Abstract
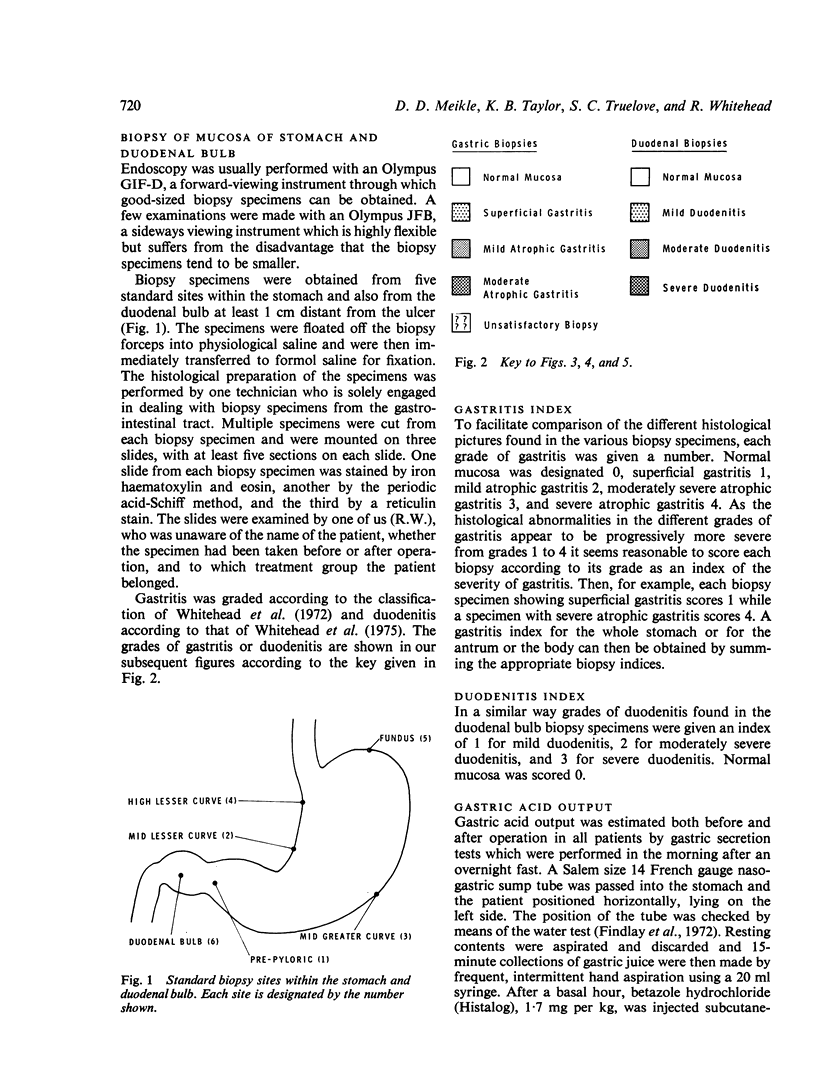
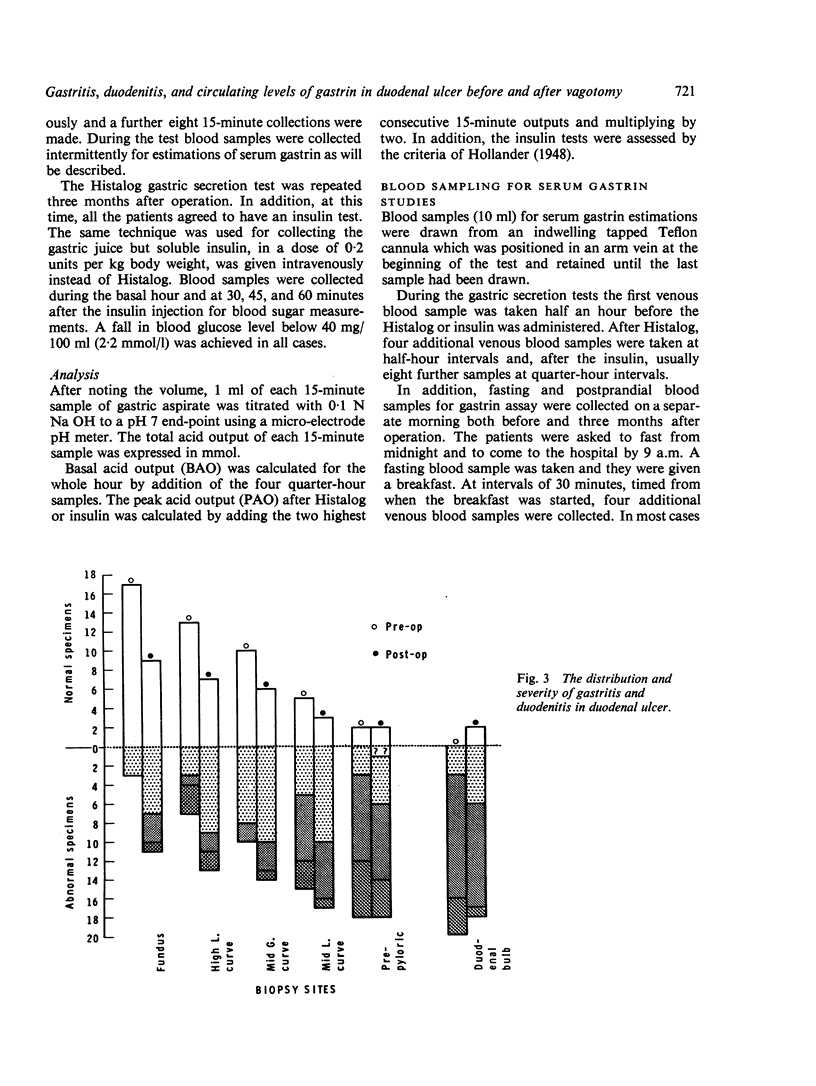
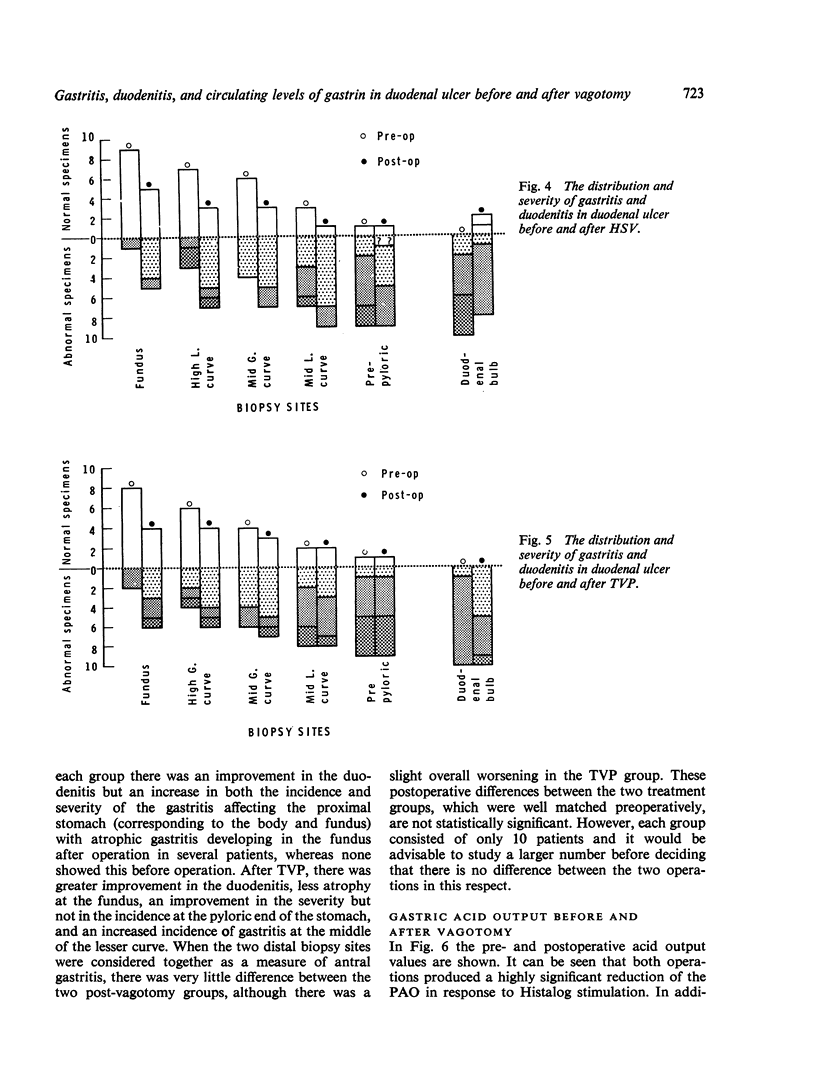
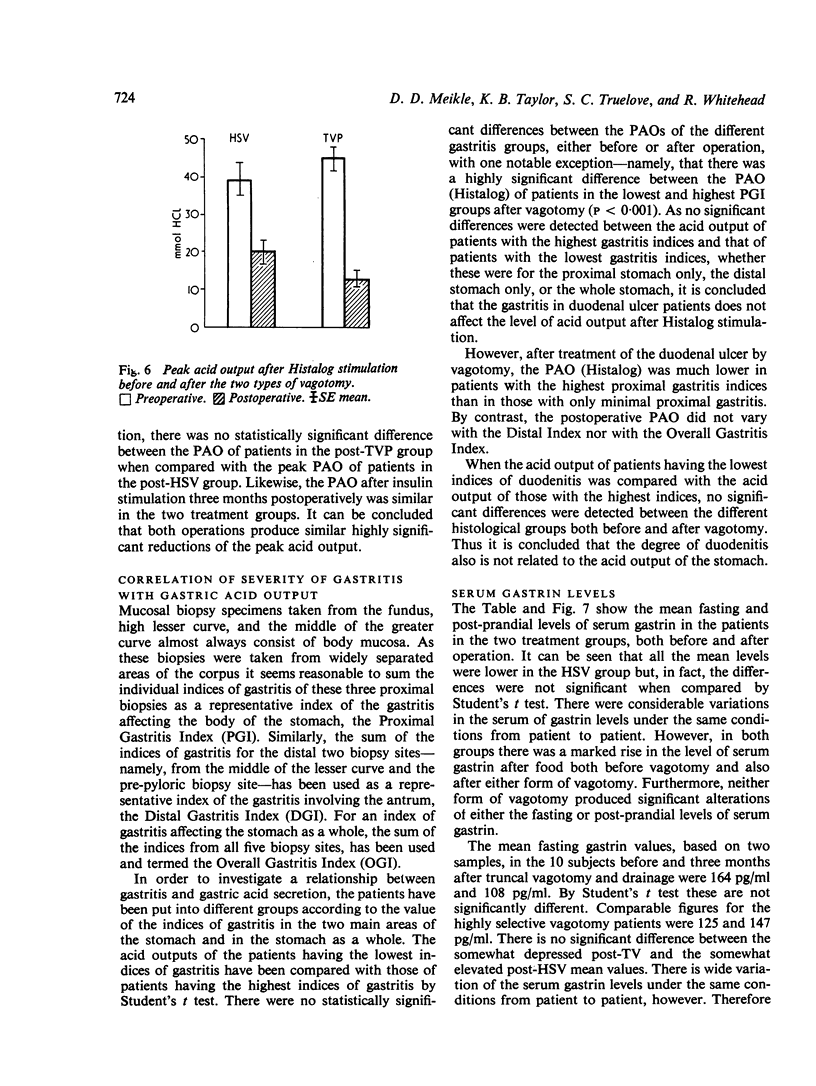
Biopsy specimens have been taken from five standard sites in the stomach and from the duodenal bulb in order to investigate the association of gastritis and duodenitis with duodenal ulcer. Twenty patients with chronic duodenal ulcer were investigated in this manner and in addition had gastric secretion tests and a radio-immune assay of serum gastrin under differing conditions. The patients were then treated either by a truncal vagotomy and pyloroplasty (TVP) or by a highly selective vagotomy without a drainage procedure (HSV). All the investigations were repeated three months postoperatively. Duodenal ulcer was usually associated with gastriitis, although this varied in extent and severity from patient to patient. In nearly all the patients, gastritis was present at the pyloric end of the stomach and along the lesser curve. In more than half of the patients, gastritis was also present in the body of the stomach but the fundus was usually spared. Chronic duodenitis was found in the duodenal bulb in all these patients. After vagotomy there was a marked increase in both the extent and severity of the proximal gastritis in both treatment groups but the distal gastritis remain almost unchanged. There was little change in the incidence of duodenitis after vagotomy but its severity was lessened. No correlation was found between the peak acid output (PAO) in response to Histalog and the severity of the gastritis or the duodenitis either before or after operation, with one exception. The postoperative PAO was significantly less in those patients who developed a severe proximal gastritis after vagotomy. No relationship was found between the severity of the distal gastritis and the levels of serum gastrin. No correlation was found between either the basal or peak acid output and the corresponding serum gastrin levels before or after vagotomy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. F., Cox A. G., Kennedy E. H., Thompson J. Effect of medical and surgical vagotomy on intrinsic factor secretion. Br Med J. 1967 Aug 19;3(5563):473–476. doi: 10.1136/bmj.3.5563.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H. D., Reeder D. D., Thompson J. C. Effect of truncal vagotomy with pyloroplasty or with antrectomy on food-stimulated gastrin values in patients with duodenal ulcer. Surgery. 1973 Oct;74(4):580–586. [PubMed] [Google Scholar]
- Bitsch V., Christiansen P. M., Faber V., Rodbro P. Gastric secretory patterns before and after vagotomy. Lancet. 1966 Jun 11;1(7450):1288–1291. doi: 10.1016/s0140-6736(66)91200-1. [DOI] [PubMed] [Google Scholar]
- COX A. G., BOND M. R., PODMORE D. A., ROSE D. P. ASPECTS OF NUTRITION AFTER VAGOTOMY AND GASTROJEJUNOSTOMY. Br Med J. 1964 Feb 22;1(5381):465–469. doi: 10.1136/bmj.1.5381.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Yip B. S., Jordan P. H., Jr Radio-immunoassay of gastrin using antiserum to porcine gastrin. Proc Soc Exp Biol Med. 1970 Jun;134(2):380–385. doi: 10.3181/00379727-134-34796. [DOI] [PubMed] [Google Scholar]
- Findlay J. M., Prescott R. J., Sircus W. Comparative evaluation of water recovery test and fluoroscopic screening in positioning a nasogastric tube during gastric secretory studies. Br Med J. 1972 Nov 25;4(5838):458–461. doi: 10.1136/bmj.4.5838.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear M. W., Truelove S. C., Whitehead R. Gastric ulcer and gastritis. Gut. 1971 Aug;12(8):639–645. doi: 10.1136/gut.12.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- McGuigan J. E., Trudeau W. L. Serum gastrin levels before and after vagotomy and pyloroplasty or vagotomy and antrectomy. N Engl J Med. 1972 Jan 27;286(4):184–188. doi: 10.1056/NEJM197201272860404. [DOI] [PubMed] [Google Scholar]
- Rosenquist G. L., Holmquist A. M. The specificity of antibodies directed to porcine gastrin. Immunochemistry. 1974 Aug;11(8):489–494. doi: 10.1016/0019-2791(74)90120-7. [DOI] [PubMed] [Google Scholar]
- Sanders G. B., Mecray P. M. PSEUDOGASTRITIS OF OPERATIVE ORIGIN. Ann Surg. 1941 Dec;114(6):986–996. doi: 10.1097/00000658-194112000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalsberg H., Taksdal S. Stomach cancer following gastric surgery for benign conditions. Lancet. 1971 Nov 27;2(7735):1175–1177. doi: 10.1016/s0140-6736(71)90489-2. [DOI] [PubMed] [Google Scholar]
- Stern D. H., Walsh J. H. Gastrin release in postoperative ulcer patients: evidence for release of duodenal gastrin. Gastroenterology. 1973 Mar;64(3):363–369. [PubMed] [Google Scholar]
- Trudeau W. L., McGuigan J. E. Serum gastrin levels in patients with peptic ulcer disease. Gastroenterology. 1970 Jul;59(1):6–12. [PubMed] [Google Scholar]
- Wesdorp R. I., Fischer J. E. Plasma-gastrin and acid secretion in patients with peptic ulceration. Lancet. 1974 Oct 12;2(7885):857–860. doi: 10.1016/s0140-6736(74)91199-4. [DOI] [PubMed] [Google Scholar]
- Whitehead R., Roca M., Meikle D. D., Skinner J., Truelove S. C. The histological classification of duodenitis in fibreoptic biopsy specimens. Digestion. 1975;13(3):129–136. doi: 10.1159/000197701. [DOI] [PubMed] [Google Scholar]
- Whitehead R., Truelove S. C., Gear M. W. The histological diagnosis of chronic gastritis in fibreoptic gastroscope biopsy specimens. J Clin Pathol. 1972 Jan;25(1):1–11. doi: 10.1136/jcp.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size and charge distinctions between endogenous human plasma gastrin in peripheral blood and heptadecapeptide gastrins. Gastroenterology. 1970 May;58(5):609–615. [PubMed] [Google Scholar]


