gE in gE/gI complexes, or MAb 3104, which recognizes gI in gE/gI complexes. Samples were subjected to electrophoresis in polyacrylamide gels, and radiolabeled proteins were identified by using a PhosphorImager. The positions of gE (mature gE expressed by wild-type HSV-1), gEΔCT/gI (expressed by F-gEΔCT and by coinfection with AdgEΔCT and AdtetgI), gI (mature gI), and pgI (immature gI) and molecular mass markers of 97, 67, 45, and 31 kDa are indicated.
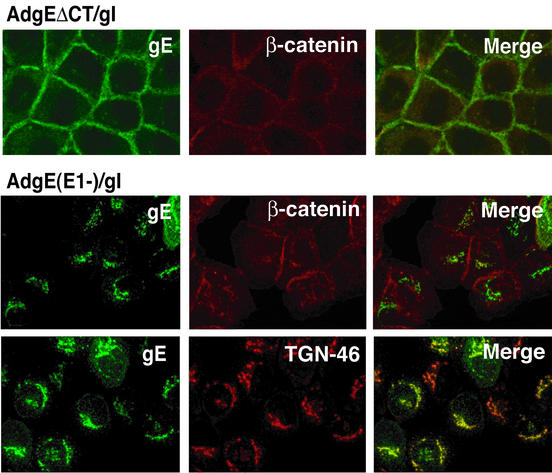
The subcellular distribution of gEΔCT was determined by confocal immunofluorescence microscopy. As in previous experiments, wild-type gE/gI expressed by coinfecting cells with AdgE(E1−) and AdgI(E1−) colocalized extensively with the TGN marker TGN46 (Fig. 2). There was little wild-type gE/gI at cell junctions stained with β-catenin antibodies. By contrast, gEΔCT/gI was observed extensively at cell junctions, colocalizing with β-catenin (Fig. 2).
FIG. 2.
Confocal immunofluorescence microscopy of cells expressing gEΔCT/gI. HaCaT cells were infected with either AdgEΔCT (150 PFU/cell), AdtetgI (150 PFU/cell) and AdTet-trans (80 PFU/cell), or AdgE(E1−) and AdgI(E1−) with 150 PFU of each per cell. After 20 to 24 h the cells were fixed, permeabilized, and stained for gE with MAb 3114 and simultaneously with anti-β-catenin or anti-TGN46 antibodies.
Expression of gEΔCT/gI in trans reduces cell-to-cell spread.
Previously, we found that HSV cell-to-cell spread in a human keratinocyte cell line, HaCaT, was dependent on gE/gI, so that five- to eightfold-fewer cells were infected by a gE− HSV-1 mutant (48). To test the effects of expression of gEΔCT/gI on HSV cell-to-cell spread, HaCaT cells were infected with various doses of replication-defective Ad vectors AdgEΔCT and AdtetgI for various times. The cells were then infected with small amounts of HSV (50 to 200 PFU/dish), and plaques were stained after 2 days. These experiments demonstrated that transduction of HaCaT cells with AdgEΔCT and AdtetgI with 100 to 300 PFU/cell (measured on complementing 293 cells) for 12 h followed by infection of the cells with HSV-1 with ∼100 PFU/dish for 48 h gave optimal results. It is important to note that these E1− Ad vectors do not replicate significantly on human cells other than complementing 293 cells, although at higher doses (≥500 PFU/cell) or after longer periods cytopathic effects associated with limited E1-independent replication do occur. However, after 12 h even with 300 PFU/cell, there was little or no cytopathic effect and, from previous observations, HSV replication occurred normally in the cells (see also below).
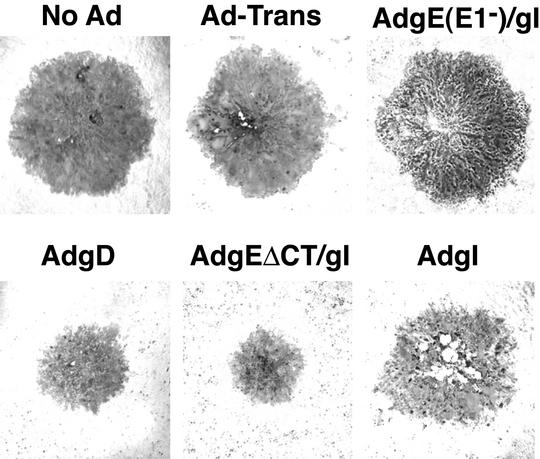
HSV plaques formed on AdgEΔCT/gI-transduced HaCaT cells were significantly smaller than those formed on cells infected with control Ad vector, AdTet-trans, or on cells not infected with any Ad vector (Fig. 3). There was no reduction in the sizes of HSV plaques when cells were transduced with gI alone (AdtetgI) or with wild-type gE and gI [AdgE(E1−)/gI]. However, gD delivered with AdgD reduced plaques significantly (Fig. 3). When ∼20 randomly chosen plaques were photographed and analyzed digitally, the diameters of HSV plaques formed on AdgEΔCT/gI-infected cells were approximately 35% the size of those formed on control cells infected with AdTet-trans or not infected with an Ad vector (Table 1). Thus, there were approximately 87% fewer cells infected by HSV when gEΔCT/gI was expressed in cells than when it was expressed in controls. Similarly, gD expression reduced plaque size by approximately 50%, so that 72% fewer cells were infected compared with controls. There was no further reduction in the sizes of HSV plaques when both gD and gEΔCT/gI were coexpressed in HaCaT cells, compared with expression of gEΔCT/gI alone (Table 1), although lower doses of each Ad vector were used to avoid cytopathic effects. There was no significant reduction in plaque size when wild-type gE/gI (which accumulates in the TGN) or gI alone was expressed. Moreover, plaques produced by the gE− mutant F-gEβ were not reduced in size by prior expression of gEΔCT/gI (Table 1).
FIG. 3.
HSV plaques produced on cells expressing gEΔCT/gI. HaCaT cells were left uninfected (No Ad) or infected with AdTet-trans (Ad-Trans); with AdgE(E1−) and AdgI(E1−) with 150 PFU of each per cell [AdgE(E1−)/gI]; with AdgD with 300 PFU/cell; with AdgEΔCT (150 PFU/cell), AdtetgI (150 PFU/cell), and AdTet-trans with 80 PFU/cell (AdgEΔCT/gI); or with AdtetgI (AdgI) with 300 PFU/cell for 12 h. The cells were then infected with wild-type HSV-1 F, with ∼100 PFU/dish, and after 2 to 2.5 days the cells were fixed and stained with anti-HSV polyclonal antibodies and peroxidase-conjugated secondary antibodies.
TABLE 1.
Interference of HSV cell-to-cell spread by expression of gEΔCT and gI in cells
| HSV | Ad vectora | Size of HSV plaquesb | No. of plaquesc | Yield of HSVd |
|---|---|---|---|---|
| Wild type | No Ad | 100 | 100 | 320 |
| AdTet-trans | 94.4 ± 2.8 | 96 | ND | |
| AdtetgI | 94.6 ± 7.8 | 116 | ND | |
| AdgE(E1−)/gI | 96.1 ± 4.4 | 96 | 350 | |
| AdgEΔCT/gI | 35.5 ± 3.9 | 92 | 280 | |
| AdgD | 52.7 ± 5.6 | 11 | ND | |
| AdgEΔCT/gI and AdgD | 30.1 ± 3.4 | 9 | ND | |
| F-gEβ | No Ad | 29.7 ± 0.6 | 100 | ND |
| AdgEΔCT/gI | 30.2 ± 0.4 | 95 | ND |
HaCaT cells were infected with various Ad vectors or combinations of vectors with 300 PFU/cell for each of the single infections with AdTet-trans, AdtetgI, and AdgD; 150 PFU of each virus per cell for the dual infections, AdgE(E1−)/gI and AdgEΔCT/gI; and 100, 100, and 200 PFU/cell, respectively, for the AdgEΔCT-AdtetgI-AdgD infections. After 12 h, the cells were infected with wild-type HSV-1 strain F or the gE-null mutant, F-gEβ, with ∼100 PFU/dish for 48 to 60 h.
The cell monolayers were fixed and stained with polyclonal anti-HSV antibodies, secondary peroxidase-conjugated antibodies, and peroxidase substrate. The diameter of ∼20 plaques was measured, and standard deviations are shown.
The numbers of plaques produced on cells not infected with Ad vectors were arbitarily set at 100, and plaques formed on other monolayers were compared to this.
Cells infected with Ad vectors were infected with HSV with 10 PFU/cell for 18 to 20 h, then the cells and media were harvested and sonicated, and infectious HSV titers were determined on Vero cell monolayers. Yield of HSV indicates PFU per cell. ND, not determined.
The number of plaques formed on cells expressing gD was reduced by ∼10-fold, consistent with previous observations that gD expression blocks entry (4, 24, 36). Inhibition of entry, i.e., reduced numbers of plaques, was not observed with gEΔCT/gI or gE/gI. We concluded that expression of gEΔCT/gI in HaCaT cells significantly reduced the sizes of HSV plaques to the sizes of those produced by a gE− mutant; however, there was no obvious inhibition of entry of HSV.
HSV replication on HaCaT cells transduced with gEΔCT/gI.
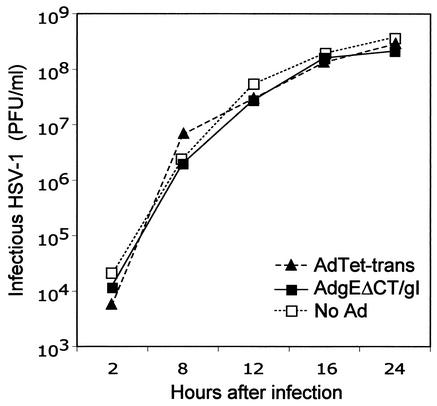
In order to determine whether there was inhibition of HSV replication, HaCaT cells were transduced with AdgEΔCT/gI for 12 h and then infected with HSV-1 (10 PFU/cell) for 17 h. The cells and media were harvested, and infectious HSV was assayed. There were no large differences in the yields of infectious HSV when cells were first infected with either AdgEΔCT/gI or AdgE(E1−)/gI compared to control cells not infected with Ad vectors (Table 1). To establish this further, single-step growth analyses were performed. Cells were transduced with Ad vectors and then infected with HSV-1 for various times before infectious HSV was harvested and its titers were determined. There were no differences in the yields of HSV produced at early or late times, whether the cells were infected with AdgEΔCT/gI or AdTet-trans or not infected with Ad vectors (Fig. 4). Therefore, replication of HSV is not compromised by transduction of cells with AdgEΔCT/gI or other Ad vectors.
FIG. 4.
Replication of HSV-1 on cells expressing gEΔCT/gI. HaCaT cells were left uninfected (No Ad) or infected with AdTet-trans (300 PFU/cell) or AdgEΔCT and AdtetgI (150 PFU of each per cell) for 12 h. The cells were then infected with HSV-1 (10 PFU/cell). Cells and cell culture supernatants were collected at various times and sonicated, and infectious HSV was assayed by plaque assays.
Accumulation of HSV particles at cell junctions.
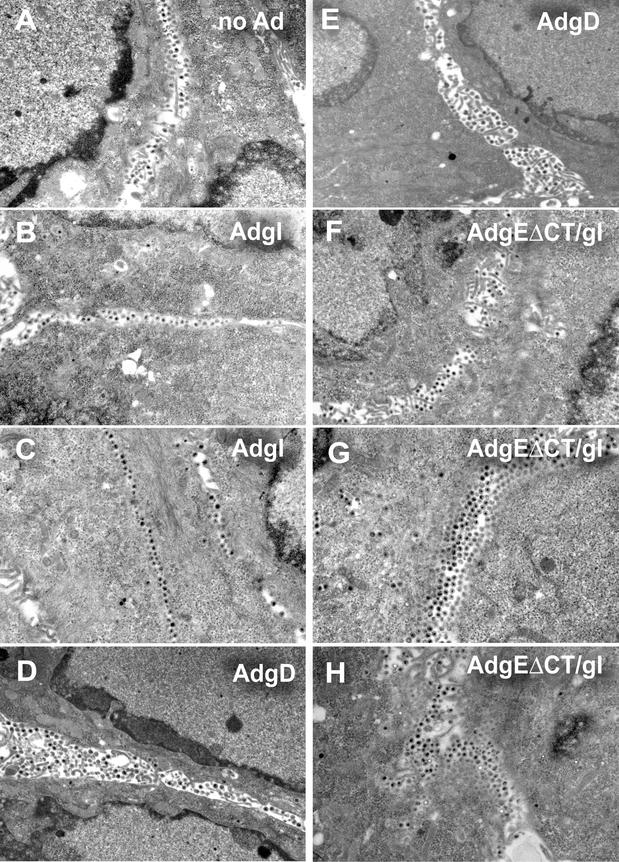
Given the effects of gE/gI on sorting of virus particles described previously (23), gEΔCT/gI expression might potentially cause intracellular accumulation or mislocalization of HSV particles. This appeared unlikely given that wild-type gE/gI, a glycoprotein complex that accumulates at intracellular sites, did not reduce cell-to-cell spread. However, to characterize this further, electron microscopic analyses were performed. As in previous experiments with HEC-1A cells, HSV-infected HaCaT cells (not infected with Ad vectors) displayed numerous virus particles that accumulated at cell junctions (Fig. 5A). With HaCaT cells it was more difficult to discern apical versus lateral versus basal surfaces, as the morphology of these cells is less cuboidal and more squamous. However, as before, there were relatively few intracellular particles and large-scale accumulation of virions in the spaces between closely apposed cells. Cells transduced with AdtetgI before HSV infection also displayed large numbers of virus particles present in the spaces between cells (Fig. 5B and C). Similarly, HSV particles were found at cell junctions of cells transduced with AdgD (Fig. 5D and E). There was no reduction of the numbers of virus particles observed when the cells were infected with AdtetgI or AdgD compared with those for cells that were not infected with an Ad vector. Thus, there were no negative effects of Ad infection or of expression of gD or gI on production of HSV particles and accumulation of these particles at cell junctions.
FIG. 5.
Electron microscopic analyses of cells expressing AdgEΔCT and then infected with HSV. HaCaT cells were left uninfected (no Ad) (A) or infected with AdtetgI (300 PFU/cell; designated AdgI) (B and C), AdgD (300 PFU/cell) (D and E), or AdgEΔCT and AdtetgI (150 PFU of each per cell) and AdTet-trans (80 PFU/cell) (F to H), in each case for 12 h. The cells were then infected with HSV-1 with 3 PFU/cell (A, B, D, and F) or with 0.3 PFU/cell (C, E, G, and H), and at 17 h the cells were washed, fixed with 2.5% glutaraldehyde, stained, sectioned, and viewed by electron microscopy.
Cells transduced with AdgEΔCT/gI before infection with 3 PFU of HSV-1 per cell also displayed extensive accumulation of virus particles at cell junctions (Fig. 5F). Importantly, there was not abnormal accumulation of HSV particles in the cytoplasm of gEΔCT/gI-expressing cells. HSV particles were also found at cell junctions when cells were transduced with AdgEΔCT/gI and then infected with HSV with lower multiplicities, e.g., 0.3 PFU/cell, where some of the cells were not obviously producing virus particles (Fig. 5G and H). In a significant fraction of AdgEΔCT/gI-infected cells, there was accumulation of a double, triple, or quadruple row of virus particles between cells. This was also observed occasionally in cells infected with AdgD (Fig. 5D and E). By contrast, multiple rows of virions in the space between cells were more rarely observed in cells transduced with AdtetgI (Fig. 5B and C) or when no Ad vectors were applied (Fig. 5A). We concluded from these experiments that gEΔCT/gI expression does not cause accumulation of HSV particles in the cytoplasm. Instead, particles accumulate largely at cell junctions and, in some cases, in multiple rows of enveloped virions.
DISCUSSION
HSV and PRV gE/gI are particularly interesting molecules because they promote cell-to-cell spread, but the effects of these glycoproteins are specific to cells that are polarized or that form extensive cell junctions, i.e., biologically relevant epithelial cells and neurons. In epithelial and neuronal tissues gE/gI is similarly important for virus spread. However, gE/gI does not facilitate entry of extracellular virions at apical surfaces. It is not yet clear how gE/gI functions to mediate cell-to-cell spread, although we have some clues. gE and gI are structurally and genetically related to gD, a viral glycoprotein that is absolutely required for entry of extracellular virus particles into cells. All three of these glycoproteins appear to have been derived from a common ancestor (30). Given that VZV has no gD (10) and appears to rely on gE/gI for entry and cell-to-cell spread, it is likely that the ancestral glycoprotein was more closely related to gE or gI than to gD. HSV gD is essential for both virus entry and cell-to-cell spread (13, 28) and binds nectin-1, a cell adhesion molecule found largely at epithelial cell junctions (reviewed in reference 40). By contrast, PRV gD is not required for cell-to-cell spread, either in cultured cells or in vivo; once virus can enter into cells, it can spread without gD (17, 32, 35, 38).
One mechanism by which HSV and PRV gE/gI can promote cell-to-cell spread in cultured epithelial cells involves sorting of nascent virions to cell junctions (23). The relatively large cytoplasmic tails of gE and gI affect concentration of gE/gI in TGN compartments, which apparently leads to sorting to epithelial cell junctions. The notion that gE/gI can promote envelopment in the TGN is supported by recent observations that PRV gE− gM− double mutants and an HSV gE− gD− double mutant fail to produce enveloped virions and accumulate unenveloped capsids in the cytoplasm (2; Farnsworth et al., unpublished). It was proposed previously that gE/gI promotes envelopment of nascent HSV particles into subdomains of the TGN that are sorted preferentially to lateral surfaces of polarized epithelial cells, a process that would promote movement to adjacent cells (21, 23).
There is also evidence that the extracellular domains of gE and gI participate in cell-to-cell spread by binding to components of cell junctions (21, 23, 31, 48). gE/gI preferentially accumulates at lateral cell surfaces, not on apical surfaces, at late stages of HSV infection (14) and when gE lacking the CT domain was coexpressed with gI with Ad vectors in this report. Small insertion mutations in the ET domain of gE inhibited cell-to-cell spread as significantly as was observed when gE was deleted entirely, and yet these gE ET domain mutants folded normally, bound gI, and reached the cell surface (Goldsmith et al., submitted). In this report we describe interference with cell-to-cell spread: when gEΔCT/gI was expressed in keratinocytes, plaque sizes were reduced so that only 12 to 15% the number of cells was infected compared with controls. This inhibition in cell-to-cell spread was similar to that observed when gE was deleted. However, another form of gE/gI, wild-type gE/gI, which accumulates largely in the TGN, did not affect plaque size. Thus, this interference apparently occurs at the cell surface. Moreover, expression of gEΔCT/gI did not lead to inhibition of HSV replication and virions accumulated not in the cytoplasm but on cell surfaces, largely at cell junctions. There was often, although not always, a double, triple, or even quadruple row of virions at the junctions of cells expressing gEΔCT/gI. This was less frequently observed in cells expressing gI alone, suggesting that interference caused a virion traffic jam as virus particles moved across cell junctions.
Together these observations are consistent with the notion that gEΔCT/gI acts at the cell surface, and not at intracellular sites, to block HSV cell-to-cell spread. One might argue that gEΔCT/gI does not accurately reflect the properties of wild-type gE/gI, which accumulates extensively in the TGN. In HSV-infected cells, gE/gI accumulates in the TGN at early times but later moves to lateral surfaces of epithelial cells (31). The virion envelope includes gE/gI that could interact with gE/gI receptors at cell junctions, perhaps in a receptor mechanism to facilitate HSV entry into an adjacent cell. To confirm and extend these observations, stably transfected cells are being constructed. However, in this case, use of Ad vectors had several major advantages over transfected cells because (i) clones of transfected cells differ among themselves in many ways, (ii) it was possible to test several different cell types, and (iii) we could coexpress gE/gI and gD in the same cells.
Previous observations that HSV and PRV gD expression in cells blocked entry of HSV and PRV were interpreted in two ways. First, it was suggested that gD expressed in cells, either at the cell surface or internally, could sequester receptors required for HSV or PRV entry (4, 24, 36). This was similar to the interference first described for avian retroviruses where infection of cells with one retrovirus blocked subsequent infection by another retrovirus utilizing the same receptor (39, 41, 42). The notion of cell surface gD receptors was first proposed to explain results involving HSV gD− mutants that adsorbed onto, but did not enter, cells and which lacked the ability to interact with a limited set of virus receptors (22, 28). These receptors were enumerated in studies involving soluble forms of gD that blocked virus entry but not adsorption (18). Other glycoproteins, e.g., gB, did not appear to possess saturable receptors (18). A second model for gD-mediated interference was proposed later and suggested that cell-associated gD interacts with gD or some other viral protein in the virion (5, 11). This was based on observations with mutant gD molecules involving residue 25 or 27 that rendered HSV resistant to interference by wild-type gD or that reduced interference activity when transfected into cells. Given our present state of knowledge of gD receptors, it appears likely that this second model is not correct; more likely mutations at the N terminus of gD affected affinities with various gD receptors (8, 47).
Our observations have important implications for understanding cell-to-cell spread and the role of gE/gI in this process. The results support a model in which gE/gI mediates interactions with cellular molecules, receptors that enhance cell-cell spread so that expression of gEΔCT/gI in trans interferes with this interaction. gD also interfered with cell-to-cell spread, although in HaCaT cells induced to express gD by using these Ad vectors this inhibition was less extensive than that with gEΔCT/gI. Coexpression of both gD and gEΔCT/gI in cells did not reduce plaque size beyond that observed with gEΔCT/gI. It is not clear from these results whether gD and gEΔCT/gI bind molecules that are identical, although these molecules appear to function similarly at cell surfaces and to promote entry into adjacent cells. We have been unable to find any evidence that gE/gI binds the gD receptor nectin-1 or HveA in enzyme-linked immunosorbent assays involving purified, soluble gE/gI and gD receptors (26, 27; M. Huber, C. Kummenacher, G. Cohen, R. Eisenberg, and D. C. Johnson, unpublished results). However, it is important to note that gD is absolutely required for cell-to-cell spread in HaCaT cells (17). Without gD, HSV does not spread beyond a single infected cell. Therefore, to explain the incomplete nature of the gD interference that we observed, it is likely that the Ad vectors do not express sufficient quantities of gD or that there is insufficient access to all the gD receptors in HaCaT cells which have high levels of nectin-1 (17).
In summary, we observed that expression of gE/gI at cell junctions blocks HSV cell-to-cell spread, causing accumulation of virions at junctions. These observations support the hypothesis that gE/gI binds to components of cell junctions, gE/gI receptors, to promote movement of virus across epithelial cell junctions.
Acknowledgments
We are most grateful to Todd Wisner and Claire Dunn for advice and technical help. Michael Webb provided invaluable assistance in the electron microscopic experiments, and Aurelie Snyder made indispensable contributions in the confocal microscopy experiments.
This work was supported by NIH grant CA73996.
REFERENCES
- 1.Balan, P., N. Davis-Poynter, S. Bell, H. Atkinson, H. Browne, and T. Minson. 1994. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J. Gen. Virol. 75:1245-1258. [DOI] [PubMed] [Google Scholar]
- 2.Brack, A. R., B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetti, C. R., K. S. Dingwell, C. Wale, F. L. Graham, and D. C. Johnson. 1998. Herpes simplex virus gD and virions accumulate in endosomes by mannose 6-phosphate-dependent and -independent mechanisms. J. Virol. 72:3330-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume, G., M. Arsenakis, F. Farabegoli, and B. Roizman. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campadelli-Fiume, G., S. Qi, E. Avitabile, L. Foa-Tomasi, R. Brandimarti, and B. Roizman. 1990. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J. Virol. 64:6070-6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card, J. P., and L. W. Enquist. 1995. Neurovirulence of pseudorabies virus. Crit. Rev. Neurobiol. 9:137-162. (Erratum, 9:310.) [PubMed]
- 7.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 9.Chevalier, M. S., G. M. Daniels, and D. C. Johnson. 2002. Binding of human cytomegalovirus US2 to major histocompatibility complex class I and II proteins is not sufficient for their degradation. J. Virol. 76:8265-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 11.Dean, H. J., S. S. Terhune, M. T. Shieh, N. Susmarski, and P. G. Spear. 1994. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology 199:67-80. [DOI] [PubMed] [Google Scholar]
- 12.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dingwell, K. S., and D. C. Johnson. 1998. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alpha-herpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 16.Heffner, S., F. Kovacs, B. G. Klupp, and T. C. Mettenleiter. 1993. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J. Virol. 67:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 75:10309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, D. C., R. L. Burke, and T. Gregory. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J. Virol. 64:2569-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Johnson, D. C., and P. G. Spear. 1983. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell 32:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, R. M., and P. G. Spear. 1989. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J. Virol. 63:819-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura, H., S. E. Straus, and R. K. Williams. 1997. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology 233:382-391. [DOI] [PubMed] [Google Scholar]
- 26.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeoch, D. J., A. Dolan, S. Donald, and D. H. Brauer. 1986. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 14:1727-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 31.McMillan, T., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulder, W., J. Pol, T. Kimman, G. Kok, J. Priem, and B. Peeters. 1996. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J. Virol. 70:2191-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulder, W. A., L. Jacobs, J. Priem, G. L. Kok, F. Wagenaar, T. G. Kimman, and J. M. Pol. 1994. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J. Gen. Virol. 75:3095-3106. [DOI] [PubMed] [Google Scholar]
- 34.Ng, P., R. J. Parks, D. T. Cummings, C. M. Evelegh, U. Sankar, and F. L. Graham. 1999. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum. Gene Ther. 10:2667-2672. [DOI] [PubMed] [Google Scholar]
- 35.Peeters, B., J. Pol, A. Gielkens, and R. Moormann. 1993. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J. Virol. 67:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovskis, E. A., A. L. Meyer, and L. E. Post. 1988. Reduced yield of infectious pseudorabies virus and herpes simplex virus from cell lines producing viral glycoprotein gp50. J. Virol. 62:2196-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajcani, J., U. Herget, and H. C. Kaerner. 1990. Spread of herpes simplex virus (HSV) strains SC16, ANG, ANGpath and its GlyC minus and GlyE minus mutants in DBA-2 mice. Acta Virol. 34:305-320. [PubMed] [Google Scholar]
- 38.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 64:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson, H. L., S. M. Astrin, A. M. Senior, and F. H. Salazar. 1981. Host susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J. Virol. 40:745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alpha-herpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 41.Steck, F. T., and H. Rubin. 1966. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology 29:628-641. [DOI] [PubMed] [Google Scholar]
- 42.Steck, F. T., and H. Rubin. 1966. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology 29:642-653. [DOI] [PubMed] [Google Scholar]
- 43.Tirabassi, R. S., and L. W. Enquist. 1998. Role of the envelope protein gE in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tirabassi, R. S., and L. W. Enquist. 2000. Role of the pseudorabies virus gI cytoplasmic tail in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J. Virol. 74:3505-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cell. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 46.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willis, S. H., A. H. Rux, C. Peng, J. C. Whitbeck, A. V. Nicola, H. Lou, W. Hou, L. Salvador, R. J. Eisenberg, and G. H. Cohen. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J. Virol. 72:5937-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao, Z., W. Jackson, B. Forghani, and C. Grose. 1993. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J. Virol. 67:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuckermann, F. A., T. C. Mettenleiter, C. Schreurs, N. Sugg, and T. Ben-Porat. 1988. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J. Virol. 62:4622-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]