Abstract
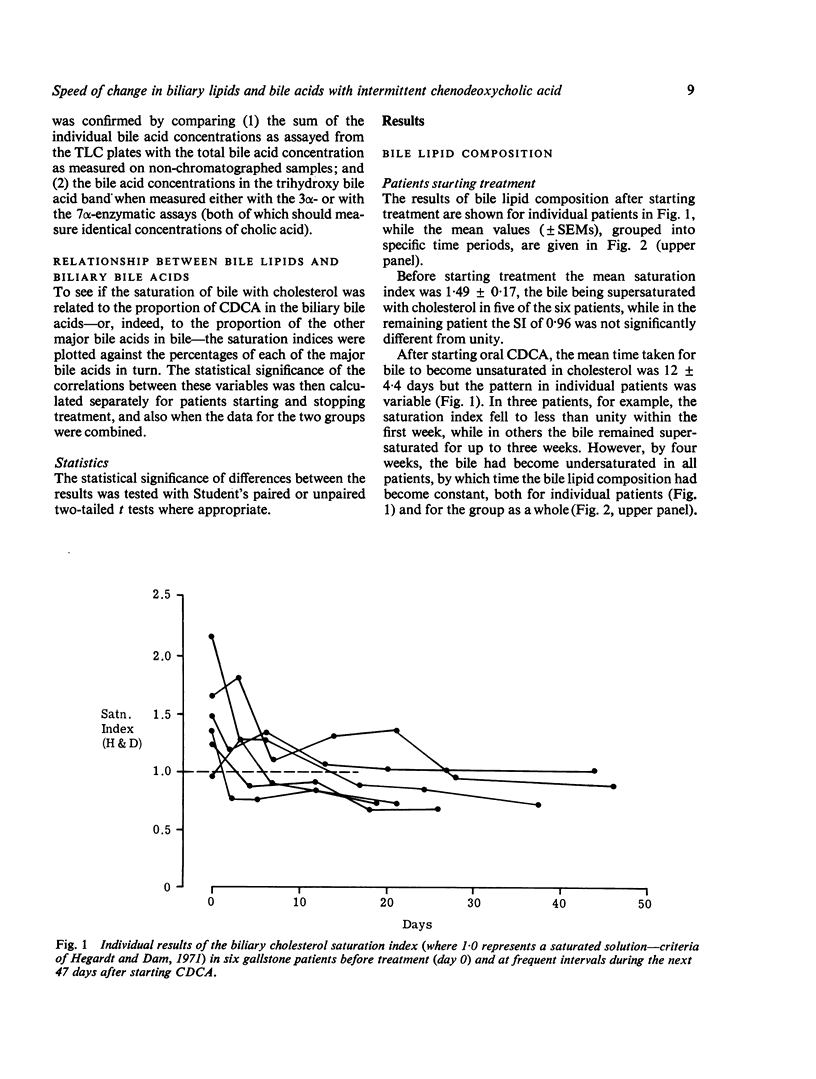
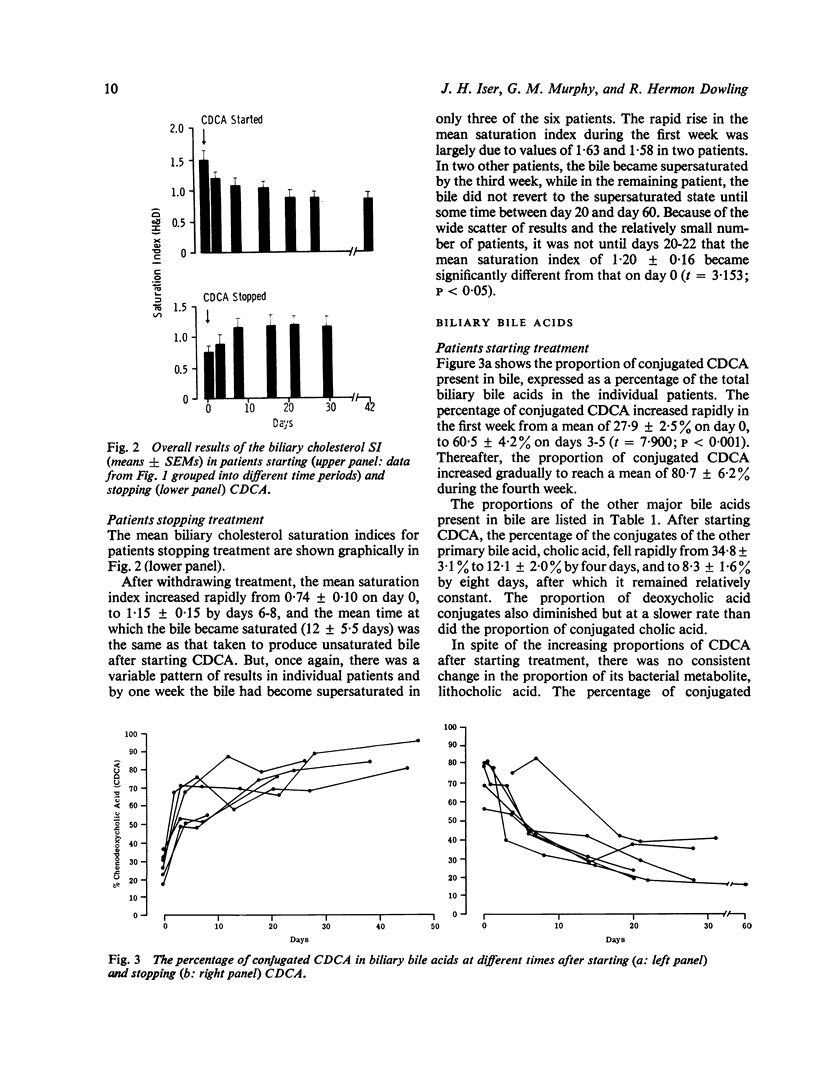
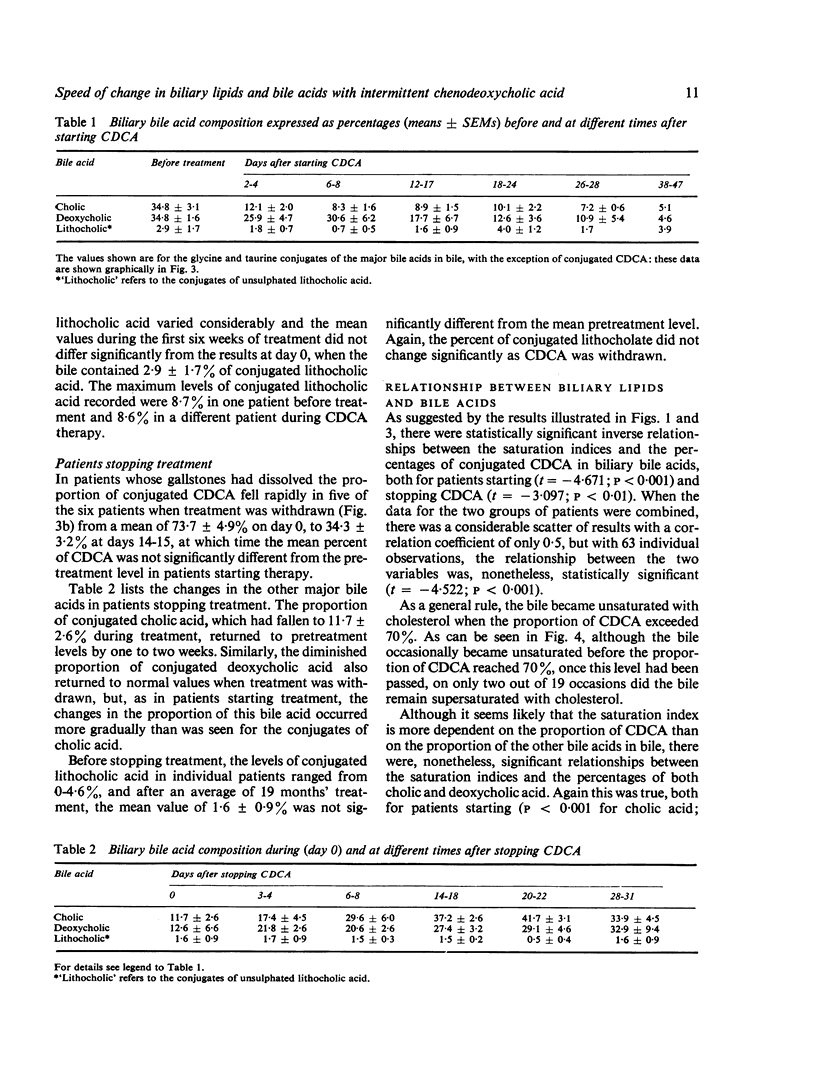
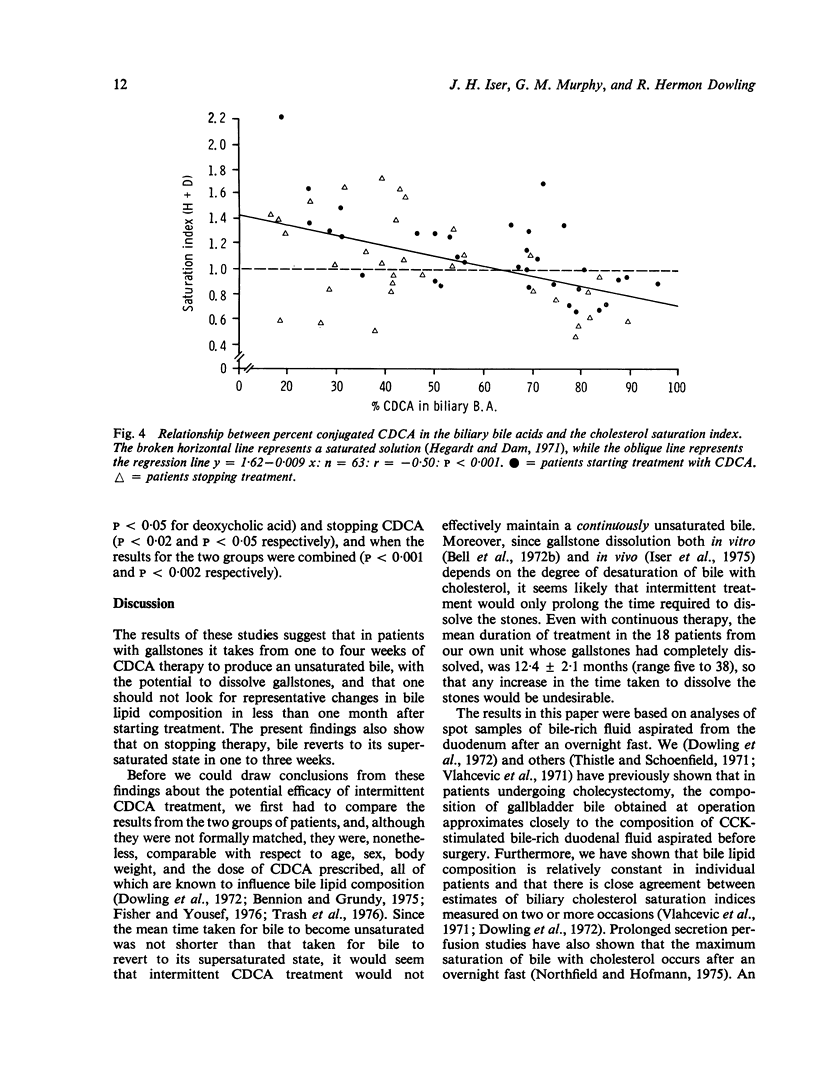
To see whehter intermittent chenodeoxycholic acid (CDCA) therapy is a potential alternative to continous treatment for gallstone dissolution, the speed of change in bile lipid composition was studied after starting and stopping CDCA therapy. In addition, the relationship between bile lipid composition and the proportions of the bile acids was examined. Bile-rich duodenal fluid was collected twice in the first week and then at approximately weekly intervals for four to six weeks, from six gallstone patients starting 13-15 mg CDCA.kg BW-1 day-1 and from another group of six patients whose treatment was stopped after gallstone dissolution. After starting treatment, the mean biliary cholesterol saturation index (based on criteria of Hegardt and Dam, 1971) decreased from 1-49 +/- SEM 0-17 to 0-92 +/- 0-13 at three weeks and 0-88 +/- 0-10 at four weeks, by which time bile lipid composition had become relatively constant. In patients whose treatment was stopped, bile reverted to its supersaturated state within one week, changing from an on-treatment mean saturation index of 0-74 +/- 0-10 to 1-15 +/- 0-15 in six to eight days after withdrawing CDCA. The proportion of conjugated CDCA in the biliary bile acids increased from 27-9 +/- 2-5% to 60-5 +/- 4-2% within four days and to 80-7 +/- 6-2% by four weeks after starting CDCA. When treatment was stopped, the proportion of CDCA reverted to pretreatment levels by two to three weeks. The saturation index was significantly related (P less than 0-001) to the percent of conjugated CDCA present, such that when the proportion of CDCA exceeded 70%, bile was almost invariably unsaturated. Since the mean time taken for bile to become unsaturated was not shorter than the time taken for bile to revert to its supersaturated state, it seems that intermittent treatment would not be adequate to maintain an unsaturated bile and is, therefore, unlikely to be as effective as continuous treatment in dissolving gallstones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. D., Bennion L. J., Duane W. C., Grundy S. M. Effects of low dose chenodeoxycholic acid feeding on biliary lipid metabolism. Gastroenterology. 1975 Feb;68(2):326–334. [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. D., Sutor D. J., Whitney B., Dowling R. Factors influencing human gallstone dissolution in monkey, dog, and human bile. Gut. 1972 Oct;13(10):836–836. [PubMed] [Google Scholar]
- Bell G. D., Whitney B., Dowling R. H. Gallstone dissolution in man using chenodeoxycholic acid. Lancet. 1972 Dec 9;2(7789):1213–1216. doi: 10.1016/s0140-6736(72)92266-0. [DOI] [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975 Oct;56(4):996–1011. doi: 10.1172/JCI108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmelgaard A., Pedersen L. Bile acids in bile during long-term chenodeoxycholic acid treatment. Scand J Gastroenterol. 1976;11(2):161–165. [PubMed] [Google Scholar]
- Bruusgaard A. Quantitative determination of the major 3-hydroxy bile acids in biological material after thin-layer chromatographic separation. Clin Chim Acta. 1970 Jun;28(3):495–504. doi: 10.1016/0009-8981(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Carey M. C. Editorial: Cheno and urso: what the goose and the bear have in common. N Engl J Med. 1975 Dec 11;293(24):1255–1257. doi: 10.1056/NEJM197512112932412. [DOI] [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W., Coffin S. B. Metabolism of lithocholate in healthy man. II. Enterohepatic circulation. Gastroenterology. 1975 Jul;69(1):67–76. [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W. Metabolism of lethocholate in healthy man. I. Biotransformation and biliary excretion of intravenously administered lithocholate, lithocholylglycine, and their sulfates. Gastroenterology. 1975 Jul;69(1):59–66. [PubMed] [Google Scholar]
- Coyne M. J., Bonorris G. G., Chung A., Goldstein L. I., Lahana D., Schoenfield L. J. Treatment of gallstones with chenodeoxycholic acid and phenobarbital. N Engl J Med. 1975 Mar 20;292(12):604–607. doi: 10.1056/NEJM197503202921202. [DOI] [PubMed] [Google Scholar]
- Danzinger R. C., Hofmann A. F., Thistle J. L., Schoenfield L. J. Effect of oral chenodeoxycholic acid on bile acid kinetics and biliary lipid composition in women with cholelithiasis. J Clin Invest. 1973 Nov;52(11):2809–2821. doi: 10.1172/JCI107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzinger R. G., Hofmann A. F., Schoenfield L. J., Thistle J. L. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972 Jan 6;286(1):1–8. doi: 10.1056/NEJM197201062860101. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Bell G. D., White J. Lithogenic bile in patients with ileal dysfunction. Gut. 1972 Jun;13(6):415–420. doi: 10.1136/gut.13.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. I., Bonorris G. G., Coyne M. J., Schoenfield L. J. Persistent effects of chenodeoxycholic acid on biliary lipids in the hamster. J Lab Clin Med. 1975 Jun;85(6):1032–1041. [PubMed] [Google Scholar]
- Gregg J. A. New solvent systems for thin-layer chromatography of bile acids. J Lipid Res. 1966 Jul;7(4):579–581. [PubMed] [Google Scholar]
- Haslewood G. A., Murphy G. M., Richardson J. M. A direct enzymic assay for 7 -hydroxy bile acids and their conjugates. Clin Sci. 1973 Jan;44(1):95–98. doi: 10.1042/cs0440095. [DOI] [PubMed] [Google Scholar]
- Hegardt F. G., Dam H. The solubility of cholesterol in aqueous solutions of bile salts and lecithin. Z Ernahrungswiss. 1971 Apr;10(3):223–233. doi: 10.1007/BF02020933. [DOI] [PubMed] [Google Scholar]
- Iser J. H., Dowling H., Mok H. Y., Bell G. D. Chenodeoxycholic acid treatment of gallstones. A follow-up report and analysis of factors influencing response to therapy. N Engl J Med. 1975 Aug 21;293(8):378–383. doi: 10.1056/NEJM197508212930804. [DOI] [PubMed] [Google Scholar]
- LaRusso N. F., Hoffman N. E., Hofmann A. F., Northfield T. C., Thistle J. L. Effect of primary bile acid ingestion on bile acid metabolism and biliary lipid secretion in gallstone patients. Gastroenterology. 1975 Dec;69(6):1301–1314. [PubMed] [Google Scholar]
- Low-Beer T. S., Pomare E. W. Can colonic bacterial metabolites predispose to cholesterol gall stones? Br Med J. 1975 Feb 22;1(5955):438–440. doi: 10.1136/bmj.1.5955.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low-Beer T. S., Tyor M. P., Lack L. Effects of sulfation of taurolithocholic and glycolithocholic acids on their intestinal transport. Gastroenterology. 1969 Apr;56(4):721–726. [PubMed] [Google Scholar]
- Mok H. Y., Bell G. D., Dowling R. H. Effect of different doses of chenodeoxycholic acid on bile-lipid composition and on frequency of side-effects in patients with gallstones. Lancet. 1974 Aug 3;2(7875):253–257. doi: 10.1016/s0140-6736(74)91415-9. [DOI] [PubMed] [Google Scholar]
- Mok H. Y., Perry P. M., Dowling R. H. The control of bile acid pool size: effect of jejunal resection and phenobarbitone on bile acid metabolism in the rat. Gut. 1974 Apr;15(4):247–253. [PMC free article] [PubMed] [Google Scholar]
- Northfield T. C., Hofmann A. F. Biliary lipid output during three meals and an overnight fast. I. Relationship to bile acid pool size and cholesterol saturation of bile in gallstone and control subjects. Gut. 1975 Jan;16(1):1–11. doi: 10.1136/gut.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northfield T. C., LaRusso N. F., Hofmann A. F., Thistle J. L. Biliary lipid output during three meals and an overnight fast. II. Effect of chenodeoxycholic acid treatment in gallstone subjects. Gut. 1975 Jan;16(1):12–17. doi: 10.1136/gut.16.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. H., Bolt M. G. Bile acid sulfates. I. Synthesis of lithocholic acid sulfates and their identification in human bile. J Lipid Res. 1971 Nov;12(6):671–679. [PubMed] [Google Scholar]
- Pomare E. W., Heaton R. W., Lowbeer T. S., White C. Proceedings: Effect of wheat bran on bile salt metabolism and bile composition. Gut. 1974 Oct;15(10):824–825. [PubMed] [Google Scholar]
- Salen G., Tint G. S., Eliav B., Deering N., Mosbach E. H. Increased formation of ursodeoxycholic acid in patients treated with chenodeoxycholic acid. J Clin Invest. 1974 Feb;53(2):612–621. doi: 10.1172/JCI107596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehl A., Raedsch R., Kommerell B. Increased sulfation of lithocholate in patients with cholesterol gallstones during chenodeoxycholate treatment. Digestion. 1975;12(2):105–110. doi: 10.1159/000197660. [DOI] [PubMed] [Google Scholar]
- Thistle J. L., Schoenfield L. J. Induced alterations in composition of bile of persons having cholelithiasis. Gastroenterology. 1971 Oct;61(4):488–496. [PubMed] [Google Scholar]
- Thomas P. J., Hofmann A. F. Letter: A simple calculation of the lithogenic index of bile: expressing biliary lipid composition on rectangular coordinates. Gastroenterology. 1973 Oct;65(4):698–700. [PubMed] [Google Scholar]
- Trash D. B., Ross P. E., Murison J., Bouchier I. A. Proceedings: The influence of age on cholesterol saturation of bile. Gut. 1976 May;17(5):394–394. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Juttijudata P., Swell L. Bile-rich duodenal fluid as an indicator of biliary lipid composition and its applicability to detection of lithogenic bile. Am J Dig Dis. 1971 Sep;16(9):797–802. doi: 10.1007/BF02239307. [DOI] [PubMed] [Google Scholar]


