Abstract
The immune response to cutaneous herpes simplex virus type 1 (HSV-1) infection begins with remarkable rapidity. Activation of specific cytotoxic T lymphocytes (CTL) begins within hours of infection, even though the response within the draining lymph nodes peaks nearly 5 days later. HSV gene products are classified into three main groups, α, β, and γ, based on their kinetics and requirements for expression. In C57BL/6 mice, the immunodominant epitope from HSV is derived from glycoprotein B (gB498-505). While gB is considered a γ or “late” gene product, previous reports have indicated that some level of gene expression may occur soon after infection. Using brefeldin A as a specific inhibitor of viral antigen presentation to major histocompatibility complex class I-restricted CTL, we have formally addressed the timing of gB peptide expression in an immunologically relevant manner following infection. Presentation of gB peptide detected by T-cell activation was first observed within 2 h of infection. Comparison with another viral epitope expressed early during infection, HSV-1 ribonucleotide reductase, demonstrated that gB is presented with the same kinetics as this classical early-gene product. Moreover, this rapidity of gB expression was further illustrated via rapid priming of naïve transgenic CD8+ T cells in vivo after HSV-1 infection of mice. These results establish that gB is expressed rapidly following HSV-1 infection, at levels capable of effectively stimulating CD8+ T cells.
Herpes simplex virus type 1 (HSV-1) is a linear double-stranded DNA virus that infects epidermal or mucosal tissues while also entering local sensory neurons and establishing a latent infection. The HSV lytic cycle lasts approximately 18 h (18). Initial infection by HSV involves a complex pattern of viral gene expression with three classes of polypeptides synthesized in a sequential coordinately regulated manner (20). These classes include the immediate-early (α) proteins, which regulate viral gene expression during the lytic phase; the early (β) polypeptides, which are involved in viral DNA replication; and the late (γ) gene products, which encode structural peptides such as glycoprotein B (gB), gC, and gD, which have been implicated as important targets in adaptive immunity to HSV infection (40).
Cutaneous footpad infection of C57BL/6 mice with HSV-1 elicits an H-2Kb-restricted cytotoxic T-lymphocyte (CTL) response that is directed almost entirely to an immunodominant epitope from gB (gB498-505). This seems at odds with the general notion that in viruses with regulated gene expression, T cells are directed to earlier gene products (10). However, in HSV, the γ genes are divided into two groups (γ1 and γ2) depending on their timing of expression. Experiments using DNA synthesis blockers have determined a requirement for DNA synthesis for gC expression, but low-level production of gB and gD occurs in the presence of such blockers (33, 35). Indeed, gB and gD (γ1, termed “leaky-late” gene products) mirror the β polypeptides until DNA replication has commenced, when expression is dramatically increased (33). Moreover, it is clear from various reports that at least some expression of gB mRNA and polypeptide can be detected biochemically within 3 h of infection with HSV-1 in vitro (34, 36, 41), although it does not appear on the surface of infected cells as intact antibody-detectable protein until around 6 h after infection (50).
It is not clear from these reports, however, whether any such early low-level expression of gB is immunologically relevant. Given that we have found CTL priming within hours of infection (29), we sought to formally address the timing of T-cell- detectable gB peptide expression following HSV-1 infection. In this study, we found significant direct presentation of the major histocompatibility complex (MHC) class I-restricted gB498-505 determinant within 2 h of infection and also found that this presentation requires de novo antigen synthesis. Our results demonstrate that gB is synthesized with rapid kinetics following HSV-1 infection, at levels sufficient for direct T-cell activation and generation of an effector CTL population.
MATERIALS AND METHODS
Mice.
C57BL/6 and the gB-specific, T-cell receptor (TCR) transgenic MHC class I-restricted gBT-I (gBT-I.1) mice were obtained from the Department of Microbiology and Immunology, University of Melbourne, animal house and kept under specific-pathogen-free conditions. The gBT-I transgenic mice express a Vα2/Vβ8.1+ TCR from a CTL clone (HSV-2.3) (21) that recognizes the HSV-1 gB498-505 determinant complexed with H-2Kb. The generation and characterization of these mice are described in detail elsewhere (29).
Viruses, peptides, and cell lines.
The KOS strain of HSV-1 was propagated and subjected to titer determination using Vero cells grown in minimal essential medium (MEM) plus 10% fetal calf serum (FCS) (MEM-10). Briefly, Vero cells were infected at a multiplicity of infection of 1/300 for 3 days before sonication of the cells and supernatant and centrifugation to remove the cell debris. UV-inactivated KOS (UV-KOS) was generated by placing virus in a 30-mm petri dish 10 cm from a UV light source for 30 min. Both before and after UV treatment, a sample of virus was taken and subjected to titer determination by a PFU assay on Vero cells to confirm inactivation. The KOS-derived gB deletion mutant KΔ318 and KΔ5C viruses were propagated and subjected to titer determination using the gB-producing D6 · Vero cell line, which complements the gene inactivation (14). The KΔ5C mutant lacks amino acids 43 to 595 of gB, which encompasses the gB498-505 epitope, while the KΔ318 deletion lacks only amino acids 616 to 711 and retains the ability to synthesize the determinant (44). The WSN/NA/OVA (7) and WSN/NA/gB (4) recombinant influenza viruses expressing the ovalbumin OVA257-264 or gB498-505 epitopes, respectively, in the neuraminidase (NA) stalk were propagated on Madin-Darby canine kidney (MDCK) cells in MEM containing 4% bovine serum albumin and 1 μg of tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin per ml (Worthington Biochemicals, Lakewood, N.J.). Viral titers were obtained by an assay of plaque formation on monolayers of MDCK cells (42). The H-2Kb-restricted HSV-1 gB498-505 (SSIEFARL) (5, 17), the H-2Kb-restricted HSV-1 ribonucleotide reductase peptide RR822-829 (QTFDFGRL) (37), and the H-2Db-restricted influenza A virus nucleoprotein (NP) peptide NP366-374 (ASNENMETM) (43) were obtained from Auspep (Parkville, Australia). Primary mouse embryonic fibroblasts (PMEFs) were produced from embryonic C57BL/6 mice and maintained in Dulbecco MEM plus 10% FCS. C57BL/6 spleen-derived dendritic cells (DC) were grown in vitro in our laboratory from spleen precursors in NIH 3T3 supernatant and granulocyte-macrophage colony-stimulating factor, as described previously (46).
Adoptive Transfers, Virus Infections and Peptide Immunizations.
Lymph node cells obtained from naive gBT-I mice containing approximately 106 CD8+ T cells as determined by flow cytometry were transferred into C57BL/6 mice. At 24 h later, the mice were injected intravenously with 5 × 104 PFU of HSV-1 KOS, KΔ318, or KΔ5C or with an equal volume of UV-KOS in phosphate-buffered saline. For peptide injection, mice were immunized intravenously with 1 μg of SSIEFARL (gB498-505) peptide in phosphate-buffered saline. The spleens were removed, and single-cell suspensions were produced and counted by trypan blue exclusion prior to antibody staining for fluorescence-activated cell sorter (FACS) analysis.
Monoclonal antibodies.
Single-cell suspensions prepared from the spleen were stained with anti-CD8α-APC (53-6.7) and anti-CD69-PE (H1.2F3) obtained from PharMingen (San Diego, Calif.). Dead cells were excluded by using propidium iodide, and live events were collected on a Becton Dickinson FACSort apparatus.
Kinetic intracellular IFN-γ assays.
The kinetic intracellular gamma interferon (IFN-γ) assays were based on methods described previously (8). Antigen-experienced memory CD8+ T cells for the in vitro intracellular IFN-γ assays were generated from gBT-I splenocytes by coculturing with equal numbers of gB498-505 peptide-pulsed C57BL/6 splenocytes (pulsed at 1 μg/ml for 45 min at 37°C) in the presence of 25 U of interleukin-2 per ml for 8 to 10 days. Polyclonal NP−, gB−, or RR-specific memory T cells were generated from splenocytes from mice primed with 5 × 104 PFU of WSN/NA/gB (for anti-NP) or HSV-1 (for anti-gB and anti-RR specificity) 7 to 9 days previously. These splenocytes were cocultured for 8 to 10 days in the presence of 25 U of interleukin-2 (IL-2) per ml with irradiated C57BL/6 splenocytes pulsed with the NP366-374 (43), RR822-829 (37) or gB498-505 (5, 17) peptide, respectively, at 1 μg/ml. A total of 105 antigen-presenting cells (APC) (PMEFs or DC) were added to round-bottom 96-well tissue culture plates and infected with a relevant virus (HSV-1 KOS, UV-KOS, KΔ318, KΔ5C, WSN/NA/gB, or WSN/NA/OVA) at a multiplicity of infection of 5 for 10 min. A total of 105 T cells (gBT, NP, RR, or gB specific) were added to each well in 200 μl of RPMI 1640 plus 10% FCS, including no-virus control wells, along with 105 C57BL/6 splenocytes to minimize nonspecific activation of the T cells. Brefeldin A (BFA), diluted in phosphate-buffered saline, was added to different wells at various intervals (0, 30, 60, 90, 120, 150, 180, 240, and 360 min) to a final concentration of 5 μg/ml. The culture was allowed to continue for 2 h after the final addition of BFA to permit IFN-γ accumulation. The cells were then washed twice before being stained with anti-CD8α-APC in the plate for 30 min on ice. Excess antibody was washed off before the cells were fixed with 1% paraformaldehyde in phosphate-buffered saline at room temperature for 20 min. Following two more washes, the cells were stained for 45 to 60 min on ice with anti-IFN-γ-phycoerythrin (Pharmingen) diluted in 0.2% saponin (Calbiochem). Each well was then harvested into tubes for analysis by flow cytometry on a Becton Dickinson FACSort apparatus.
RESULTS
Kinetics of presentation of gB following infection with HSV-1.
To assess the kinetics of gB498-505-Kb appearing on the surface of virus infected cells, MHC egress from the endoplasmic reticulum (ER) to the cell surface was blocked by the addition of BFA at increasing intervals after infection (31, 49). BFA causes collapse of the Golgi network within 15 min of addition (15), as such, any surface MHC-peptide complexes must have formed and passed beyond the Golgi before the time of drug addition for cell surface expression to occur. In our assay, the surface-expressed gB498-505 was detected by adding antigen-experienced, or memory, gB-specific T cells derived from T-cell receptor (TCR) transgenic mice and measuring the appearance of intracellular IFN-γ. Since memory or effector-memory T cells express IFN-γ swiftly on restimulation (2, 24, 53), they enable the rapid and sensitive detection of antigen presentation in our system.
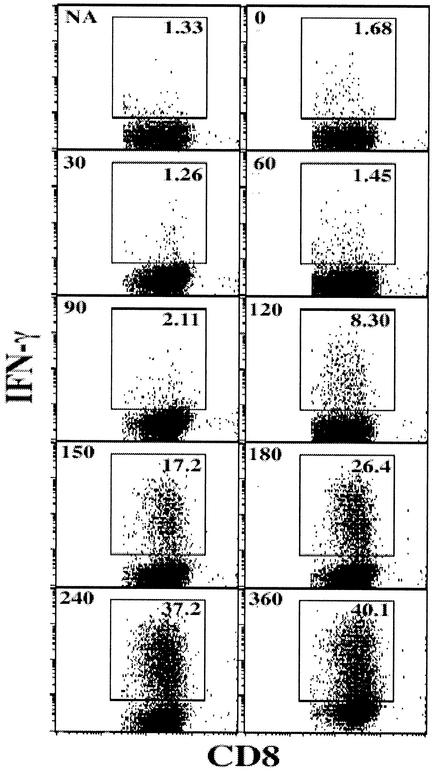
The kinetics of antigen presentation following in vitro infection with HSV-1 KOS is shown in Fig. 1. The first complexes of gB498-505-Kb had progressed beyond the “Golgi gate” between 90 and 120 min after infection with HSV-1. Since addition of BFA before this time completely blocked detectable surface expression of gB-Kb complexes in the infected cells, gB protein expression must have occurred prior to this, given that it takes about 20 min for Kb to be synthesized, loaded, and ready to leave the ER (13). A four to fivefold increase in IFN-γ+ cells was observed within a further 2 to 4 h of first detection (Fig. 1), representing increased production of peptide-MHC complexes to near maximum levels (see below).
FIG. 1.
HSV-1 gB498-505 is expressed and presented to CD8+ T cells within 2 h of infection in vitro. CD8+ T cells from gBT-I mice were cultured with HSV-1 infected PMEFs, and BFA was added at intervals. The cells were then stained with anti-CD8-APC and anti-IFN-γ-phycoerythrin antibodies for FACS analysis. Numbers in the top left of each plot represent BFA addition time (in minutes); NA, no-virus control. Numbers in the top right within the gated regions represent the percentage of IFN-γ+ CD8+ T cells. Results of one representative experiment of six are shown.
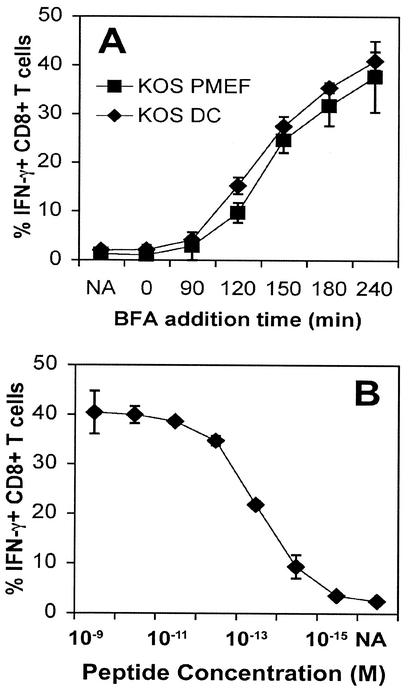
While it is understood that different peptides can be processed from the same protein with markedly different efficiencies (1, 39), it is highly likely that cells may also differ considerably in the kinetics and efficiency of peptide processing and presentation. To determine whether differences in the kinetics of gB498-505 presentation occur between cell types, we compared presentation by PMEFs which would mimic targets of effector T cells and an immature DC line (46). Both these cells are primary cultured lines. The stimulation of CD8+ T cells was identical following infection of DCs and of PMEFs. Maximal levels of T-cell activation of about 40% of total T cells in the assay, as shown in Fig. 2B, were reached within 4 h of infection of both cell lines. Together, these results indicate that gB is expressed at levels capable of activating T cells no later than 2 h following infection and that presentation reaches maximal levels around 1 to 2 h later.
FIG. 2.
Professional and nonprofessional APCs present gB498-505 with the same kinetics after infection with HSV-1. (A) The assay was performed as described in the legend to Fig. 1, with either spleen-derived DCs or PMEFs as APCs in combination with gBT T cells. (B) To determine the maximal possible levels of IFN-γ production by the readout gBT-I T cells, PMEFs were pulsed with serial dilutions of gB498-505 peptide and BFA was added after 2 h of culture. CD8+ T cells were subjected to FACS analysis for IFN-γ production. Results of one representative experiment of three are shown.
HSV-1 gB is presented with kinetics similar to early-gene products.
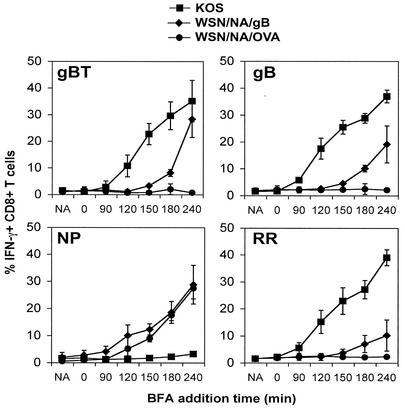
The above data suggest that presentation of gB is occurring with kinetics consistent with early-gene expression. To more clearly illustrate the rapidity of gB presentation during HSV-1 infection, we compared its expression with that of an H-2Kb-restricted epitope from the large subunit of HSV-1 ribonucleotide reductase (RR822-829) (37). RR (ICP6) is an early (β)-gene product expressed at a significant level prior to DNA synthesis. The kinetics of RR822-829 presentation was not significantly faster than that of gB498-505 (Fig. 3). This formally demonstrates that immunologically detectable presentation of gB498-505 occurs with early-antigen kinetics following HSV-1 infection in vitro.
FIG. 3.
gB is presented with the kinetics of an early-gene product. PMEFs were infected with HSV-1 KOS or either of the recombinant viruses WSN/NA/gB or WSN/NA/OVA and incubated with gBT-specific, gB-specific (gB498-505), NP-specific (NP366-374) or RR-specific (RR822-829) T cells prior to addition of BFA at increasing intervals. IFN-γ production was determined by flow cytometric analysis of gated CD8+ T cells. NA, no virus. Results of one representative experiment of four are shown.
We next wanted to compare presentation of the natural gB determinant with other forms of presentation of rapidly induced viral antigens. To this end, we made use of recombinant influenza viruses expressing either the gB498-505 epitope or an irrelevant Kb-restricted peptide from ovalbumin (OVA257-264) in the NA stalk (4, 7). Since influenza virus gene expression is not sequentially regulated, synthesis of all antigens should begin at the start of infection and presentation of gB498-505 and the endogenous nucleoprotein epitope (NP366-374) would be expected to occur concurrently. As shown in Fig. 3, presentation of the nucleoprotein epitope occurred with similar kinetics to that of gB, reinforcing the notion that gB498-505 presentation occurs very soon after infection. Notably, presentation of the gB determinant from the recombinant influenza virus was delayed compared to the presentation after infection with HSV-1. This may reflect inefficient expression or peptide generation from the recombinant NA protein.
As expected, no activation of gBT- or RR-specific T cells was seen following infection of APCs with the OVA-expressing recombinant virus (WSN/NA/OVA). However, the RR-specific T cells were stimulated to produce IFN-γ 3 to 4 h after WSN/NA/gB infection. Since we used a short-term polyclonal T-cell line specific for the subdominant RR determinant derived from HSV-1-immune mice, it is likely that a small cohort of the more dominant gB-specific T cells were present within the cultures and stimulated to produce IFN-γ. Since both the NP- and RR-specific T cells were generated from immune C57BL/6 mice rather than transgenic mice, we also examined presentation using polyclonal gB-specific cells generated in the same manner. These demonstrated activation kinetics identical to that for the transgenic gBT T cells following infection with HSV-1 KOS or either of the influenza virus recombinants (Fig. 3). Overall, these data support the contention that the gB determinant is presented in a class I-restricted manner very shortly after HSV-1 infection, with kinetics equivalent to those for an early-gene product.
De novo synthesis of viral peptide is required for in vitro presentation of gB498-505 to CD8+ T cells.
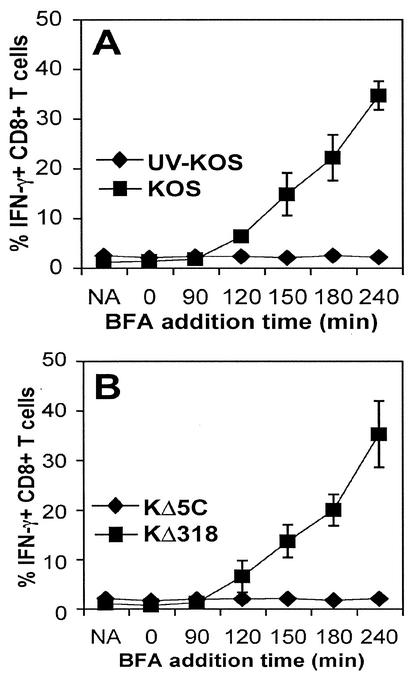
The rapidity of gB expression in vitro raised the possibility that antigen was processed directly from the introduced virions rather than that synthesized de novo following infection. To assess this, we measured IFN-γ expression following infection with UV-inactivated KOS (UV-KOS) (Fig. 4A). The inactivation of replicating input virus to less than <101 PFU/ml resulted in total abrogation of IFN-γ production. To further demonstrate the requirement for de novo synthesis of gB, we used two mutant strains of HSV-1 KOS (KΔ318 and KΔ5C) that express defective forms of the gB protein (14). Both mutants are grown and packaged in vitro in the presence of the wild-type gB protein, but they each express distinct truncated forms of gB, with only KΔ318 capable of synthesizing the gB498-505 epitope. Following infection with KΔ318 and KΔ5C, it was clear that in the absence of gB498-505 synthesis, there was no stimulation of gB-specific CTL (Fig. 4B). Thus, the swift presentation of gB following in vitro infection most probably requires de novo synthesis of viral gene products.
FIG. 4.
De novo synthesis of viral proteins is required for in vitro antigen presentation. These experiments were set up as described in the legend to Fig. 1; however, PMEFs were infected with either HSV-1 or UV-inactivated HSV-1 (A) or the recombinant HSV-1 strains KΔ318 or KΔ5C (B). BFA was added to the cultures at various intervals following infection, before flow cytometric analysis of CD8+ T cells for IFN-γ production. NA, no virus. Results reflect three independent experiments.
Direct presentation of gB induces rapid T-cell priming in vivo following HSV-1 infection.
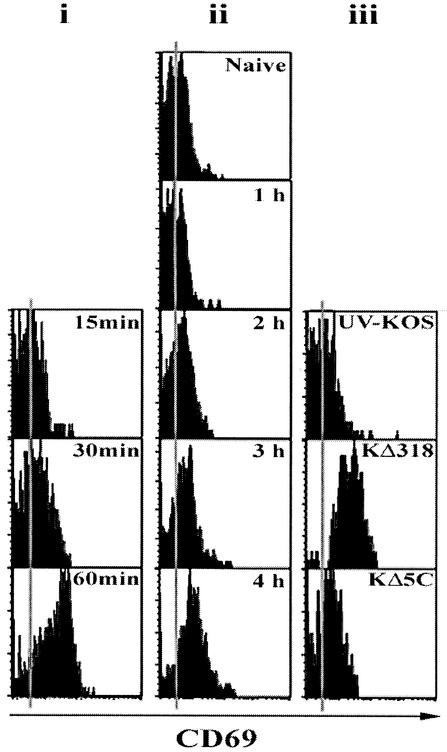
It is clear from the above data that gB is expressed rapidly on in vitro infection and is presented in a form capable of stimulating memory T cells to produce IFN-γ. We next sought to examine the relationship between gB expression and naive CD8+ T-cell priming in vivo following infection with HSV-1. To this end, CD8+ T cells from naïve transgenic mice were analyzed for expression of the early activation marker CD69 after adoptive transfer into the C57BL/6 host and intravenous virus inoculation. This marker is expressed soon after TCR engagement (51). To ascertain the delay between activation and CD69 upregulation, mice were immunized intravenously with 1 μg of gB498-505 peptide, eliminating any delays caused by the synthesis and presentation of proteins. CD69 appeared on the gBT-I CD8+ T cells between 30 and 60 min after the start of stimulation (Fig. 5i). In contrast, intravenous injection of HSV-1 KOS resulted in CD69 upregulation by 4 h of infection (Fig. 5ii). Given the delay of 30 to 60 min between TCR engagement and CD69 upregulation, this suggests that activation of naïve T cells probably occurs within 3 h of initial intravenous infection. Finally, infection with UV-KOS, KΔ5C, or KΔ318 indicated that de novo synthesis of gB is also required for upregulation of the CD69 marker on the naïve transgenic T cells (Fig. 5iii). Overall, these data demonstrate that expeditious presentation of gB498-505 on the surface of infected cells in vivo can potentially prime specific naïve CD8+ T cells.
FIG. 5.
Rapid priming of naive T cells in vivo closely mirrors in vitro antigen presentation kinetics. Mice adoptively transferred with naive gBT-I T cells were sacrificed after intravenous immunization with gB498-505 peptide (i), HSV-1 KOS (ii), or either of KΔ318, KΔ5C, or UV-inactivated KOS (iii), and CD8+ T cells were analyzed for the expression of CD69 at 15 to 60 min (i), 1 to 4 h (ii), or 6 h (iii) after immunization. Similar results were obtained in three additional experiments.
DISCUSSION
We have used BFA as a specific inhibitor of MHC class I transport from the ER (31, 49) to examine the kinetics of gB presentation following HSV-1 infection. The data suggest that gB is presented very shortly after initiation of infection, as early as expression of the early-gene products and probably only marginally behind the bulk of the immediate-early antigens. Previous work by Yewdell, Nuchtern, and coworkers (31, 48), using BFA, demonstrated presentation of NP to CD8+ T cells within 2 to 3 h of influenza virus infection, which is consistent with the results presented here.
The class I antigen-processing pathway is a coordinated intracellular mechanism. Antigenic peptides presented on the cell surface in the context of MHC class I are usually derived from proteins degraded in the cytosol. These peptides are transported to the ER by transporters associated with antigen processing, which also facilitate the binding of peptides to class I molecules (25). Following binding, a conformational change in the class I molecule is thought to induce the release and transport of the stable complex via the Golgi apparatus to the cell surface (52). The efficiency of antigen presentation appears to be limited by the rate of MHC egress to the cell surface rather than by levels of cell surface expression (11). Examination of transport rates for MHC class I indicates that marked variation occurs between different H-2 molecules (45). In particular, the average time for egress of Kb from the ER is approximately 20 min, while others (Kd, Db and Ld) may take greater than 60 min (13). More specifically, the overall rate of presentation is limited at the stages of peptide-MHC binding, or earlier during peptide generation (28), and depends directly on the amounts of processed peptides available (47). Together, these intrinsic rate-limiting factors may account for the majority of the 2-h delay observed from infection to presentation.
The rapid activation of gB-specific T cells so soon after infection has some relevance in terms of the CTL priming. The fast presentation kinetics may, in part, explain why gB can attain such a dominant status in the overall antiviral CTL response (44). Simply, it would not make sense for this to occur if this protein were expressed very late in the replication cycle. Indeed, it has been shown with vaccinia virus, which also has a regulated pattern of gene expression, that late-gene products are very poor class I-restricted antigens (10). Thus, while we do not dispute that gB levels increase at later times in the replication cycle, especially after initiation of virus DNA replication, this enhanced level of expression is not necessary for optimal presentation of this antigen to T cells.
In addition to the priming event, these data provide some insight into antigen presentation during the different phases of HSV infection. Of particular interest is the possibility that gB could be an effective target as HSV emerges from latent infection. There have been reports suggesting that CD8+ T cells are important in maintaining latency (26). At that time, it was proposed that the target of these T cells would be immediate- early gene products, and these antigens were detected during latent infection, although more recent work has found that most CD8+ T cells in the latently infected ganglion are gB specific (R. L. Hendricks, personal communication). There is also some suggestion of continuous reactivation of virus (16), which further emphasizes that lytic antigens may form appropriate targets of latency-maintaining T cells. Since gB can be expressed fairly early during lytic replication, it is a target worthy of consideration in this process, especially in experiments involving the C57BL/6 strain of mouse, where this antigen forms the dominant T-cell target (44). Although one could argue that there is a gradual change in specificity as the acute primary infection phase subsides, latency begins within days of infection of the sensory ganglia innervating the site of inoculation (9), at a time when gB-specific T-cell numbers are still at their peak. Moreover, the gB specificity appears to dominate the memory pool specific for HSV in convalescent animals (32), which further emphasizes that gB could well be an important target through all phases of infection with this virus.
Finally, these results provide some insight into the course of infection necessary to initiate T-cell responses following mucosal or cutaneous infections. It is expected that resident Langerhans' cells within these tissues migrate via afferent lymphatics to the draining lymph nodes, where they initiate the antigen-specific immune responses (3, 12, 22). These migrating APCs would have to take up exogenous viral antigen from infected cells and “cross-present” this to CD8+ T cells in secondary lymphoid organs (6, 19, 23, 38) or themselves be infected with HSV-1 for direct presentation of viral antigens to CTL. Alternatively, virus could simply drain to the lymph nodes immediately after infection where they could infect APCs, resulting in direct presentation of the gB determinant. Here we have shown that direct presentation occurs within a few hours of intravenous infection and at least 2 to 3 h earlier than we have found following cutaneous infection (30). While this delay may reflect a delay in the movement of virus to the lymph nodes, we think it simply reflects the time taken for DC migration coupled with the transfer of antigen from infected epithelium. Earlier studies on contact sensitization showed that skin-derived DCs reach draining lymph nodes within 30 min of application of the irritant (27), which fits readily within the time frame of the lag between infection and the presentation seen in draining lymph nodes (30).
In summary, we have addressed the kinetics of gB expression following HSV-1 infection both in vitro and in vivo. Via T-cell stimulation, we show that gB is presented on the surface of infected cells with the kinetics of an early-gene product. Moreover, we demonstrate rapid antigen presentation that is linked to immunologically relevant, functional priming of specific CD8+ T cells in vivo. This may have important implications for understanding the mechanisms underlying the CD8+ T-cell-mediated control of viral replication during both acute and latent phases of infection.
Acknowledgments
This work was supported in part by grants from the National Health and Medical Research Council (NHMRC) of Australia, the National Institutes of Health (AI43347 to W.R.H.), and a Howard Hughes Medical Institute International Fellowship (to W.R.H.).
We thank Satvir Tevethia for supplying the WSN/NA/gB virus and Catherine Keech for assisting with the intracellular cytokine staining assays.
REFERENCES
- 1.Anton, L. C., J. W. Yewdell, and J. R. Bennink. 1997. MHC class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J. Immunol. 158:2535-2542. [PubMed] [Google Scholar]
- 2.Bachmann, M. F., M. Barner, A. Viola, and M. Kopf. 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29:291-299. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Blaney, J. E., Jr., E. Nobusawa, M. A. Brehm, R. H. Bonneau, L. M. Mylin, T. M. Fu, Y. Kawaoka, and S. S. Tevethia. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567-9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonneau, R. H., T. M. Fu, and S. S. Tevethia. 1993. In vivo priming and activation of memory cytotoxic T-lymphocytes (CTL) by a chimeric simian virus 40 T antigen expressing an eight amino acid residue herpes simplex virus gB CTL epitope. Virology 197:782-787. [DOI] [PubMed] [Google Scholar]
- 6.Carbone, F. R., C. Kurts, S. R. Bennett, J. F. Miller, and W. R. Heath. 1998. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol. Today 19:368-373. [DOI] [PubMed] [Google Scholar]
- 7.Castrucci, M. R., S. Hou, P. C. Doherty, and Y. Kawaoka. 1994. Protection against lethal lymphocytic choriomeningitis virus (LCMV) infection by immunization of mice with an influenza virus containing an LCMV epitope recognized by cytotoxic T lymphocytes. J. Virol. 68:3486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I- restricted T cell responses to viruses. Immunity 12:83-93. [DOI] [PubMed] [Google Scholar]
- 9.Cook, M. L., and J. G. Stevens. 1973. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect. Immun. 7:272-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coupar, B. E., M. E. Andrew, G. W. Both, and D. B. Boyle. 1986. Temporal regulation of influenza hemagglutinin expression in vaccinia virus recombinants and effects on the immune response. Eur. J. Immunol. 16:1479-1487. [DOI] [PubMed] [Google Scholar]
- 11.Cox, J. H., J. W. Yewdell, L. C. Eisenlohr, P. R. Johnson, and J. R. Bennink. 1990. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science 247:715-718. [DOI] [PubMed] [Google Scholar]
- 12.Cumberbatch, M., R. J. Dearman, and I. Kimber. 1997. Langerhans cells require signals from both tumour necrosis factor alpha and interleukin 1 beta for migration. Adv. Exp. Med. Biol. 417:125-128. [DOI] [PubMed] [Google Scholar]
- 13.Degen, E., and D. B. Williams. 1991. Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility molecules. J. Cell Biol. 112:1099-1115. [DOI] [PMC free article] [PubMed]
- 14.Desai, P., F. L. Homa, S. Person, and J. C. Glorioso. 1994. A genetic selection method for the transfer of HSV-1 glycoprotein B mutations from plasmid to the viral genome: preliminary characterization of transdominance and entry kinetics of mutant viruses. Virology 204:312-322. [DOI] [PubMed] [Google Scholar]
- 15.Doms, R. W., G. Russ, and J. W. Yewdell. 1989. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 109:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke, T., F. L. Graham, K. L. Rosenthal, and D. C. Johnson. 1991. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 65:1177-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay, J., and W. T. Ruyechan. 1992. Regulation of herpes simplex virus type 1 gene expression. Curr. Top. Microbiol. Immunol. 179:1-14. [DOI] [PubMed] [Google Scholar]
- 19.Heath, W. R., and F. R. Carbone. 1999. Cytotoxic T lymphocyte activation by cross-priming. Curr. Opin. Immunol. 11:314-318. [DOI] [PubMed] [Google Scholar]
- 20.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, C. M., S. C. Cose, and F. R. Carbone. 1997. Evidence for cooperation between TCR V region and junctional sequences in determining a dominant cytotoxic T lymphocyte response to herpes simplex virus glycoprotein B. Int. Immunol. 9:1319-1328. [DOI] [PubMed] [Google Scholar]
- 22.Kupiec-Weglinski, J. W., J. M. Austyn, and P. J. Morris. 1988. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J. Exp. Med. 167:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurts, C., M. Cannarile, I. Klebba, and T. Brocker. 2001. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 166:1439-1442. [DOI] [PubMed] [Google Scholar]
- 24.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehner, P. J., and P. Cresswell. 1996. Processing and delivery of peptides presented by MHC class I molecules. Curr. Opin. Immunol. 8:59-67. [DOI] [PubMed] [Google Scholar]
- 26.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macatonia, S. E., S. C. Knight, A. J. Edwards, S. Griffiths, and P. Fryer. 1987. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J. Exp. Med. 166:1654-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montoya, M., and M. Del Val. 1999. Intracellular rate-limiting steps in MHC class I antigen processing. J. Immunol. 163:1914-1922. [PubMed] [Google Scholar]
- 29.Mueller, S. N., W. Heath, J. D. McLain, F. R. Carbone, and C. M. Jones. 2002. Characterization of two TCR transgenic mouse lines specificfor herpes simplex virus. Immunol. Cell Biol. 80:156-163. [DOI] [PubMed] [Google Scholar]
- 30.Mueller, S. N., C. M. Jones, C. M. Smith, W. R. Heath, and F. R. Carbone. 2002. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuchtern, J. G., J. S. Bonifacino, W. E. Biddison, and R. D. Klausner. 1989. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature 339:223-226. [DOI] [PubMed] [Google Scholar]
- 32.Nugent, C. T., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Analysis of the cytolytic T-lymphocyte response to herpes simplex virus type 1 glycoprotein B during primary and secondary infection. J. Virol. 68:7644-7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peake, M. L., P. Nystrom, and L. I. Pizer. 1982. Herpesvirus glycoprotein synthesis and insertion into plasma membranes. J. Virol. 42:678-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pederson, N. E., S. Person, and F. L. Homa. 1992. Analysis of the gB promoter of herpes simplex virus type 1: high-level expression requires both an 89-base-pair promoter fragment and a nontranslated leader sequence. J. Virol. 66:6226-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rafield, L. F., and D. M. Knipe. 1984. Characterization of the major mRNAs transcribed from the genes for glycoprotein B and DNA-binding protein ICP8 of herpes simplex virus type 1. J. Virol. 49:960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvucci, L. A., R. H. Bonneau, and S. S. Tevethia. 1995. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J. Virol. 69:1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigal, L. J., S. Crotty, R. Andino, and K. L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398:77-80. [DOI] [PubMed] [Google Scholar]
- 39.Sijts, A. J., A. Neisig, J. Neefjes, and E. G. Pamer. 1996. Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies. J. Immunol. 156:683-692. [PubMed] [Google Scholar]
- 40.Simmons, A., D. Tscharke, and P. Speck. 1992. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr. Top. Microbiol. Immunol. 179:31-56. [DOI] [PubMed] [Google Scholar]
- 41.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 42.Tannock, G. A., J. A. Paul, and R. D. Barry. 1984. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect. Immun. 43:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Townsend, A. R., J. Rothbard, F. M. Gotch, G. Bahadur, D. Wraith, and A. J. McMichael. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44:959-968. [DOI] [PubMed] [Google Scholar]
- 44.Wallace, M. E., R. Keating, W. R. Heath, and F. R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 73:7619-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams, D. B., S. J. Swiedler, and G. W. Hart. 1985. Intracellular transport of membrane glycoproteins: two closely related histocompatibility antigens differ in their rates of transit to the cell surface. J. Cell Biol. 101:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winzler, C., P. Rovere, M. Rescigno, F. Granucci, G. Penna, L. Adorini, V. S. Zimmermann, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, B., and T. J. Braciale. 1995. Characteristics of ATP-dependent peptide transport in isolated microsomes. J. Immunol. 155:3889-3896. [PubMed] [Google Scholar]
- 48.Yewdell, J., C. Lapham, I. Bacik, T. Spies, and J. Bennink. 1994. MHC-encoded proteasome subunits LMP2 and LMP7 are not required for efficient antigen presentation. J. Immunol. 152:1163-1170. [PubMed] [Google Scholar]
- 49.Yewdell, J. W., and J. R. Bennink. 1989. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science 244:1072-1075. [DOI] [PubMed] [Google Scholar]
- 50.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama, W. M., F. Koning, P. J. Kehn, G. M. Pereira, G. Stingl, J. E. Coligan, and E. M. Shevach. 1988. Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J. Immunol. 141:369-376. [PubMed] [Google Scholar]
- 52.York, I. A., and K. L. Rock. 1996. Antigen processing and presentation by the class I major histocompatibility complex. Annu. Rev. Immunol. 14:369-396. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann, C., A. Prevost-Blondel, C. Blaser, and H. Pircher. 1999. Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences. Eur. J. Immunol. 29:284-290. [DOI] [PubMed] [Google Scholar]