Abstract
The envelope (E) protein of Japanese encephalitis virus (JEV) is associated with viral binding to cellular receptors, membrane fusion, and the induction of protective neutralizing-antibody responses in hosts. Most previous studies have not provided detailed molecular information about the spatial configuration of the functional epitopes on domain III of the E protein. Here site-directed mutagenesis was performed to demonstrate that the functional epitope determinants at Ser331 and Asp332 on domain III of the JEV E protein interacted with neutralizing monoclonal antibody (MAb) E3.3. Bacterial expression of the recombinant Fab E3.3 confirmed the molecular interactions of Arg94 in complementary determining region H3 with Ser331 and Asp332 on domain III. This study elucidates the detailed molecular structures of the neutralizing epitope determinants on JEV domain III, which can provide useful information for designing new vaccines.
Japanese encephalitis virus (JEV) (family Flaviviridae, genus Flavivirus) is a single-stranded, positive-sense RNA virus with a genome size of approximately 11 kb. The genome contains a single open reading frame encoding three structural proteins (capsid [C], premembrane [prM/M], and envelope [E]) and seven nonstructural proteins, and two nontranslating regions flank the 5′ and 3′ ends (17, 23, 25). The flavivirus E protein has approximately 500 amino acids with six conserved disulfide bonds to maintain its conformational structure (20, 29). The E protein structures have been determined for the tick-borne encephalitis (TBE) virus by using X-ray crystallography (24) and recently have been used for a low-resolution map of whole dengue virions (13). The E protein is a head-to-tail homodimer with three structurally distinct domains: a central β-barrel (domain I), an elongated dimerization region (domain II), and a C-terminal immunoglobulin (Ig)-like module (domain III) (24). The E protein is associated with viral attachment, fusion, hemagglutination, cellular tropism, viral virulence, and the induction of protective immune response (17).
The domain III protein contains one disulfide bond to maintain its conformational structure and can be independently folded as a trypsin-resistant core protein (16, 20, 29). Neutralizing epitopes on the lateral surface of domain III have been identified, including residues 333 (1), 373 to 399 (28), and 306, 331, and 387 of JEV (30); 308, 310, and 311 of louping ill virus (8); 384 and 386 of TBE (6, 7); 305 of yellow fever virus (27); and 383 to 389 (5), 333 to 351 (26), and 307 (15) of the dengue viruses. However, most studies have not provided detailed molecular information about the spatial configuration of the epitopes on domain III of the flavivirus E proteins, where the epitopes are defined as a few contact residues that are energetically important for binding affinity in the antibody-antigen complex (9, 21). In this study, domain III of the JEV E protein was expressed in Escherichia coli to investigate the functional epitope determinants for a JEV-specific neutralizing monoclonal antibody (MAb), E3.3 (30). The alteration of proteins with single or double site-directed mutagenesis was conducted at positions 306 (Glu→Gly), 331 (Ser→Arg), and 387 (Met→Arg). Also, mutations at or near position 331 of the JEV E protein were replaced with alanine or other hydrophobic and charged amino acids. By using complementary structural modeling (4, 14, 22), the structure of the domain III MAb E3.3 complex was constructed. Specific interactions of the functional epitope determinants on domain III were confirmed by further mutating the combining sites of MAb E3.3 Fab antibody fragment. This study elucidates the detailed molecular structures of the neutralizing epitope determinants on the JEV domain III protein, which can provide useful information for designing new vaccines.
MATERIALS AND METHODS
Virus, cells, and media.
The JEV attenuated variant CH2195LA was plaque purified in Vero cells from a Taiwanese isolate (30) and was propagated in Vero cells by using M199 medium (Invitrogen) containing 10% fetal bovine serum (FBS) (Invitrogen). MAb E3.3-producing hybridoma cells were grown in Iscove's modified Dulbecco's medium (Invitrogen) with 10% FBS.
Cloning, expression, purification, and site-directed mutagenesis of E and domain III fusion protein.
Viral RNAs were extracted from the culture supernatants of infected Vero cells by using a TRIzol kit (Invitrogen), and the reverse transcriptase-PCR (RT-PCR) was conducted by use of Superscript II RT and Elongase mix enzymes (Invitrogen) to obtain JEV E gene fragments. Oligonucleotide primers used included (i) the full-length E gene with the forward primer 5′-ATGCGCGGATCCTTCAACTGTCTGGGAAT-3′ and the reverse primer 5′-GGGGAAGCTTAGCATGCACATTGGTGG-3′, (ii) the domain I and II protein gene (residues 1 to 291) with the forward primer 5′-ATGCGCGGATCCTTCAACTGTCTGGGAAT-3′ and the reverse primer 5′-GGGGAAGCTTCATTTTTAGCCTGCATTTCAG-3′, and (iii) the domain III protein gene (residues 292 to 402) with the forward primer 5′-ATGCGCGGATCCGACAAACTGGCCCTGAA-3′ and the reverse primer 5′-GGGGAAGCTTCGTGCTTCCAGCTTTGTGCC-3′. The BamHI-HindIII restriction sites are underlined. Site-directed mutagenesis was conducted by using paired complementary oligonucleotides for the desired point mutations to generate specific mutations into the JEV domain III protein. The correct sequence of each mutant of domain III fusion protein constructs was confirmed by DNA sequence analysis. All of these forward primers contained a BamHI restriction site, and the reverse primers contained a HindIII restriction site. Each RT-PCR product was digested with BamHI and HindIII and then was ligated into the BamHI/HindIII sites of the pET32a vector (Novagen) and expressed as a thioredoxin (Trx) fusion protein. The E. coli strain BL21(DE3) with the pET32a plasmid containing the E gene fragment was cultured overnight and induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 25°C for 4 h. Bacteria cells were harvested by centrifugation for sonication, and the expressed proteins were purified by using the Ni-nitrilotriacetic acid agarose column (Qiagen). The eluted recombinant fusion protein was further dialyzed, and the protein concentration was determined by using the Bio-Rad protein assay reagent.
SDS-PAGE and Western blotting.
Samples from each lysate of transformed cells were dissolved in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer without 2-mercaptoethanol and were boiled for 10 min. Proteins were resolved on SDS-12% PAGE gels and were electrophoretically transferred to nitrocellulose papers. The resultant blots were blocked with 5% skim milk and then were reacted with appropriately diluted MAb E3.3 for a 1-h incubation. The blots were then washed with Tris-buffered saline (TBS) three times and were overlaid with a 1/1,000 dilution of rabbit anti-mouse IgG antibodies conjugated with alkaline phosphatase (KPL). Following another 1-h incubation at room temperature, the blots were developed with 5′-bromo-4-chloro-3-indolyl phosphate nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolylphosphate (BCIP) (Invitrogen).
ELISA affinity assay.
The wells of a 96-well microtiter plate were coated with 100 μl of diluted rabbit anti-Trx antibodies (Sigma) and were incubated overnight at 4°C. Following each incubation and subsequent layer of the enzyme-linked immunosorbent assay (ELISA), the wells were washed three times with TBS containing 0.05% Tween 20 (TBST). After being blocked by incubation with 5% skim milk in TBST for 2 h at room temperature (200 μl per well), 100 μl of wild-type or mutant recombinant domain III fusion proteins (10 μg/ml) was captured onto rabbit anti-Trx-coated wells for 2 h of incubation. Following this, serial dilutions of purified MAb E3.3 were added and incubated at room temperature for 1 h. The bound antibodies were detected after incubation with the anti-mouse IgG conjugated to peroxidase (KPL) for 1 h at room temperature. The ELISA products were developed by using a chromogen solution containing 2,2Ν-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) and hydrogen peroxide and then were measured at A405. The steady-state equilibrium affinity constant, Kd, was estimated from the concentration of MAb E3.3 corresponding to 50% maximal binding.
Recombinant Fab antibody.
The cDNA genes of the Fab region of MAb E3.3 were amplified by use of a 3′ primer of heavy-chain IgG2a Fd (5′-GTTCTGACTAGTATGATGATGATGATGATGGGGCCCTCTGGGCTCAATTTTCTTGTC-3′) and the κ light-chain 3′ primer (5′-GCGCCGTCTAGAATTAACACTCATTCCTGTTGAA-3′). Nine 5′ primers for Fd and seven 5′ primers for κ were paired with the above-described 3′ primers in order to test for the ability to amplify the variable-region genes of light and heavy chains of MAb E3.3 (11). The 3′ primer of heavy-chain IgG2a Fd mentioned above contained extra nucleotides encoding six-histidine residues. The resultant PCR products of heavy-chain Fd and light-chain genes were digested with XhoI/SpeI and SacI/XbaI, respectively, and then were cloned into XhoI/SpeI and SacI/XbaI sites of the phagemid expression vector pComb3H (provided by Scripps Research Institute, La Jolla, Calif.). The ligation product was transformed into the competent E. coli XL-1 Blue cells (Stratagene). The pComb3H vectors containing the cDNAs of heavy- and light-chain variable regions were digested with SpeI and NheI to remove the gene III fragment for producing the soluble recombinant Fab, after which the resulting vectors were transformed into E. coli XL-1 Blue cells. Bacteria from the transformed colonies were cultured and induced by the addition of 1 mM IPTG. The recombinant Fab were obtained from the periplasm by using six consecutive freeze-thawing steps and were purified through a Ni-nitrilotriacetic acid agarose column and were dialyzed against phosphate-buffered saline.
Antibody modeling and antigen-antibody complex prediction.
Three-dimensional structures of the light- and heavy-chain variable regions of MAb E3.3 were separately constructed on the basis of the known homologous antibody structures by using the Swiss Modeling Workstation (http://expasy.hcuge.ch/swissmod/SWISS-MODEL.html). The designed model for MAb E3.3 was constructed by merging the structures of the light and heavy chains in the appropriate position on the basis of the crystal structures of a Fab fragment of MAb 8f5 (Protein Data Base [PDB] code, 1A3R). The resulting structure of MAb E3.3 was energy-minimized with the consistent valence forcefield parameterized process by using a Discover/Insight II (Biosym Co., Ltd.) on an Indigo2 Workstation (SGI Co., Ltd.). The modeled structure of the E protein of the JEV CH2195LA strain was based upon the X-ray crystallographic structure of the E protein of TBE virus (PDB code, 1SVB) by using the Swiss Modeling Workstation. Visual docking for identified epitope determinants of domain III into the combining site of the E3.3 Fab was performed after the complementary structural modeling (4, 14, 22). A selection of appropriate shape and electrostatic complementarity options between domain III and MAb E3.3 was performed by using Discovery/Insight II. The structural model of the domain III-MAb E3.3 complex was defined through optimizing the atom contact, hydrogen bonds, ion pairs, and hydrophobic interactions by using the Builder and Discovery programs on the Insight II. Thus, the model was made by using 300 steps of energy minimizing for 0.3 ps and 50,000 steps of dynamic for 50 ps in a consistent valence forcefield. The buried surface area upon complex formation, the number of contacts, and the number of hydrogen bonds between domain III and MAb E3.3 were measured by using the Insight II.
RESULTS AND DISCUSSION
MAb E3.3 binding on domain III of the JEV E protein.
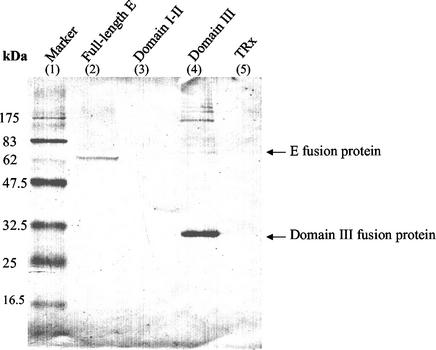
Neutralizing MAb E3.3 was previously demonstrated by comparing sensitive and resistant variants that four amino acids in the E protein at residues 85 (domain II), 306 (domain III), 331 (domain III), and 387 (domain III) are related to the antibody binding to JEV, as shown in Fig. 1 (30). In this study, we further characterized the neutralizing epitope determinants by expressing different E protein fragments in E. coli. Total protein lysates were prepared from the recombinant E. coli and were analyzed in SDS-PAGE. Western blotting of these expressed proteins demonstrated that a 75-kDa band for the full-length E fusion protein and a 32-kDa band for the domain III fusion protein specifically reacted with MAb E3.3 (Fig. 2, lanes 2 and 4). No reactivity was found for the domain I and II fusion protein in the Western blot (Fig. 2, lane 3).
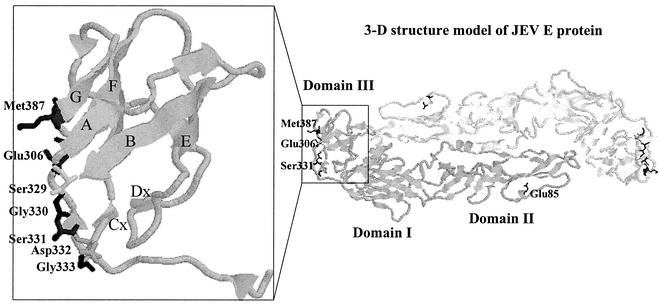
FIG. 1.
Structural model of the ectodomain of the JEV E protein. The model was built on the basis of the X-ray crystallographic structure of the E protein of TBE virus (PDB code, 1SVB) by using a Swiss Modeling Workstation. The overall illustration of the three-dimensional structure of the JEV E protein (right) and the close-up view of domain III (left) were represented by using the RasMol 2.6 Molecular Graphics Visualization Program (R. Sayle, Glaxo Wellcome).
FIG. 2.
Western blot analysis of the recombinant JEV E-Trx fusion proteins from the transformed cell lysate. The blot was probed with MAb E3.3 and was developed with an alkaline phosphatase-conjugated secondary antibody and NBT/BCIP substrates.
On the basis of the three-dimensional structure of TBE E protein (24), the structural model of JEV E protein (Fig. 1) indicates that the ABED sheets of domain III contact both the domain I and the cd-loop of domain II. It is thus possible that other residues within the domain I and II region can participate in the binding of MAb E3.3 to domain III. However, according to the structure model shown in Fig. 1, the residues at 306, 331, and 387 are all located in the loops of AAx, BCx, and FG. The neutralizing epitope of MAb E3.3 are located only on the outer lateral surface of domain III, where the three amino acids at 306, 331, and 387 are located.
Single and double site mutations on the domain III protein.
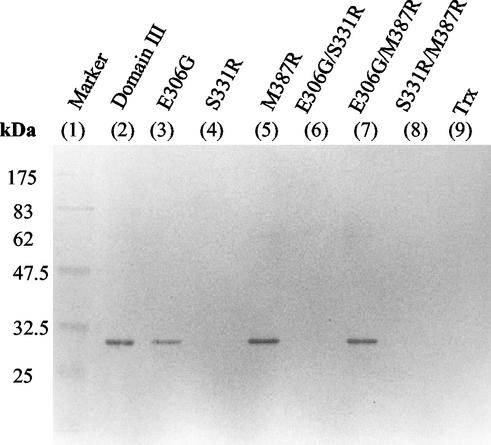
Site-directed mutagenesis was further performed by generating mutant proteins of domain III with single or double mutations at positions 306 (E→G), 331 (S→R), and 387 (M→R). Six clones were obtained to express the mutant domain III fusion proteins (E306G, S331R, M387R, E306G/S331R, E306G/M387R, and S331R/M387R). Immunoblots of these mutant proteins reacted with MAb E3.3, which shows that S331R, S331R/M387R, and E306G/S331R mutants lost their binding to MAb E3.3 (Fig. 3, lanes 4, 6, and 8). In contrast, the single site mutations of E306G and M387R and the double site mutation of E306G/M387R retained their binding activities with MAb E3.3 (Fig. 3, lanes 3, 5, and 7). The negative results with some of the mutations at residue 331 can be interpreted as identifying key amino acids, if these mutations do not affect the overall folding and structure of domain III, to constitute the neutralizing epitope for MAb E3.3.
FIG. 3.
Western blot analysis of mutant domain III with single or double site-directed mutagenesis at positions 306, 331, and 387. The wild-type and mutant domain III fusion proteins from each lysate of transformed cells reacted with MAb E3.3 and developed with an alkaline phosphatase-conjugated secondary antibody and NBT/BCIP substrates.
Alanine mutations at or near position 331 of domain III.
Subsequent mutations at or near position 331 of the E protein were further performed to determine the effects of the amino acid side chains on MAb E3.3 binding. The residues at Ser329, Ser331, and Asp332 were replaced with alanine (i.e., S329A, S331A, and D332A). Mutations on Gly330 and Gly333 were not conducted due to the lack of a side chain of Gly. The binding affinities of each mutant protein to MAb E3.3 were determined by using ELISA (Fig. 4). Compared with the binding affinity measured on wild-type domain III, D332A has the greatest decrease in the binding affinity for MAb E3.3. S331A exhibits a slightly decreased affinity and S329A gives an insignificant affinity change to MAb E3.3 (Fig. 4A, Table 1). Side chain removal of Ser329, Ser331, and Asp332 with Ala resulted in reducing the binding affinities to MAb E3.3, ranking as D332A (25.6-fold decrease) > S331A (1.6-fold decrease) > S329A (1.1-fold decrease). Therefore, the negatively charged side chain of Asp332 is also involved in constituting the functional epitope for MAb E3.3.
FIG. 4.
Relative immunoreactivities of the mutant domain III proteins to MAb E3.3 determined by ELISA. The wild-type, alanine substitution mutants (A), Ser-331 mutants (B), and Asp-332 mutants (C) were captured onto 96-well plates with rabbit anti-Trx antibodies, followed by incubation with a serial dilution of MAb E3.3. Bound MAb E3.3 was detected by using goat anti-mouse IgG-horseradish peroxidase conjugate and ABTS/H2O2 substrates. OD (405 nm), optical density at 405 nm.
TABLE 1.
Relative affinities of mutant and wild-type domain III fusion proteins for MAb E3.3 determined by ELISA
| Mutant and wild-type domain III proteins | Kd (nM)a | Affinity reduction (fold)b |
|---|---|---|
| Wild type | 0.83 ± 0.21 | |
| Alanine substitution | ||
| S329A | 0.89 ± 0.42 | 1.07 |
| S331A | 1.31 ± 0.06 | 1.58 |
| D332A | 21.22 ± 0.85 | 25.57 |
| Substitution at residue 331 | ||
| S331L | 0.93 ± 0.23 | 1.12 |
| S331Q | 0.93 ± 0.35 | 1.12 |
| S331K | >100 | >100 |
| S331R | >100 | >100 |
| S331E | >100 | >100 |
| Substitution at residue 332 | ||
| D332L | >100 | >100 |
| D332Q | 13.15 ± 0.64 | 15.84 |
| D332K | >100 | >100 |
| D332R | >100 | >100 |
| D332E | 7.55 ± 0.08 | 9.10 |
Kd values of each mutant domain III for MAb E3.3 were determined from the concentration giving 50% maximal binding in affinity ELISA assays.
The affinity reduction of each mutant domain III was calculated by dividing the Kd of the individual mutant domain III by that of wild-type domain III.
Amino acid replacements at position 331 of domain III.
Further analysis was carried out to determine whether substitution of Ser331 by other amino acids had any effect on the binding of domain III to MAb E3.3. Five mutant domain III fusion proteins that exhibited Ser331 replaced by the glutamic acid (E, negatively charged), arginine (R, positively charged), lysine (K, positively charged), leucine (L, hydrophobic), and glutamine (Q, neutral polar) were constructed. Positively and negatively charged mutations of S331E, S331K, and S331R decreased the binding affinities by more than 100-fold (Fig. 4B, Table 1), suggesting that the interposition of a negative or positive charge at position 331 is important. Since a negative or positive charge at position 331 is close to the functional residue Asp (D) at 332, it is possible that the repulsion or attraction of the charged groups between residues 331 and 332 influence the conformation of antigen-antibody recognition as previously reported (18). In contrast, hydrophobic and neutral polar mutations of S331L and S331Q only decreased the binding affinities by 1.1-fold (Fig. 4B, Table 1), suggesting that either a large polar glutaminyl group or a long hydrophobic aliphatic side chain at position 331 has no significant effect for MAb E3.3 binding.
Amino acid replacements at position 332 of domain III.
Five altered proteins at Asp332 were also obtained by using replacements with the same residues (D332E, D332K, D332R, D332Q, and D332L). The mutants of D332K (positively charged), D332R (positively charged), and D332L (hydrophobic) yielded binding affinities decreased by more than 100-fold. However, D332Q (neutral polar) and D332E (negatively charged) mutant proteins resulted in a 16- and 9-fold reduction in their binding affinities to MAb E3.3, respectively (Fig. 4C, Table 1). The order of the reduced affinity of Asp332-substituted mutants was D332K (>100-fold), D332R (>100-fold), D332L (>100-fold), D332Q (15.8-fold), and D332E (9.1-fold) (Fig. 4C, Table 1). These results indicated that the change from negative- to positive-charge residues (D332K, D332R) was the most significant factor for the domain III-MAb E3.3 interaction. However, the change to another negatively charged residue (D332E) was less significant. An explanation for this observation is that the negative charge of Glu at position 332 might involve salt bridges and hydrogen bonds with MAb E3.3. In contrast, the change to a large hydrophobic side chain (D332L) was more significant than that to a polar amide group (D332Q), suggesting that a hydrogen bond formation occurs in the interactions between domain III and the combining sites of MAb E3.3.
Computer modeling of the interactions among positions 331 and 332 of domain III with the combining sites of MAb E3.3.
For constructing the structural model of MAb E3.3 bound with domain III, the light- and heavy-chain variable-region sequences of MAb E3.3 were sequenced from the E3.3 Fab-producing bacterial clone. The initial structural model of the domain III-MAb E3.3 complex was constructed by docking Ser331 and Asp332 on domain III to fit in the combining sites of MAb E3.3 and then was optimized by using an energy minimization and molecular dynamic. The structural model of the domain III-MAb E3.3 complex (Fig. 5) shows rational contact residues within the complementary shapes between domain III and the combining sites of MAb E3.3. Ser331 and Asp332 in the BC loop of domain III were directed toward the cleft between complementary determining regions (CDR) H1 and H3. According to this structural model, Ser331 was found to be closely contacted with Arg H94 in MAb E3.3, and Asp332 was closely contacted with Tyr H27, Tyr H32, and Arg H94 (Table 2). The model also predicts that Asp332 on domain III can form two hydrogen bonds and a salt bridge with Arg H94 in MAb E3.3.
FIG. 5.
General shape complementarity of the E3.3 Fab-domain III complex is shown as a close-up view of interaction surfaces. The E3.3 epitope of domain III is indicated in the stick model, and CDRs of the E3.3 Fab are shown in the space-filling model. White, blue, green-blue, cyan, purple, violet, and magenta are present as the E3.3 epitope and CDRs H1, H2, H3, L1, L2, and L3 of E3.3 Fab, respectively.
TABLE 2.
Close contacts between JEV domain III and MAb E3.3 in the structural model (up to 4.5Å)
| Domain III residue | Antibody residue | CDRa |
|---|---|---|
| Loop AxA | ||
| Glu306 | Tyr L49 | L2 |
| Lys L53 | L2 | |
| Ala L55 | L2 | |
| Tyr H100 | H3 | |
| Lys307 | Tyr L49 | L2 |
| Tyr H100 | H3 | |
| Phe308 | Tyr L49 | L2 |
| Lys L53 | L2 | |
| Tyr H100 | H3 | |
| Ser309 | Tyr L32 | L1 |
| Tyr L49 | L2 | |
| Asp L50 | L2 | |
| Trp H99 | H3 | |
| Tyr H100 | H3 | |
| Loop BCx | ||
| Ser327 | Trp H99 | H3 |
| Tyr328 | Tyr H32 | H1 |
| Ser H96 | H3 | |
| Trp H99 | H3 | |
| Asp H101 | H3 | |
| Ser329 | Arg H94 | H3 |
| Leu H95 | H3 | |
| Ser H96 | H3 | |
| Trp H99 | H3 | |
| Tyr H100 | H3 | |
| Phe H100A | H3 | |
| Gly330 | Tyr H100 | H3 |
| Phe H100A | H3 | |
| Asp H101 | H3 | |
| Ser331 | Arg H94 | H3 |
| Asp332 | Tyr H27 | H1 |
| Tyr H32 | H1 | |
| Arg H94 | H3 | |
| Gly333 | Tyr H27 | H1 |
| Loop DxE | ||
| Ser364 | Tyr H27 | FRb H1 |
| Thr H28 | FR H1 | |
| Tyr H32 | H1 | |
| Ser365 | Asn H31 | H1 |
| Tyr H32 | H1 | |
| Ser H96 | H3 | |
| Ala366 | Asn H31 | H1 |
| Tyr H27 | FR H1 | |
| Ser H96 | H3 | |
| Asn367 | Asn H31 | H1 |
| Ser H96 | H3 | |
| Tyr H97 | H3 | |
| Trp H99 | H3 | |
| Ser368 | Tyr H97 | H3 |
| Trp H99 | H3 | |
| Loop FG | ||
| Met387 | Lys53 | L2 |
| Leu54 | L2 | |
| Ala55 | L2 | |
| Ser56 | L2 | |
| Gly388 | Lys53 | L2 |
| Asp389 | Lys53 | L2 |
CDRs of the light- and heavy-chain variable regions of MAb E3.3 were determined based on the Kabat numbering scheme (10).
FR, framework region.
Our observations that the model of the domain III-MAb E3.3 complex involves four loops of domain III interacting with four antibody CDRs and that 19 amino acids in domain III are closely in contact with 21 amino acids of MAb E3.3 (Table 2), 12 hydrogen bonds, one salt linkage, and 1,091 Å2 of total buried surface area would appear to be in close agreement with the properties of the higher-resolution crystal structures of the antibody-protein complex (2, 3). Furthermore, CDR H3 of MAb E3.3 demonstrates the most-extensive interactions with domain III and appears to fit particularly well within the BCx and DxE loops, subsequently contributing significantly to the interactions of domain III-MAb E3.3.
Mutation at Arg H94 in the recombinant Fab of MAb E3.3.
To confirm whether Asp332 in domain III forms a salt bridge pair with Arg H94 in MAb E3.3, mutations were conducted at these two sites. A mutation at Arg H94 in E3.3 Fab replaced by glutamic acid was constructed (E3.3 Fab-R94E). E3.3 Fab and E3.3 Fab-R94E were used to test their binding properties with wild-type domain III and the D332R domain III mutant. A proportion of the binding property reflects that E3.3 Fab-R94E resulted in a decreased intensity by at least 90% compared to that of E3.3 Fab (Fig. 6). However, the mutant E3.3 Fab-R94E combined with the D332R mutant (double mutant combination) reveals a substantial restoration of the binding ability for E3.3 Fab-R94E and wild-type domain III (Fig. 6). This implies that Asp332 on domain III forms a salt bridge pair with Arg H94 in MAb E3.3.
FIG. 6.
Binding capacity determined by ELISA for double mutations of Asp332 to Arg in domain III and Arg H94 to Glu in MAb E3.3. Wild-type (WT) domain III and the D332R mutant were captured onto 96-well plates with rabbit anti-Trx antibodies, followed by incubation with 800 nM E3.3 Fab and the mutant E3.3 Fab-R94E. Bound E3.3 Fab was detected by using goat anti-mouse IgG F(ab′)2-horseradish peroxidase conjugate and ABTS/H2O2 substrates. OD405nm, optical density at 405 nm.
The critical role of Arg H94 in MAb E3.3 was ascertained according to fact that the double mutant combination (E3.3 Fab-R94E and D332R mutants) restored the binding interaction in the domain III-E3.3 Fab complex (Fig. 6). Our finding was contrary to that of a previous report that Arg H94 at the base of CDR H3 usually has hydrogen bonding with Asp H101 for forming a bulged CDR H3 loop (19). However, another study reported that Arg H94 and Asp H101 in the anti-single-stranded DNA Fab did not appear to form a salt bridge (12). Therefore, the binding capacity for double mutations of domain III and E3.3 Fab led us to assume that Asp332 was the essential structural and functional epitope determinants on domain III of the JEV E protein for MAb E3.3 binding.
In conclusion, site-directed mutagenesis was performed to identify and characterize the functional epitope determinants at Ser331 and Asp332 of the JEV domain III protein. Bacterial expression of recombinant Fab antibody also confirmed the molecular interactions of Arg94 in CDR H3 with Ser331 and Asp332 on domain III. This study elucidates the detailed molecular structures of the functional epitope determinants on the JEV domain III protein, which can provide useful information for designing new flavivirus vaccines.
Acknowledgments
The authors thank C. F. Barbas, D. R. Burton, and Y.-Y. Yang for providing the pComb3H phagemid vector for Fab expression.
REFERENCES
- 1.Cecilia, D., and E. A. Gould. 1991. Nucleotide changes responsible for loss of neuroinvasiveness in Japanese encephalitis virus neutralization-resistant mutants. Virology 181:70-77. [DOI] [PubMed] [Google Scholar]
- 2.Chothia, C., A. M. Lesk, A. Tramontano, M. Levitt, S. J. Smith-Gill, G. Air, S. Sheriff, E. A. Padlan, D. Davies, W. R. Tulip, P. M. Colman, S. Spinelli, P. M. Alzari, and R. J. Poljak. 1989. Conformations of immunoglobulin hypervariable regions. Nature 342:877-883. [DOI] [PubMed] [Google Scholar]
- 3.Conte, L. L., C. Chothia, and J. Janin. 1999. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 285:2177-2198. [DOI] [PubMed] [Google Scholar]
- 4.Germain, N., K. Merienne, S. Zinn-Justin, J. C. Boulain, F. Ducancel, and A. Menez. 2000. Molecular and structural basis of the specificity of a neutralizing acetylcholine receptor-mimicking antibody, using combined mutational and molecular modeling analyses. J. Biol. Chem. 275:21578-21586. [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu, K., M. Tadano, R. Men, and C. J. Lai. 1996. Analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224:437-445. [DOI] [PubMed] [Google Scholar]
- 6.Holzmann, H., F. X. Heinz, C. W. Mandl, F. Guirakhoo, and C. Kunz. 1990. A single amino acid substitution in envelope protein E of tick-borne encephalitis virus leads to attenuation in the mouse model. J. Virol. 64:5156-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzmann, H., K. Stiasny, M. Ecker, C. Kunz, and F. X. Heinz. 1997. Characterization of monoclonal antibody-escape mutants of tick-borne encephalitis virus with reduced neuroinvasiveness in mice. J. Gen. Virol. 78:31-37. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, W. R., A. Lowe, S. Higgs, H. Reid, and E. A. Gould. 1993. Single amino acid codon changes detected in louping ill virus antibody-resistant mutants with reduced neurovirulence. J. Gen. Virol. 74:931-935. [DOI] [PubMed] [Google Scholar]
- 9.Jin, L., B. M. Fendly, and J. A. Wells. 1992. High resolution functional analysis of antibody-antigen interactions. J. Mol. Biol. 226:851-865. [DOI] [PubMed] [Google Scholar]
- 10.Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest, 5th ed. Public Health Service, National Institutes of Health, Washington, D.C.
- 11.Kang, A. S., D. R. Burton, and R. A. Lerner. 1991. Combinational immunoglobulin libraries in phage. Methods 2:11-118. [Google Scholar]
- 12.Komissarov, A. A., M. T. Marchbank, M. J. Calcutt, T. P. Quinn, and S. L. Deutscher. 1997. Site-specific mutagenesis of a recombinant anti-single-stranded DNA Fab. Role of heavy chain complementarity-determining region 3 residues in antigen interaction. J. Biol. Chem. 272:26864-26870. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus. Implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuttner, G., A. Kramer, G. Schmidtke, E. Giessmann, L. Dong, D. Roggenbuck, C. Scholz, M. Seifert, R. D. Stigler, J. Schneider-Mergener, T. Porstmann, and W. Hohne. 1999. Characterization of neutralizing anti-pre-S1 and anti-pre-S2 (HBV) monoclonal antibodies and their fragments. Mol. Immunol. 36:669-683. [DOI] [PubMed] [Google Scholar]
- 15.Lin, B., C. R. Parrish, J. M. Murray, and P. J. Wright. 1994. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 202:885-890. [DOI] [PubMed] [Google Scholar]
- 16.Mason, P. W., J. M. Dalrymple, M. K. Gentry, J. M. McCown, C. H. Hoke, D. S. Burke, M. J. Fournier, and T. L. Mason. 1989. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J. Gen. Virol. 70:2037-2049. [DOI] [PubMed] [Google Scholar]
- 17.Monath, T. P., and F. X. Heinz. 1996. Flaviviruses, p. 961-1034. In B. N. Fields, D. M. Knipe, and P. M. Howley.(ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadephia, Pa.
- 18.Monera, O. D., C. M. Kay, and R. S. Hodges. 1994. Electrostatic interactions control the parallel and antiparallel orientation of alpha-helical chains in two-stranded alpha-helical coiled-coils. Biochemistry 33:3862-3871. [DOI] [PubMed] [Google Scholar]
- 19.Morea, V., A. Tramontano, M. Rustici, C. Chothia, and A. M. Lesk. 1998. Conformations of the third hypervariable region in the VH domain of immunoglobulins. J. Mol. Biol. 275:269-294. [DOI] [PubMed] [Google Scholar]
- 20.Nowak, T., and G. Wengler. 1987. Analysis of disulfides present in the membrane proteins of the West Nile flavivirus. Virology 156:127-137. [DOI] [PubMed] [Google Scholar]
- 21.Prasad, L., S. Sharma, M. Vandonselaar, J. W. Quail, J. S. Lee, E. B. Waygood, K. S. Wilson, Z. Dauter, and L. T. Delbaere. 1993. Evaluation of mutagenesis for epitope mapping. Structure of an antibody-protein antigen complex. J. Biol. Chem. 268:10705-10708. [PubMed] [Google Scholar]
- 22.Raffai, R., K. H. Weisgraber, R. MacKenzie, B. Rupp, E. Rassart, T. Hirama, T. L. Innerarity, and R. Milne. 2000. Binding of an antibody mimetic of the human low density lipoprotein receptor to apolipoprotein E is governed through electrostatic forces. Studies using site-directed mutagenesis and molecular modeling. J. Biol. Chem. 275:7109-7116. [DOI] [PubMed] [Google Scholar]
- 23.Rauscher, S., C. Flamm, C. W. Mandl, F. X. Heinz, and P. F. Stadler. 1997. Secondary structure of the 3′-noncoding region of flavivirus genomes: comparative analysis of base pairing probabilities. RNA 3:779-791. [PMC free article] [PubMed] [Google Scholar]
- 24.Rey, F. A., F. X. Heinz, C. W. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 25.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 931-959. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadephia, Pa.
- 26.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger, J. J., S. Chapman, A. Nestorowicz, C. M. Rice, T. E. Ginocchio, and T. J. Chambers. 1996. Replication of yellow fever virus in the mouse central nervous system: comparison of neuroadapted and nonneuroadapted virus and partial sequence analysis of the neuroadapted strain. J. Gen. Virol. 77:1277-1285. [DOI] [PubMed] [Google Scholar]
- 28.Seif, S. A., K. Morita, S. Matsuo, F. Hasebe, and A. Igarashi. 1995. Finer mapping of neutralizing epitope(s) on the C-terminal of Japanese encephalitis virus E-protein expressed in recombinant Escherichia coli system. Vaccine 13:1515-1521. [DOI] [PubMed] [Google Scholar]
- 29.Winkler, G., F. X. Heinz, and C. Kunz. 1987. Characterization of a disulfide bridge-stabilized antigenic domain of tick-borne encephalitis virus structural glycoprotein. J. Gen. Virol. 68:2239-2244. [DOI] [PubMed] [Google Scholar]
- 30.Wu, S.-C., W.-C. Lian, L.-C. Hsu, and M.-Y. Liau. 1997. Japanese encephalitis virus antigenic variants with characteristic differences in neutralization resistance and mouse virulence. Virus Res. 51:173-181. [DOI] [PubMed] [Google Scholar]