Abstract
The human herpesvirus 6 (HHV-6) variant A U100 gene encodes the third component of the glycoprotein H (gH)-glycoprotein L (gL)-containing complex. Glycosidase digestion analysis showed that the U100 gene products are glycoproteins consisting of an 80-kDa protein with complex N-linked oligosaccharides and a 74-kDa protein with immature, high-mannose N-linked oligosaccharides. Based on these characteristics, we designated the U100 gene products glycoprotein Q (gQ). Only the 80-kDa form of gQ was coimmunoprecipitated with an anti-gH antibody, suggesting that the 80-kDa protein associates with the gH-gL complex in HHV-6-infected cells. Furthermore, the complex was detected in purified virions, suggesting that it may play an important role in viral entry.
Human herpesvirus 6 (HHV-6) was first isolated from the peripheral blood of patients with AIDS and lymphoproliferative disorders (5, 31). HHV-6 isolates can be classified into at least two variants, variant A (HHV-6A) and variant B (HHV-6B), based on their genetic, antigenic, and growth characteristics (1, 2). HHV-6B is the causative agent of exanthem subitum (42).
In the herpesvirus family, the envelope glycoproteins play multiple critical roles in viral infection, including attachment, penetration, cell-to-cell spread, and the envelopment and maturation of nascent viral particles. In all human and animal herpesviruses studied to date, homologs of glycoprotein H (gH) and glycoprotein L (gL) have been found (9, 14, 15, 17, 18, 21, 22, 26, 32, 34, 35, 40, 41, 43, 44). These two envelope glycoproteins, which associate to form a gH-gL complex, have been implicated as key participants in fusion events that are critical to herpesvirus infection. In general, gH is thought to be the major player in virus cell fusion while the role of gL is to serve as a chaperone, essential for the folding and transport of functional gH (7, 8, 16, 20, 27). In Epstein-Barr virus (EBV), a third viral glycoprotein, gp42, associates with the gH-gL complex (19, 38, 39). Recently, a third viral gene product of human cytomegalovirus (HCMV) was also identified as a member of the gH-gL complex (11, 12, 37). It is now known that the complex has a heterotrimeric glycoprotein structure and is composed of the products of three distinct HCMV genes: UL75 (gH), UL115(gL), and UL74(gO) (11).
Studies of the HHV-6 gH (14, 30) and gL proteins have shown them to be representative gH and gL homologs (20, 21). During their cosynthesis, gH and gL interact to form a disulfide bond-dependent gH-gL complex. Furthermore, anti-gH antibodies that mirror a neutralizing epitope on HHV-6 gH block penetration of the virus and polykaryocyte formation (6).
The products encoded by the U100 gene of HHV-6A (10) have been reported to form a complex (gp82-105) containing several polypeptides (28, 29). The gp82-105 complex has been reported to be a major component of the HHV-6 virion and a target for virus-neutralizing antibodies (28, 29). The U100 gene open reading frame is predicted to encode a 650-amino-acid protein that has a potential signal peptide and no other hydrophobic sequence long enough to be membrane spanning and includes 11 potential N-linked glycosylation sites (29). The gene has an intron-exon structure, resulting in a highly spliced mRNA transcript, and is unique to HHV-6 and HHV-7 (29, 33).
In this study, we analyzed the products encoded by the U100 gene of HHV-6A. We found that the U100 gene products are mainly composed of 80- and 78-kDa glycoproteins and, furthermore, that the 80-kDa gene product is the third glycoprotein component of the gH-gL complex in HHV-6A-infected cells.
MATERIALS AND METHODS
Cells and viruses.
T-cell lines (HSB-2 cells) were cultured in RPMI 1640 with 10% fetal calf serum. Umbilical cord blood mononuclear cells (CBMCs) were prepared as described previously (24). HHV-6A strains U1102 and GS were propagated on CBMCs, and titers of viruses were estimated using HSB-2 cells. HHV-6 cell-free virus was prepared as described previously (24). When HHV-6-infected CBMCs showed evidence of more than 80% infection by the immunofluorescent-antibody assay, the cells were lysed by freeze-thawing twice and centrifuged at 1,500 × g for 10 min. The supernatant was used as cell-free virus. Partially purified virions were isolated as follows. HSB-2 cells were infected with HHV-6, and at 72 h postinfection the cells were centrifuged at 1,500 × g for 15 min at 4°C. The supernatant from the cells was concentrated by centrifugation at 20,000 rpm for 2 h at 4°C through a 15% sucrose cushion in an SW27 rotor (Beckman). Virions were collected from the bottom.
Abs.
As described previously (3), hybridoma clones producing monoclonal antibodies (MAbs) (designated AU100-119 and AU100-124) were established from the splenocytes of mice immunized with the purified recombinant protein, AU100-N. To obtain the recombinant protein, AU100-N, for raising MAbs, the following procedure was used. A primer pair (AU100bamF [5′ ggatccACCGCAAGACTGGGCGTTATGAG-3′] and BU100R pstR [5′ ctgcag CGTGCAGGTTTCCCAATC]) was used to amplify inserts from HHV-6A cDNA (strain GS) for the amino terminus of the GS U100 gene (corresponding to the codons for amino acids 3 to 422). The PCR products were inserted into the prokaryotic expression vector pQE30 (Qiagen) at BamHI and PstI restriction sites. The resulting expression plasmid encoded the U100 gene products with an N-terminal tag containing six histidine residues (MRGSHHHHHHGS), AU100-N. The recombinant proteins were expressed in Escherichia coli and purified under denaturing conditions as specified by the manufacturer (Qiagen). A MAb of HHV-6A gH, 2E4, was generously provided by G. Campadelli-Fiume, University of Bologna, Bologna, Italy.
Mouse antisera specific for HHV-6A gH and gL were generated by immunizing mice four times with the purified proteins AgH-N (a recombinant protein corresponding to the codons for amino acids 18 to 335) and AgL (corresponding to the codons for amino acids 39 to 250), respectively. To express the recombinant proteins, the following primer pairs were used to amplify inserts from HHV-6A U1102 DNA: for AgH-N, AgHbamF (5′ggatccAGACCGTTGAACATATCGAAC-3′) and AgHsalR (5′gtcgacACATCGGTTGACAAATGAGTC), and for AgL, AgLbamF (5′ggatccGTAATAAACTGCACGAAATCC) and AbgLecoRVR (5′gatatcTTATGTGTTTCTAATCAGAAT). The PCR products were inserted into pQE30 (Qiagen). Recombinant proteins were expressed in E. coli, purified as described above, and used to raise Abs in mice.
Preparation of metabolically labeled proteins and immunoprecipitations.
HSB-2 cells were infected with HHV-6A strain U1102 at a multiplicity of infection of 0.1 or mock infected. At 72 h postinfection, the cells were incubated in methionine-free RPMI for 1 h and then radiolabeled with 20 μCi of [35S]methionine (Amersham Pharmacia Biotech) per ml for 16 h. The cells were recovered and lysed in RIPA buffer (0.01 M Tris-HCl [pH 7.4], 0.15 M NaCl, 1% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride) for 30 min on ice. After centrifugation at 200,000 × g for 1 h, the supernatants were incubated with MAb (AU100-119, AU100-124 or 2E4)-protein G-Sepharose (Amersham Pharmacia Biotech) complex at 4°C for 3 h. Immunocomplexes were washed with RIPA buffer to remove unbound proteins. Precipitated proteins were solubilized with a sample buffer (32 mM Tris-HCl [pH 6.8], 1.5% SDS, 5% glycerol, 2.5% 2-mercaptoethanol) and separated by SDS-polyacrylamide gel electrophoresis (PAGE). The gels were fixed, dried, and exposed to Kodak X-Omat films.
Glycosidase digestion.
For endoglycosidase digestion, endoglycosidase H (endo H) and peptide N-glycosidase F (PNGase F) were purchased from New England Biolabs, and immunologically precipitated materials from cells or lysed cells were resuspended in the digestion buffer and digested with endo H or PNGase F as specified by the manufacturer.
Immunoblotting.
HHV-6A strain U1102- and mock-infected cells were lysed in RIPA buffer. The lysed proteins were resolved by SDS-PAGE and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane for immunoblotting. Standard prestained molecular weight markers (Bio-Rad) were included in parallel lanes. After blocking, the membranes were incubated for 1 h with blocking buffer (10 mM Tris-HCl [pH 7.2], 0.15 M NaCl, 3% skim milk, 0.1% Tween 20) containing the MAbs or antisera. The reactive bands were visualized using a horseradish peroxidase-linked secondary conjugate and enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech).
RESULTS
Characterization of U100 gene products in HHV-6A-infected cells.
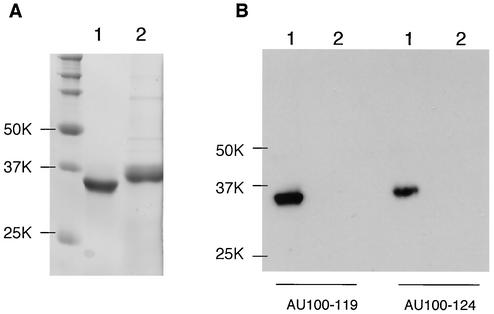
To analyze the U100 gene products, we first produced MAbs against them by immunizing mice with a prokaryotic recombinant form of the U100 protein N terminus. To confirm whether the MAbs, named AU100-119 and AU100-124, recognized the recombinant protein against which they were raised, they were used to detect the recombinant protein, AU100-N (Fig. 1A), in immunoblots. Both AU100-119 and AU100-124 reacted with AU100-N but not with a recombinant protein of HHV-6A, gH (Fig. 1B).
FIG. 1.
Immunoblotting of the prokaryotic recombinant protein. Purified recombinant proteins AU100-N (lanes 1) and AgH-N (lanes 2) were resolved by SDS-PAGE (10% polyacrylamide) and stained with Coomassie brilliant blue (A) or electrotransferred onto a PVDF membrane and the blots were reacted with the anti-HHV-6A gQ MAbs AU100-119 or AU100-124 (B). Numbers beside the panels show molecular masses in kilodaltons.
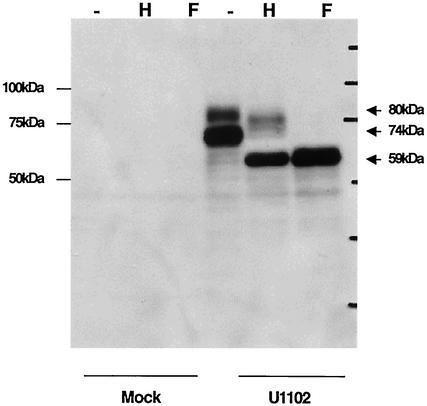
To characterize the U100 gene products in HHV-6A-infected cells by using the MAbs, HHV-6A strain U1102- or mock-infected HSB-2 cells lysates were immunoblotted by the anti-HHV-6A U100 MAbs AU100-119 or AU100-124. AU100-119 and AU100-124 specifically reacted with broadly migrating protein species: major staining was between 72 and 74 kDa and fainter staining was between 78 and 80 kDa in cells infected with either the U1102 (Fig. 2) or GS (data not shown) strain of HHV-6A.
FIG. 2.
Immunoblotting of HHV-6A-infected HSB-2 cells. HSB-2 cells were infected with HHV-6A strain U1102 at a multiplicity of infection of 0.1 or mock-infected, and the cells were lysed by RIPA buffer at 72 h postinfection. Lysates were digested with endo H (lanes H) and PNGase F (lanes F), resolved by SDS-PAGE (10% polyacrylamide) under reducing conditions, and electrotransferred onto a PVDF membrane, and the blots were reacted with the anti-gQ MAb, AU100-119. Numbers beside the panels show molecular masses in kilodaltons.
Because the HHV-6A U100 gene product is predicted to contain 11 potential N-linked glycosylation sites, we next examined whether the U100 gene products were glycosylated; this was done by treatment with endo H, which removes the immature, high-mannose asparagine (N)-linked oligosaccharides but not the mature, complex oligosaccharides, and with PNGase F, which removes both the high-mannose and complex N-linked oligosaccharides. The lysates from HHV-6A-infected cells were digested with endo H or PNGase F. Figure 2 shows the digested proteins analyzed by SDS-PAGE under reducing conditions. The 78- to 80-kDa proteins shifted in electrophoretic mobility to approximately 76 to 78 kDa after endo H treatment and to 58 to 59 kDa after PNGase F digestion, while the 72- to 74-kDa proteins shifted to 58 to 59 kDa after either endo H treatment or PNGase F digestion. Thus, the results indicate that the 78- to 80-kDa protein (80 kDa) contained complex N-linked oligosaccharides and the 72- to 74-kDa protein (74 kDa) contained immature, high-mannose N-linked oligosaccharides.
Genes encoding glycoproteins are given a “gp” designation along with the molecular mass. To maintain consistency with the naming of other herpesvirus envelope glycoproteins, we chose to designate this glycoprotein with a “g” preceding a capital letter. The last herpesvirus glycoprotein to be named was gO of HCMV (11), and there is a positional homolog gene of HCMV gO, U47, in HHV-6 (4, 11). However, the next name, gP, could be confused with the abbreviation for glycoprotein itself; therefore, we have designated the U100 gene products glycoprotein Q (gQ).
HHV-6A gQ associates with the gH-gL complex.
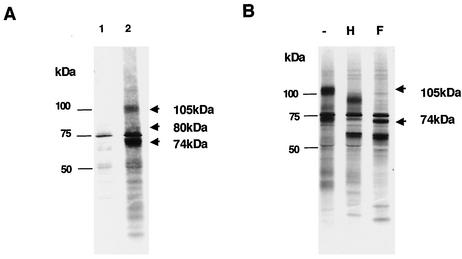
As mentioned above, the U100 gene product has been reported to exist as a gp82-105 complex (28, 29). However, in this study we could not detect a 105-kDa protein in HHV-6A (strains U1102 and GS)-infected cell lysates by immunoblotting with the MAbs against gQ. Therefore, to investigate whether the 105-kDa protein is produced by the U100 gene, lysates from metabolically labeled HHV-6A-infected cells were immunoprecipitated with AU100-119. Several proteins of about 100 to 105, 78 to 80, and 72 to 74 kDa were the major immunoprecipitates from the lysates when AU100-119 was used (Fig. 3A). To test whether the 105-kDa protein reacted with AU100-119, unlabeled precipitates were immunoblotted with this MAb. AU100-119 reacted with the 80- and 74-kDa proteins but not with the 105-kDa protein (data not shown). Therefore, we hypothesize that the 105-kDa protein was a different protein that coprecipitates with gQ.
FIG. 3.
Immunoprecipitation of metabolically labeled HHV-6A (strain U1102)-infected cells. (A) Mock-infected (lane 1) and HHV-6A-infected (lane 2) HSB-2 cells were metabolically labeled with [35S]methionine for 16 h, lysed, and immunoprecipitated with the MAb AU100-119. The proteins that were immunoprecipitated with AU100-119 were resolved by SDS-PAGE (4 to 12% Tris-glycine gel; Invitrogen). (B) The immunoprecipitates of U1102-infected cells were incubated in the absence (lane −) and presence of endo H (lane H) or PNGase F (lane F). Numbers beside the panels show molecular masses in kilodaltons.
To identify the 105-kDa protein immunoprecipitated by MAb AU100-119, the immunoprecipitates were digested with endo H and PNGase F. The 105-kDa protein shifted to 74 kDa following PNGase F digestion (Fig. 3B) but did not completely shift with endo H treatment, supporting the idea that the protein might contain N-linked complex oligosaccharides. A search was then made for predicted ORFs of the HHV-6A genome that could code for a protein(s) with a mass of 74 kDa. The best candidate was U48, which codes for gH. This glycoprotein consists of 694 amino acid residues and contains 12 potential N-linked glycosylation sites (10, 21). The mature form of HHV-6 gH has molecular masses of 98 to 102 kDa (20, 25, 30, 36). The AU100-119-immunoprecipitated lysates from HHV-6A-infected cells were then immunoblotted with a mouse anti-HHV-6A gH antiserum.
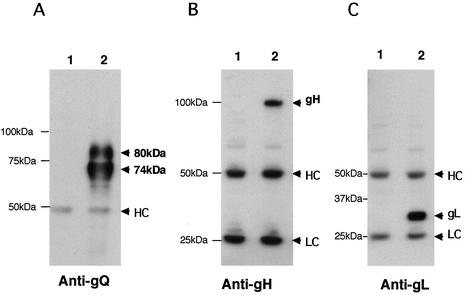
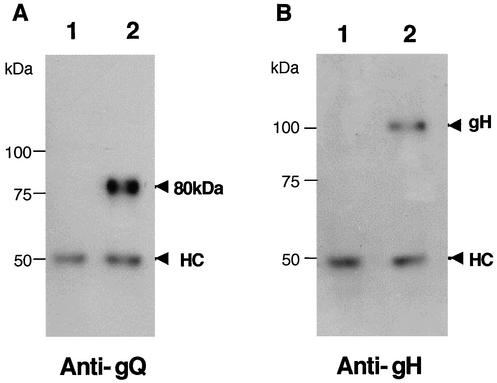
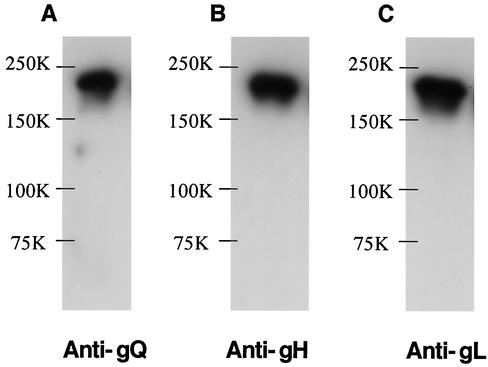
The 105-kDa protein reacted in immunoblots with the anti-gH Ab (Fig. 4B) but not with AU100-119 (Fig. 4A) or preimmune serum (data not shown), supporting the idea that the 105-kDa component corresponds to the product of the gH gene and that gQ associates with gH in HHV-6A-infected cells. In HHV-6 as well as in the other herpesviruses, gH and gL interact to form a gH-gL complex (21). Therefore, to investigate the interaction between the gH-gL complex and gQ, immunoprecipitates of HHV-6A-infected cells obtained with the AU100-119 MAb were immunoblotted with the anti-HHV-6A gL antiserum. As expected, gL was detected with the anti-gL antiserum (Fig. 4C) but not with preimmune serum (data not shown), supporting the idea that HHV-6A gQ associates with the gH-gL complex. To further elucidate the nature of the complex, lysates from HHV-6A-infected cells were immunoprecipitated with an anti-HHV-6 gH MAb, 2E4 (6). The precipitates were then immunoblotted with AU100-119. Interestingly, the AU100-119 MAb reacted only with the 80-kDa gQ protein (Fig. 5A), which was sensitive to PNGase F but not to endo H. These findings indicated that only the 80-kDa form of gQ interacts with the gH-gL complex. Accordingly, it was clear that the 80-kDa gQ could be coprecipitated with gH (Fig. 5B) and that the association among these glycoproteins was stable, even in the presence of 0.1% SDS. To examine whether the complex was disulfide bond dependent, the AU100-119-immunoprecipitated lysates from HHV-6A-infected cells were immunoblotted with MAb AU100-119 and anti-HHV-6A gH and gL antisera under nonreducing conditions. Under these conditions, MAb AU100-119 and the anti-gH and anti-gL antisera detected the same high-molecular-mass proteins, of approximately 200 to 220 -kDa, in the lysates (Fig. 6), indicating that HHV-6A gH, gL, and gQ interact to form a disulfide bond-dependent complex.
FIG. 4.
Detection of a gH-gL-gQ complex in HHV-6A (strain U1102)-infected HSB-2 cells by immunoblotting. Mock-infected (lanes 1) and U1102-infected (lanes 2) HSB-2 cell lysates were immunoprecipitated with a MAb against HHV-6 gQ (AU100-119), and the precipitated proteins were subjected to SDS-PAGE (8% polyacrylamide) under reducing conditions. The gels were electrotransferred to PVDF membranes and probed with the anti-gQ-119 MAb (A), anti-gH monospecific antiserum (B), or anti-gL monospecific antiserum (C). HC, immunoglobulin heavy chain of the immunoprecipitating antibody. LC, immunoglobulin light chain of the immunoprecipitating antibody. Numbers beside the panels show molecular masses in kilodaltons.
FIG. 5.
Detection of the 80-kDa form of gQ in the gH-gL-gQ complex of HHV-6A (strain U1102)-infected HSB-2 cells by immunoblotting. Mock-infected (lanes 1) and U1102-infected (lanes 2) HSB-2 cell lysates were immunoprecipitated with a MAb against HHV-6 gH (2E4), and the precipitated proteins were subjected to SDS-PAGE (10% polyacrylamide) under reducing conditions. The gels were then electrotransferred to PVDF membranes and probed with the anti-gQ-119 MAb (A) or anti-gH monospecific antiserum (B). HC, immunoglobulin heavy chain of the immunoprecipitating antibody. Numbers beside the panels show molecular masses in kilodaltons.
FIG. 6.
Formation of a gH-gL-gQ complex in HHV-6A-infected HSB-2 cells. HHV-6A strain U1102-infected HSB-2 cell lysates were immunoprecipitated with MAb AU100-119, and the precipitated proteins were subjected to SDS-PAGE (8% polyacrylamide) under nonreducing conditions. The gels were electrotransferred to PVDF membranes and probed with the anti-gQ MAb, AU100-119 (A) the anti-gH monospecific antiserum (B), or the anti-gL monospecific antiserum (C). Numbers beside the panels show molecular masses in kilodaltons.
gH-gL-gQ complex is present in purified HHV-6A virions.
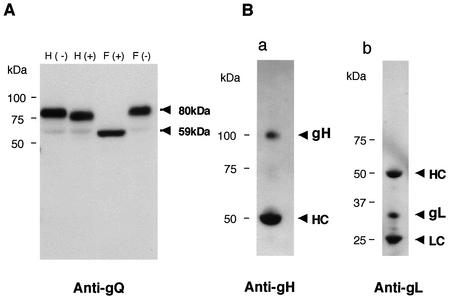
The finding that the 80-kDa form of gQ was sensitive to PNGase F but not to endo H raised the possibility that the 80-kDa gQ is packaged into HHV-6 particles. To test this possibility, the partially purified virions were lysed. The presence of gQ in the mature viral particle was verified by immunoblotting the lysate from purified HHV-6A virions with the AU100-119 MAb. Figure 7A shows that only the 80-kDa, and not the 74-kDa, form of gQ was detected in virions. To determine whether the gH-gL-gQ complex is present in virions as well as in virus-infected cells, immunoprecipitation of purified HHV-6A virions was performed with MAb AU100-119. The precipitated proteins were then immunoblotted with AU100-119 or with anti-gH or anti-gL serum. Figure 7B shows that the 105-kDa gH protein and 30-kDa gL protein derived from the immunoprecipitates were detected with the anti-gH and anti-gL sera, respectively. Based on these data, only the 80-kDa form of gQ was detected in virions, where it was associated with gH-gL.
FIG. 7.
Immunoblotting of purified HHV-6A virions. (A) Purified virions were lysed in TNE buffer (10 mM Tris-HCl [pH 7.8], 1% Nonidet P-40, 0.15 M NaCl, 1 mM EDTA), digested with endo H (lanes H) or PNGase F (lanes F), and resolved by SDS-PAGE, and the gels were electrotransferred to PVDF membranes. The blots were probed with the anti-gQ MAb, AU100-119. (B) Lysates of purified virions were immunoprecipitated with AU100-119, electroblotted, and probed with anti-gH antiserum (a) or anti-gL antiserum (b). HC, immunoglobulin heavy chain of the immunoprecipitating antibody. LC, immunoglobulin light chain of the immunoprecipitating antibody. Numbers beside the panels show molecular masses in kilodaltons.
DISCUSSION
We show here that an 80-kDa HHV-6A envelope glycoprotein associates with gH and gL to form the viral envelope complex and that this 80-kDa glycoprotein component is one of the HHV-6A U100 gene products. Thus, we have designated the HHV-6 U100 glycoprotein gQ, consistent with the nomenclature established for herpesvirus glycoproteins.
This identification of the third component of the HHV-6A gH-gL complex mirrors recent findings for the EBV gH-gL complex, which associates with a third viral gene product, gp42 (19, 38, 39), and for the HCMV gH-gL complex, which associates with gO (11, 12, 37). Interestingly, it has been shown for EBV that the gH-gL-gp42 complex is needed for the entry of the virus into B cells but not for its entry into epithelial cells (39). However, so far, it is unknown whether there is any cell specificity for the gH-gL-gQ complex and HHV-6. Comparisons of gp42 of EBV and gO of HCMV with gQ of HHV-6 do not reveal any obvious similarities. The gp42, gO, and gQ proteins may have similar functions that are important for viral entry.
It is interesting that although there is a positional and sequence homolog of the third component of the HCMVgH-gL complex in HHV-6 (named U47 or gO), the third component of the HHV-6gH-gL complex is not U47 but U100.
Recently, we showed that HHV-6A induces fusion from without in the target cells through human CD46 (23) and, furthermore, that anti-gH and anti-gB MAbs inhibit the cell-cell fusion induced by HHV-6A (23). MAbs against the gp105-82 complex neutralize HHV-6 infectivity (28, 29). Based on these data, the HHV-6A gH-gL-gQ complex may play a critical role in the life cycle of HHV-6, most probably by facilitating the viral fusion machinery. As a prerequisite to elucidating how the HHV-6 gH-gL-gQ complex participates in fusion events, it will be necessary to better understand the structure of this complex in its functional form, as it exists in the virion. Efforts are under way to determine the specific molecular functions of gQ and of the gH-gL-gQ complex in HHV-6 infection.
Interestingly, gQ positional homologs have also been reported in human HHV-7, as HHV-7 gp65 (33). In that study, gp65 was detected in HHV-7 virions and interacted with heparin and heparan sulfate proteoglycans, and gp65-specific antiserum neutralized the infectivity of virus inocula (33). These findings suggested that HHV-7 gp65 may contribute to the attachment of the virus to cell surface proteoglycans (33).
Here we investigated only gQ of HHV-6 variant A. However, the predicted HHV-6A and HHV-6B U100 gene products have 79.9% amino acid identity (4, 10, 13), which is much lower than the similarity between other glycoproteins, such as gB, gH, gL, and gM. Glycoproteins are important determinants of the specificity of the initial physical interaction between the virus and the host cell. Because the two viral variants HHV-6 A and HHV-6B display different host ranges, the gH-gL-gQ complex may confer different biological properties on the variants that cause them to target different cells.
Acknowledgments
This study was supported in part by a grant-in-aid for COE research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
We thank G. Campadelli-Fiume (University of Bologna, Bologna, Italy) for providing the 2E4 MAb and Yun Bao Jiang (Osaka University Medical School) for help in producing MAbs.
REFERENCES
- 1.Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. [DOI] [PubMed] [Google Scholar]
- 2.Chandran, B., S. Tirawatnapong, B. Pfeiffer, and D. V. Ablashi. 1992. Antigenic relationships among human herpesvirus-6 isolates. J. Med. Virol. 37:247-254. [DOI] [PubMed] [Google Scholar]
- 3.Dhepakson, P., Y. Mori, Y. B. Jiang, H. L. Huang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J. Gen. Virol. 83:847-854. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez, G., T. R. Dambaugh, F. R. Stamey, S. Dewhurst, N. Inoue, and P. E. Pellett. 1999. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J. Virol. 73:8040-8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downing, R. G., N. Sewankambo, D. Serwadda, R. Honess, D. Crawford, R. Jarrett, and B. E. Griffin. 1987. Isolation of human lymphotropic herpesviruses from Uganda. Lancet ii:390.. [DOI] [PubMed] [Google Scholar]
- 6.Foa-Tomasi, L., A. Boscaro, S. di Gaeta, and G. Campadelli-Fiume. 1991. Monoclonal antibodies to gp100 inhibit penetration of human herpesvirus 6 and polykaryocyte formation in susceptible cells. J. Virol. 65:4124-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forghani, B., L. Ni, and C. Grose. 1994. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology 199:458-462. [DOI] [PubMed] [Google Scholar]
- 8.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gompels, U. A., M. A. Craxton, and R. W. Honess. 1988. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J. Gen. Virol. 69:2819-2829. [DOI] [PubMed] [Google Scholar]
- 10.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29-51. [DOI] [PubMed] [Google Scholar]
- 11.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73:3886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isegawa, Y., T. Mukai, K. Nakano, M. Kagawa, J. Chen, Y. Mori, T. Sunagawa, K. Kawanishi, J. Sashihara, A. Hata, P. Zou, H. Kosuge, and K. Yamanishi. 1999. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J. Virol. 73:8053-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs, S. F., D. V. Ablashi, S. Z. Salahuddin, L. L. Jagodzinski, F. Wong-Staal, and R. C. Gallo. 1991. Identification of the human herpesvirus 6 glycoprotein H and putative large tegument protein genes. J. Virol. 65:5597-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 16.Keller, P. M., A. J. Davison, R. S. Lowe, M. W. Riemen, and R. W. Ellis. 1987. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology 157:526-533. [DOI] [PubMed] [Google Scholar]
- 17.Khattar, S. K., S. van Drunen Littel-van den Harke, S. K. Attah-Poku, L. A. Babiuk, and S. K. Tikoo. 1996. Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH. Virology 219:66-76. [DOI] [PubMed] [Google Scholar]
- 18.Klupp, B. G., J. Baumeister, A. Karger, N. Visser, and T. C. Mettenleiter. 1994. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J. Virol. 68:3868-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, D. X., U. A. Gompels, L. Foa-Tomasi, and G. Campadelli-Fiume. 1993. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology 197:12-22. [DOI] [PubMed] [Google Scholar]
- 21.Liu, D. X., U. A. Gompels, J. Nicholas, and C. Lelliott. 1993. Identification and expression of the human herpesvirus 6 glycoprotein H and interaction with an accessory 40K glycoprotein. J. Gen. Virol. 74:1847-1857. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch, D. J., and A. J. Davison. 1986. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 14:4281-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori, Y., T. Seya, H. L. Huang, P. Akkapaiboon, P. Dhepakson, and K. Yamanishi. 2002. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 76:6750-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori, Y., H. Yagi, T. Shimamoto, Y. Isegawa, T. Sunagawa, R. Inagi, K. Kondo, Y. Tano, and K. Yamanishi. 1998. Analysis of human herpesvirus 6 U3 gene, which is a positional homolog of human cytomegalovirus UL 24 gene. Virology 249:129-139. [DOI] [PubMed] [Google Scholar]
- 25.Okuno, T., H. Sao, H. Asada, K. Shiraki, M. Takahashi, and K. Yamanishi. 1990. Analysis of a glycoprotein of human herpesvirus 6 (HHV-6) using monoclonal antibodies. Virology 176:625-628. [DOI] [PubMed] [Google Scholar]
- 26.Pachl, C., W. S. Probert, K. M. Hermsen, F. R. Masiarz, L. Rasmussen, T. C. Merigan, and R. R. Spaete. 1989. The human cytomegalovirus strain Towne glycoprotein H gene encodes glycoprotein p86. Virology 169:418-426. [DOI] [PubMed] [Google Scholar]
- 27.Peeters, B., N. de Wind, R. Broer, A. Gielkens, and R. Moormann. 1992. Glycoprotein H of pseudorabies virus is essential for entry and cell-to- cell spread of the virus. J. Virol. 66:3888-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer, B., Z. N. Berneman, F. Neipel, C. K. Chang, S. Tirwatnapong, and B. Chandran. 1993. Identification and mapping of the gene encoding the glycoprotein complex gp82-gp105 of human herpesvirus 6 and mapping of the neutralizing epitope recognized by monoclonal antibodies. J. Virol. 67:4611-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer, B., B. Thomson, and B. Chandran. 1995. Identification and characterization of a cDNA derived from multiple splicing that encodes envelope glycoprotein gp105 of human herpesvirus 6. J. Virol. 69:3490-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian, G., C. Wood, and B. Chandran. 1993. Identification and characterization of glycoprotein gH of human herpesvirus-6. Virology 194:380-386. [DOI] [PubMed] [Google Scholar]
- 31.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 32.Scott, S. D., G. D. Smith, N. L. Ross, and M. M. Binns. 1993. Identification and sequence analysis of the homologues of the herpes simplex virus type 1 glycoprotein H in Marek's disease virus and the herpesvirus of turkeys. J. Gen. Virol. 74:1185-1190. [DOI] [PubMed] [Google Scholar]
- 33.Skrincosky, D., P. Hocknell, L. Whetter, P. Secchiero, B. Chandran, and S. Dewhurst. 2000. Identification and analysis of a novel heparin-binding glycoprotein encoded by human herpesvirus 7. J. Virol. 74:4530-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology 193:853-861. [DOI] [PubMed] [Google Scholar]
- 35.Stokes, A., D. G. Alber, J. Greensill, B. Amellal, R. Carvalho, L. A. Taylor, T. R. Doel, R. A. Killington, I. W. Halliburton, and D. M. Meredith. 1996. The expression of the proteins of equine herpesvirus 1 which share homology with herpes simplex virus 1 glycoproteins H and L. Virus Res. 40:91-107. [DOI] [PubMed] [Google Scholar]
- 36.Takeda, K., M. Haque, T. Sunagawa, T. Okuno, Y. Isegawa, and K. Yamanishi. 1997. Identification of a variant B-specific neutralizing epitope on glycoprotein H of human herpesvirus-6. J. Gen. Virol. 78:2171-2178. [DOI] [PubMed] [Google Scholar]
- 37.Theiler, R. N., and T. Compton. 2002. Distinct glycoprotein O complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells. J. Virol. 76:2890-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glyco-protein gp42 can bind to B cells but is not able to infect. J. Virol. 72:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, J., P. B. Dallas, P. A. Lyons, G. R. Shellam, and A. A. Scalzo. 1992. Identification of the glycoprotein H gene of murine cytomegalovirus. J. Gen. Virol. 73:1849-1854. [DOI] [PubMed] [Google Scholar]
- 41.Xu, J., A. A. Scalzo, P. A. Lyons, H. E. Farrell, W. D. Rawlinson, and G. R. Shellam. 1994. Identification, sequencing and expression of the glycoprotein L gene of murine cytomegalovirus. J. Gen. Virol. 75:3235-3240. [DOI] [PubMed] [Google Scholar]
- 42.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed] [Google Scholar]
- 43.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, S., L. F. Lee, N. Yanagida, and K. Nazerian. 1994. Identification and characterization of a Marek's disease virus gene homologous to glycoprotein L of herpes simplex virus. Virology 204:414-419. [DOI] [PubMed] [Google Scholar]