Abstract
As do cytokine receptors and receptor tyrosine kinases, G protein-coupled receptors (GPCRs) signal to Janus kinases (Jaks) and signal transducers and activators of transcription (STATs). However, the early biochemical events linking GPCRs to this signaling pathway have been unclear. Here we show that GPCR-stimulated Rac activity and the subsequent generation of reactive oxygen species are necessary for activating tyrosine phosphorylation of Jaks and STAT-dependent transcription. The requirement for Rac activity can be overcome by addition of hydrogen peroxide. Expression of activated mutants of Rac1 is sufficient to activate Jak2 and STAT-dependent transcription, and the activation of Jak2 correlates with the ability of Rac1 to bind to NADPH oxidase subunit p67phox. We further show that GPCR agonists stimulate tyrosine phosphorylation of STAT1 and STAT3 proteins in a Rac-dependent manner. The tyrosine phosphorylation of STAT3 is biphasic; the first peak of phosphorylation is weak and correlates with rapid activation of Jaks by GPCRs, whereas the second peak is stronger and requires the synthesis of an autocrine factor. Rho also plays an essential role in the induction of STAT transcriptional activity. Our results highlight a novel role for Rho GTPases in mediating the regulatory effects of GPCRs on STAT-dependent gene expression.
Janus kinases (Jaks) are a small family of cytoplasmic tyrosine kinases that were initially identified as essential components of interferon receptor signaling (30, 56). It is now known that all cytokine receptors induce the tyrosine phosphorylation and activation of Jaks and that Jak activity is required for most cytokine responses. The Jak family consists of four members: Jak1, Jak2, and Tyk2, which are expressed ubiquitously, and Jak3, which is primarily found in hematopoietic cells (30, 73). Stimulation of cells with cytokines induces receptor oligomerization and brings about the local aggregation of associated Jaks, resulting in their activation by trans phosphorylation. Activated Jaks in turn phosphorylate the receptor cytoplasmic tails on tyrosine, providing docking sites for recruitment of specific signal transducers and activators of transcription (STATs) via their SH2 domain. Jaks then phosphorylate the recruited STAT proteins on tyrosine, inducing their dimerization and translocation to the nucleus, where they bind to target DNA sequences (12). The Jak/STAT signaling pathway regulates a wide variety of biological responses, including development, differentiation, cell proliferation and survival, immune response, and oncogenesis (32).
Other families of cell surface receptors also activate the Jaks and STATs. Early studies have shown that the G protein-coupled receptor (GPCR) agonists thrombin and angiotensin II (Ang II) stimulate tyrosine phosphorylation of Jaks and STATs and induce STAT DNA binding activity in target cells (7, 42, 53). These findings have now been substantiated and extended to other members of the GPCR family (21, 33, 41, 43, 52, 65, 70). However, unlike cytokine receptors, the cascade of events by which GPCRs activate the Jak/STAT pathway remains poorly understood. It has been reported that Jak2 physically associates with the Ang II AT1 receptor and STAT factors upon agonist binding (3, 42). The interaction of Jaks with chemokine receptors and with the platelet-activating factor receptor was also documented (41, 43, 65). In the case of the AT1 receptor, the association of Jak2 appears to be dependent on the motif YIPP present in the cytoplasmic tail of the receptor (3). However, this motif is not conserved in any of the other GPCRs known to associate with Jaks, raising questions about the significance of this observation. Available evidence indicates that Jak2 must be catalytically active to associate with the Ang II AT1 receptor and to recruit STATs to the receptor (2, 4). A kinase-inactive form of Jak2 with a mutation in subdomain VIII fails to associate with the receptor and to activate STAT1 following Ang II stimulation (2). These observations imply that autophosphorylation of Jaks occurs prior to their recruitment to the GPCR and is an obligatory step for subsequent signaling.
Recent work has implicated reactive oxygen species (ROS) in the activation of the Jak/STAT pathway (55, 60). ROS are produced in response to cytokines and growth factors, and function as second messengers in many cellular responses (19). A major source of ROS is the membrane-bound NADPH oxidase complex, which is present in phagocytic cells and in many other cell types (5). The activity of the phagocyte NADPH oxidase is regulated by the small GTPase Rac (8, 9), suggesting that Rho family GTPases may contribute to the activation of the Jak/STAT pathway.
Here we show using a combination of bacterial toxins and dominant interfering mutants that Rac activity is necessary for activation of Jaks and STATs by GPCRs. The activation of Jaks is dependent on ROS generation and the requirement for Rac can be overcome by addition of oxidants. Expression of an activated mutant of Rac1 is sufficient to activate Jak2 and STAT-dependent transcription. Furthermore, we show that Rho is essential for transcriptional activation of STATs by GPCR agonists but does not contribute to Jak activation or STAT tyrosine phosphorylation. These findings identify Rho GTPases as components of a novel pathway that link GPCRs to activation of Jak/STAT signaling.
MATERIALS AND METHODS
Reagents, antibodies, and plasmids.
Ang II was purchased from Hukabel Scientific. Thrombin, dithiothreitol (DTT), N-acetyl-l-cysteine, diphenylene iodonium (DPI), sodium orthovanadate, and actinomycin D (ActD) were from Sigma-Aldrich. Clostridium difficile toxin B, Clostridium botulinum C3 transferase, and platelet-derived growth factor BB (PDGF-BB) were from Calbiochem. The Clostridium sordellii toxins LT82 and LT9048, Iota toxin, and the fusion toxin Iota-C3 were purified as previously described (50). Rabbit polyclonal antibodies to Jak1 (sc-7228), Jak2 (sc-294), Tyk2 (sc-169), STAT1 (sc-346), STAT2 (sc-839), STAT4 (sc-486), STAT5 (sc-836), STAT6 (sc-981), phospho-STAT1(Tyr701) (sc-7988-R), phospho-STAT4(Ser721) (sc-16317), and phospho-STAT5a/b(Ser726) (sc-12893) were from Santa Cruz Biotechnology. Polyclonal antibodies to phospho-Jak1(Tyr1022/Tyr1023), phospho-Jak2(Tyr1007/Tyr1008), and phospho-STAT1(Ser727); anti-human interleukin-6 (IL-6) receptor neutralizing antibody; gamma interferon (IFN-γ); and recombinant rat IL-6 were from BioSource International. Anti-phospho-Tyk2(Tyr1054/Tyr1055), anti-phospho-STAT3(Tyr705), anti-phospho-STAT3(Ser727), anti-phospho-STAT5(Tyr694), and anti-phospho-STAT6(Tyr641) antibodies; anti-phospho-p44/42 mitogen-activated protein kinase (Thr202/Tyr204) monoclonal antibody (MAb) E10; and anti-STAT3 MAb 7D1 were from Cell Signaling Technology. The anti-Myc MAb was prepared in-house from 9E10 hybridoma-producing cells. The pGL3-2xIFP53GAS-luc reporter plasmid was kindly provided by A. Koromilas (Lady Davis Research Institute) and has been described previously (69). pEFBOS expression vectors encoding Jak2 and Jak2ΔVIII were kind gifts of D. Wojchowski (Pennsylvania State University) (74). The C3 expression vector pEF-Myc-C3 was a gift from R. Treisman (Imperial Cancer Research Fund Laboratories [29]). The pRK5 expression vectors for Myc-tagged RhoAL63, RhoAN19, Rac1L61, Rac1N17, Cdc42L61, and Cdc42N17 and effector loop mutants of Rac1L61 were generously provided by N. Lamarche (McGill University) and have been described elsewhere (39). The bacterial expression plasmid for recombinant glutathione S-transferase (GST)-Pak1 fusion protein was kindly provided by N. Lamarche.
Cell culture and transfections.
Rat vascular smooth muscle cells (SMC) were cultured and synchronized by serum starvation as described previously (58). COS-7 cells were grown in Dulbecco's modified Eagle's medium (Dulbecco's MEM) supplemented with 10% fetal bovine serum. They were synchronized by incubation for 20 to 24 h in serum-free Dulbecco's MEM-Ham's F-12 mediumcontaining 15 mM HEPES (pH 7.4), 0.1% bovine serum albumin, and transferrin. HeLa cells were grown in MEM supplemented with 10% fetal bovine serum. Vascular SMC grown in 24-well plates were transiently transfected with expression plasmids using the FuGENE 6 transfection reagent (Roche Molecular Biochemicals). Subconfluent COS-7 cells cultured in 60-mm-diameter dishes were transfected with a total of 6 μg of DNA using Lipofectamine reagent (Life Technologies).
Immunoblot analysis.
Cell lysis and immunoblot analysis were performed as described previously (58). Immunoblotting with phospho-specific antibodies was carried out according to the manufacturer's specifications.
Reporter gene assays.
For STAT-dependent reporter gene assays, vascular SMC seeded in 24-well plates were cotransfected with 500 ng of pGL-2x3IFP53-GAS-luc reporter construct, 300 ng of pcDNA3.1-His/LacZ, and various amounts of indicated constructs. The total DNA amount was kept constant at 1.2 μg with empty vector. After 24 h, the cells were serum starved for 48 h and stimulated with GPCR agonists for 24 h prior to harvest. For experiments with activated Rho GTPases, the cells were harvested after serum starvation for 18 h. The cells were washed with ice-cold phosphate-buffered saline and scraped in 130 μl of lysis buffer (50 mM Tris-HCl, [pH 7.8] 1 mM DTT, 1% Triton X-100). Luciferase activity (100 μl of extract) was assayed by addition of 100 μl of luciferase buffer (125 mM Tris-HCl [pH 7.8], 25 mM MgCl2, 5 mM ATP) and 100 μl of luciferine solution (277 μg/ml, 5 mM KH2PO4 [pH 8]) using the AutoLumat LB 953 (Berthold). Transfection efficiency was normalized by measuring β-galactosidase activity using a spectrophotometric assay.
Small GTPase activation assays.
The recombinant GST fusion protein of Pak1 (residues 56 to 272) was expressed in Escherichia coli and purified on glutathione-agarose beads as described previously (54). Vascular SMC were washed twice with phosphate-buffered saline and lysed in buffer G (25 mM HEPES [pH 7.5], 150 mM NaCl, 5% Igepal CA-630, 50 mM MgCl2, 5 mM EDTA, 10% glycerol, 1 mM sodium orthovanadate, 10−4 M phenylmethylsulfonyl fluoride, 10−6 M leupeptin, 10−6 M pepstatin A) for 30 min at 4°C. Total lysate proteins (600 μg) were incubated for 1 h at 4°C with 10 μg of GST-Pak1 (for Rac and Cdc42 assays) bound to glutathione-agarose beads in a total volume of 800 μl. The beads were washed three times with lysis buffer, and the eluted proteins were resolved by sodium dodecyl sulfate-gel electrophoresis. The amount of active GTP-loaded small GTPase bound was analyzed by immunoblotting using the following primary antibodies: monoclonal anti-Rac antibody (1 μg ml−1) and rabbit polyclonal anti-Cdc42 antibody (2 μg ml−1) (Upstate Biotechnology).
RESULTS
Activation of Jak/STAT signaling by GPCRs in vascular SMC.
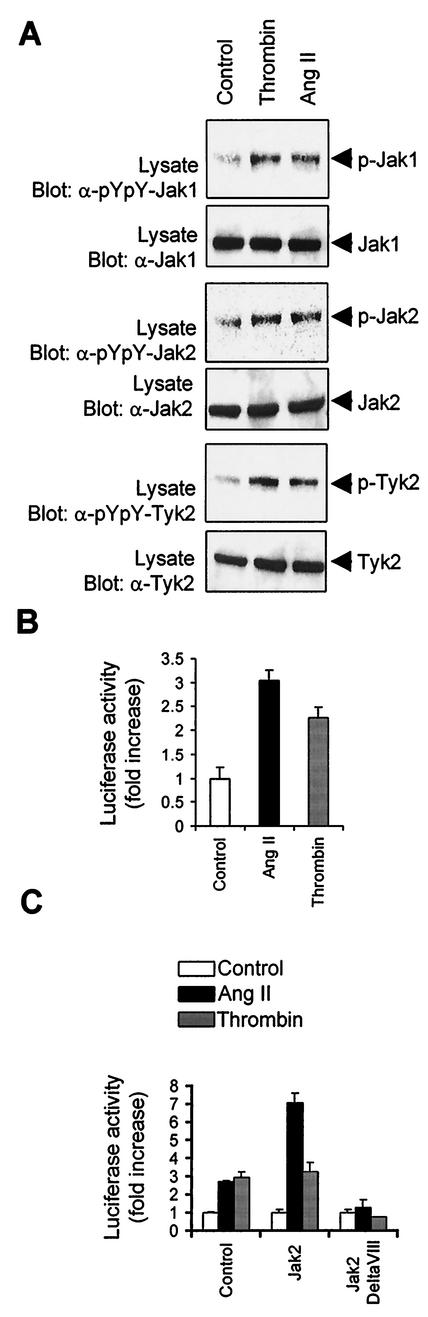
We examined the ability of representative GPCRs to activate Jak family members in normal rat vascular SMC. The activation of Jaks was monitored by immunoblotting with phospho-specific antibodies to the activation loop tyrosine residues (18, 20, 73). Treatment of cells with the GPCR agonists thrombin and Ang II resulted in the rapid activation of Jak1, Jak2, and Tyk2 (Fig. 1A). To determine whether thrombin and Ang II stimulate STAT transcriptional activity, we used a specific STAT-responsive reporter construct (pGL-2xIFP53GAS-luc) containing two copies of the IFN-γ activating sequence (GAS) element upstream of a β-globin minimal promoter (69). As shown in Fig. 1B, both GPCR agonists stimulated STAT-dependent transcription in vascular SMC, to an extent comparable to epidermal growth factor and PDGF-BB stimulation (data not shown). To evaluate the contribution of Jak2 in mediating the activation of STATs by GPCRs, we cotransfected the STAT-responsive luciferase reporter together with expression vectors for wild-type Jak2 or a catalytically inactive form of the kinase (Jak2ΔVIII) into vascular SMC. Expression of wild-type Jak2 potentiated STAT activation by Ang II, whereas the Jak2 interfering mutant completely abolished the effect of Ang II and thrombin (Fig. 1C). These observations suggest that Jak2 is required for the transcriptional activation of STATs by GPCRs in vascular SMC.
FIG. 1.
GPCR agonists stimulate Jak activity and STAT-dependent transcription in vascular SMC. (A) Quiescent rat vascular SMC were stimulated or not (Control) with thrombin (1 U/ml) or Ang II (100 nM) for 3 min. The activation of Jaks was monitored by immunoblotting of total lysate proteins with phospho-specific antibodies to activation loop tyrosine residues (pYpY). Expression levels of Jak1, Jak2, and Tyk2 were analyzed by reprobing the membrane with isoform-specific antibodies. Results are representative of five experiments. (B) Vascular SMC were transfected with pGL-2xIFP53GAS-luc reporter plasmid. After 24 h, the cells were serum starved for 48 h and stimulated with Ang II or thrombin for 24 h. The activity of luciferase was measured and normalized to that of β-galactosidase. (C) Vascular SMC were transfected with the pGL-2xIFP53GAS-luc reporter together with 500 ng of pcDNA3, pEF-BOS-Jak2, or pEF-BOS-JAk2ΔVIII. Serum-starved cells were stimulated with Ang II or thrombin, and luciferase activity was measured. The luciferase data are presented as increase over unstimulated control and represent the means of triplicate determinations (error bars, standard errors). (B and C) Results are representative of three independent experiments.
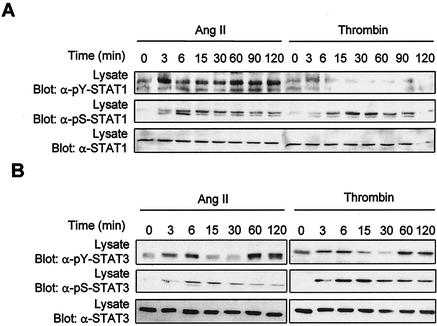
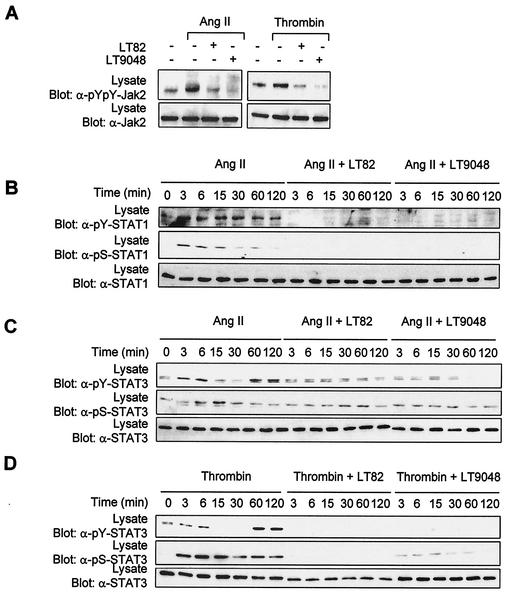
We next examined the regulation of individual STAT family members by GPCR agonists. The activation of STATs was monitored by antiphosphotyrosine immunoblotting of the precipitated protein or by immunoblotting with phospho-specific antibodies to the C-terminal tyrosine that mediates dimerization (23, 26, 28, 59, 68, 72, 73). Immunoblot analysis with isoform-specific antibodies revealed that vascular SMC express all members of the STAT family (data not shown). Addition of Ang II induced the activating tyrosine phosphorylation of STAT1 (Tyr701) and STAT3 (Tyr705) in these cells (Fig. 2). Although Ang II was previously reported to increase tyrosine phosphorylation of STAT2 (37), we were unable to detect its phosphorylation in vascular SMC, nor were we able to detect that of STAT4, STAT5, or STAT6 (data not shown). On the other hand, thrombin increased the tyrosine phosphorylation of STAT3 but not of STAT1 (Fig. 2). Control experiments using different cytokines and peroxyvanadate as stimuli confirmed that each individual STAT can be activated in these cells. Interestingly, we observed that the kinetics of STAT1 and STAT3 activation by GPCRs is different. Ang II-induced tyrosine phosphorylation of STAT1 has a rapid onset and is maintained for at least 2 h after stimulation (Fig. 2A). In contrast, GPCR-mediated tyrosine phosphorylation of STAT3 shows a biphasic profile. There is a rapid but transient peak of phosphorylation that reaches a maximum between 3 and 6 min and returns to basal levels by 15 min, followed by a second and stronger peak that appears at 60 min and persists for at least 2 h (Fig. 2B). The extent of STAT3 activation by GPCR agonists was comparable to that seen in response to epidermal growth factor treatment (data not shown).
FIG. 2.
GPCRs stimulate tyrosine and serine phosphorylation of STAT1 and STAT3 in vascular SMC. Quiescent vascular SMC were stimulated with thrombin or Ang II for the indicated times. The activation of STAT1 (A) and STAT3 (B) was monitored by immunoblotting of total lysate proteins with phospho-specific antibodies to the C-terminal tyrosine of STAT1 (Tyr701) and STAT3 (Tyr705). Serine phosphorylation of STAT1 and STAT3 was monitored by immunoblotting with phospho-specific antibodies to Ser727. The expression levels of STAT1 and -3 were analyzed by reprobing the membrane with isoform-specific antibodies. Results presented are representative of three experiments.
In addition to their phosphorylation on activating tyrosines, certain STAT isoforms are also regulated by phosphorylation of a C-terminal serine residue within the motif P-(M)-S-P (13). The functional consequence of STAT serine phosphorylation remains controversial. In some cases, mutation of this serine to alanine was found to reduce the transcriptional activity of STATs, whereas other reports suggested that it contributes to full transcriptional activation (13, 38, 67). We therefore examined the phosphorylation of these serine residues in STAT1 and STAT3 using phospho-specific antibodies. Treatment with Ang II rapidly increased the phosphorylation of STAT1 on Ser727, which persisted during the 2 h of stimulation (Fig. 2A). Thrombin also promoted Ser727 phosphorylation of STAT1, despite the fact that it does not significantly affect tyrosine phosphorylation. Similarly, both agonists stimulated phosphorylation of STAT3 on Ser727 with kinetics comparable to those for STAT1 (Fig. 2B).
Activation of Jaks by GPCRs is dependent on the generation of ROS.
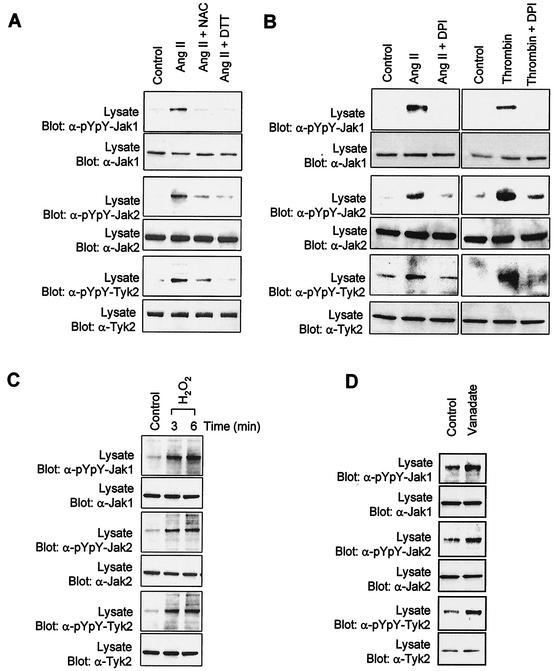
Recent studies have implicated ROS as integral components of the signaling mechanisms leading to activation of the Jak/STAT pathway (55, 60). Of interest, the GPCR agonists Ang II and thrombin have been shown to increase production of ROS via a membrane-bound NADPH oxidase system present in vascular SMC (25, 49). We therefore tested whether ROS generation is an essential step in the activation of Jaks by GPCRs. Pretreatment of vascular SMC with the antioxidants N-acetyl-l-cysteine and DTT was found to markedly inhibit Ang II-stimulated Jak1, Jak2, and Tyk2 activation (Fig. 3A). Incubation with DPI, a potent inhibitor of flavin-containing enzymes, similarly prevented the activation of Jaks by Ang II and thrombin (Fig. 3B). This effect was specific, since DPI did not interfere with IFN-γ signaling (data not shown). One mechanism by which ROS may regulate protein kinase activity is through reversible inactivation of protein tyrosine phosphatases following oxidation of their catalytic cysteine residue (14). This effect of ROS can be mimicked by hydrogen peroxide (10, 14). To test this idea, we exposed vascular SMC to H2O2 and analyzed the activation state of Jaks. Addition of H2O2 caused a rapid and robust activation of all three Jak isoforms (Fig. 3C). Consistent with these observations, inhibition of protein tyrosine phosphatase activity with vanadate also significantly increased Jaks activity (Fig. 3D). Together, these results suggest that ROS are both necessary and sufficient for activation of Jaks upon stimulation of vascular SMC by GPCRs.
FIG. 3.
Activation of Jaks by GPCRs is dependent on the production of ROS. (A) Quiescent vascular SMC were pretreated or not for 30 min with the antioxidant N-acetyl-l-cysteine (NAC) (30 mM) or DTT (30 mM) and then stimulated with Ang II for 3 min. (B) Quiescent cells were pretreated or not for 1 h with the NADPH oxidase inhibitor DPI (10 μM) and stimulated with Ang II or thrombin for 3 min. (C) Quiescent cells were incubated with 250 μM H2O2 for the indicated times. (D) Quiescent cells were incubated with 500 μM vanadate for the indicated times. The activation of Jaks was monitored by immunoblotting of total lysate proteins with phospho-specific antibodies to activating tyrosine residues. Results are representative of at least three independent experiments.
Small GTPases of the Rho family are required for activation of the Jak/STAT pathway by GPCRs.
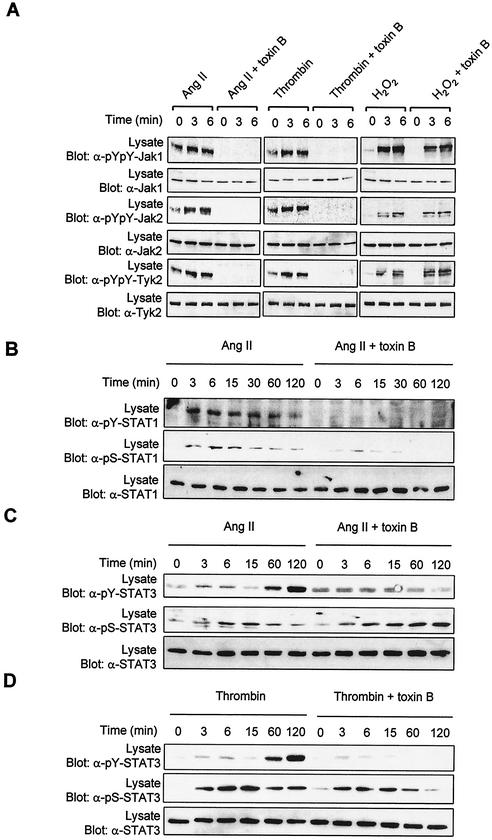
Rho family GTPases are key components of signaling pathways that control cytoskeletal organization, cell proliferation, and gene expression (8, 64). Notably, Rac1 has been implicated recently in the regulation of STAT3 transcriptional activity (17, 61). The Rac proteins are also known to regulate the catalytic activity of the NADPH oxidase complex (8, 9). All these observations prompted us to examine the role of Rho GTPases in the regulation of the Jak/STAT pathway. We used C. difficile toxin B, a bacterial toxin that selectively glucosylates Rho, Rac, and Cdc42 GTPases and inhibits their function by preventing GTP binding (1, 51). Incubation of vascular SMC with toxin B completely blocked the activation of Jak1, Jak2, and Tyk2 in response to the GPCR agonists Ang II and thrombin (Fig. 4A, left and middle columns). Importantly, treatment of cells with toxin B did not prevent the activation of Jak family members by H2O2 (Fig. 4A, right column). This result is consistent with the idea that ROS act downstream of Rho GTPases.
FIG. 4.
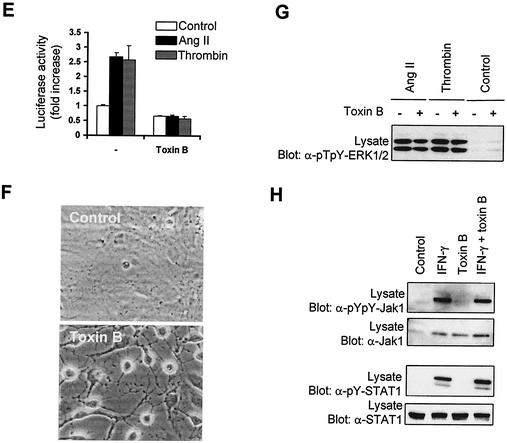
Rho GTPases are required for activation of the Jak/STAT pathway by GPCRs. (A to D) Quiescent vascular SMC were pretreated or not for 3 h with C. difficile toxin B (5 ng/ml) and then stimulated with Ang II, thrombin, or H2O2 for the indicated times. The activation of Jaks(A), STAT1 (B), and STAT3 (C and D) was monitored by immunoblotting of total lysate proteins with phospho-specific antibodies to activating tyrosine residues. (B to D) Ser727 phosphorylation of STAT1/3 was assessed by immunoblotting with phospho-specific antibodies. (E) Vascular SMC were transfected with pGL-2xIFP53GAS-luc reporter plasmid. Serum-starved cells were pretreated or not for 6 h with toxin B (0.5 ng/ml) and then stimulated with Ang II or thrombin for 24 h. The activity of luciferase was measured and normalized to that of β-galactosidase. Data are expressed as increase over unstimulated control and represent the means of triplicate determinations (error bars, standard errors). (A to E) Results are representative of three independent experiments. (F) Phase-contrast micrographs showing the effect of toxin B on the morphology of vascular SMC. (G) Quiescent cells were pretreated or not with toxin B and then stimulated with Ang II or thrombin for 5 min. The activating phosphorylation of Erk1/Erk2 was monitored by immunoblotting of lysate proteins with a phospho-specific antibody. (H) Quiescent vascular SMC were pretreated or not with toxin B and then stimulated with IFN-γ for 15 min. The activation of Jak1 and STAT1 was monitored as described above.
Incubation with toxin B suppressed Ang II-induced tyrosine phosphorylation of STAT1 and markedly attenuated its phosphorylation on Ser727 in vascular SMC (Fig. 4B). The toxin also prevented GPCR-stimulated tyrosine phosphorylation of STAT3 but did not affect the phosphorylation of the protein on Ser727 (Fig. 4, C, and D). We next evaluated the consequences of inhibiting Rho GTPases function on the transcriptional activation of STATs. As shown in Fig. 4E, incubation with toxin B completely suppressed the induction of STAT-dependent transcription by GPCR agonists. Bacterial toxins interfering with small GTPases are known to provoke dramatic changes in cell morphology (51). To ascertain that C. difficile toxin B was working effectively, we always tested its effect on the morphology of vascular SMC. As expected, incubation with C. difficile toxin B caused dramatic changes in morphology, leading to cell rounding and appearance of retraction filaments (Fig. 4F). However, under these conditions, toxin B had no effect on the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinases by Ang II and thrombin (Fig. 4G). Furthermore, incubation with the bacterial toxin did not interfere with IFN-γ-stimulated Jak1 activation or STAT1 tyrosine phosphorylation (Fig. 4H), confirming the specificity of its effect on GPCR signaling. Taken together, these data suggest that small GTPases of the Rho family are necessary for activating phosphorylation of Jak and STAT isoforms and for the resulting induction of STAT-dependent transcription in response to GPCR engagement.
Rho is required for transcriptional activation of STATs by GPCRs.
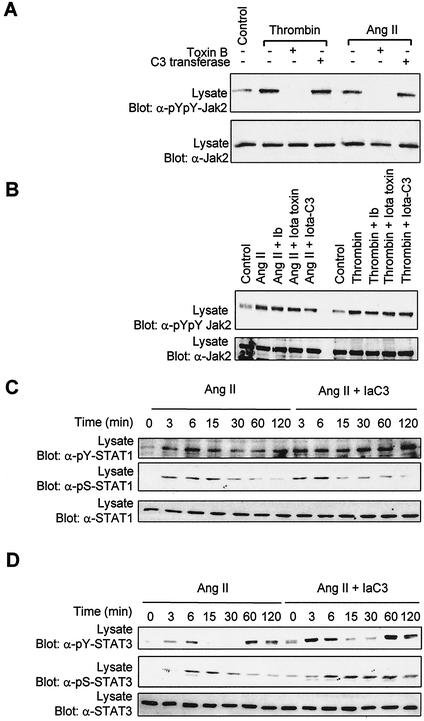
We next attempted to define the specific roles of Rho, Rac, and Cdc42 in mediating the activation of Jak/STAT signaling by GPCRs. Since individual Jak family members appear to be similarly regulated by GPCRs and Rho GTPases and because of the essential role of Jak2 in the transcriptional activation of STATs, we only monitored the activity of Jak2 in subsequent experiments. It has been previously reported that stimulation of vascular SMC with Ang II or thrombin activates Rho (57, 71). To evaluate the specific contribution of Rho proteins in Jak2 regulation we used C. botulinum C3 transferase, which ADP-ribosylates Rho at Asn41 and inhibits its translocation to the membrane (1, 51). Incubation of vascular SMC with C3 transferase failed to prevent Jak2 activation by GPCR agonists, whereas under similar experimental conditions exposure to toxin B completely abolished enzyme activity (Fig. 5A). To confirm these results, we used a fusion Iota-C3 toxin made by combining the binding subunit for Iota toxin (Ib) with a chimeric Ia-C3 transferase. This fusion toxin only exhibits C3 enzymatic activity and is internalized more rapidly and efficiently into cells via the Ib binding protein (51). We found that neither Ib alone, Iota toxin (Ia plus Ib), nor Iota-C3 fusion toxin affected Jak2 activation by Ang II or thrombin (Fig. 5B). Incubation with Iota-C3 toxin also failed to inhibit GPCR-stimulated tyrosine phosphorylation or Ser727 phosphorylation of STAT1 and STAT3 (Fig. 5C to E).
FIG. 5.
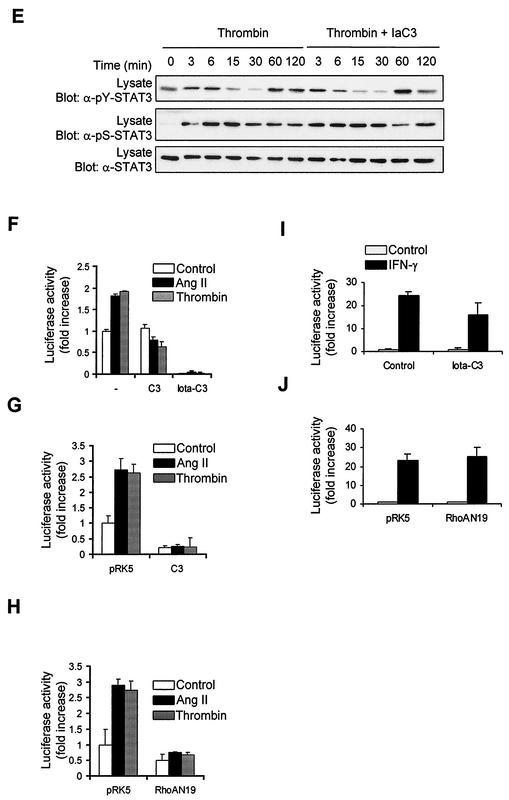
Rho is necessary for the transcriptional activation of STATs by GPCRs. (A) Quiescent vascular SMC were pretreated or not for 3 h with C. difficile toxin B (5 ng/ml) or for 48 h with C. botulinum C3 transferase (20 μg/ml). (B) Quiescent cells were pretreated or not for 24 h with Iota toxin subunit Ib (7 μg/ml), Iota toxin (Ia and Ib) (each subunit, 7 μg/ml), or Iota-C3 fusion protein (7 μg of Ia-C3 and Ib/ml). The cells were then stimulated with Ang II or thrombin for 3 min. The activation of Jak2 was monitored by immunoblotting of lysate proteins with a phospho-specific antibody to activating tyrosine residues. (C to E) Quiescent cells were pretreated or not for 24 h with Iota-C3 fusion protein, and then stimulated with Ang II or thrombin for the indicated times. The activation and serine phosphorylation of STAT1 (C) and STAT3 (D and E) were monitored by immunoblotting with phospho-specific antibodies. (F) Vascular SMC were transfected with pGL-2xIFP53GAS-luc reporter plasmid. Serum-starved cells were pretreated or not for 48 h with C3 transferase (10 μg/ml) or for 6 h with Iota-C3 fusion toxin, and then stimulated with Ang II or thrombin for 24 h. The activity of luciferase was measured and normalized to that of β-galactosidase. (G and H) Vascular SMC were transfected with the pGL-2xIFP53GAS-luc reporter together with 500 ng of pEF-Myc-C3 transferase (G) or pRK5-MycRhoAN19 (H). Serum-starved cells were stimulated with Ang II or thrombin for 24 h, and luciferase activity was measured. Luciferase data are presented as increase over unstimulated control and represent the means of triplicate determinations (error bars, standard errors). (A to H) Results arerepresentative of three independent experiments. (i) Vascular SMC were transfected with pGL-2xIFP53GAS-luc reporter plasmid. Serum-starved cells were pretreated or not with Iota-C3 fusion toxin, and then stimulated with IFN-γ for 6 h. The activity of luciferase was measured and normalized to that of β-galactosidase. (J) Vascular SMC were transfected with the GAS reporter together with 500 ng of pRK5-MycRhoAN19. Serum-starved cells were stimulated with IFN-γ for 6 h, and luciferase activity was measured. Results are representative of three experiments.
Surprisingly, incubation of vascular SMC with C3 transferase or Iota-C3 fusion protein blocked the induction of STAT-dependent transcription in response to both GPCR agonists (Fig. 5F). Consistent with this result, transfection of a dominant-negative mutant of Rho isoforms, RhoAN19, or Myc-tagged C3 transferase together with the STAT-dependent reporter completely abolished the transcriptional activation of STATs by GPCRs (Fig. 5G and H). In contrast, incubation of cells with Iota-C3 toxin or expression of RhoAN19 failed to inhibit STAT transcriptional activation induced by IFN-γ (Fig. 5I and J). These results provide strong evidence for the specific involvement of Rho in the transcriptional activation of STATs by GPCRs.
Rac is required for activation of Jaks and STATs by GPCRs.
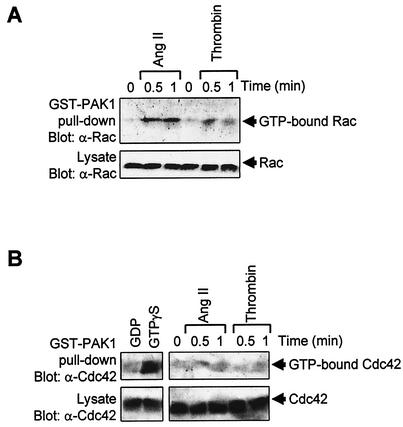
The observation that toxin B but not C3 transferase inhibits activation of Jak2 and STAT1/3 by GPCRs point at the involvement of Rac and/or Cdc42 in this process. To address this hypothesis, we first determined whether GPCR agonists could activate Rac and Cdc42 in vascular SMC. Stimulation with Ang II or thrombin caused a significant increase in GTP loading of Rac, as measured by association of the GTPase to the CRIB (Cdc42/Rac-interactive binding) domain of Pak1 (Fig. 6A). However, we were not able to detect any significant activation of Cdc42, which is well expressed in vascular SMC (Fig. 6B). Control experiments with GTPγS confirmed that the Cdc42 assay, is working effectively.
FIG. 6.
GPCR agonists activate Rac but not Cdc42 in vascular SMC. (A and B) Quiescent vascular SMC were stimulated with Ang II or thrombin for the times indicated. The activity of Rac1 (A) and Cdc42 (B) was determined by measuring the amount of GTP-loaded protein bound to GST-Pak as described in Materials and Methods. Control assays were carried out by incubating lysates of unstimulated cells with either GTPγS (positive control) or GDP (negative control) for 15 min at 25°C prior to incubation with GST-Pak1 beads. Results are representative of three experiments.
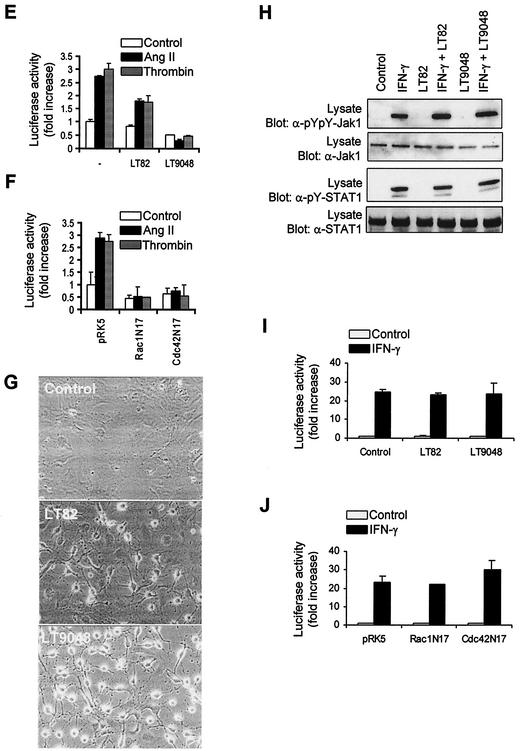
We then exploited the use of two other bacterial toxins to examine the contribution of Rac and Cdc42 in signaling to Jaks. Lethal toxin (LT) from C. sordellii strain 82 (LT82) glucosylates and inactivates Rac, Ras, Rap, Ral, and to a lesser extent R-Ras proteins, whereas LT from strain 9048 inactivates Rac, Cdc42, Ras, and (weakly) Rap and R-Ras proteins (1, 16, 51). Exposure of vascular SMC to either LT82 or LT9048 completely suppressed the activating tyrosine phosphorylation of Jak2 (Fig. 7A) and STAT1/3 (Fig. 7B to D) upon stimulation with Ang II or thrombin. The two toxins also eliminated the stimulatory effect of GPCRs on Ser727 phosphorylation of STAT1 and STAT3 (Fig. 7B to D). We next tested the effect of the toxins on STAT transcriptional activity. Incubation of cells with low concentrations of LT82 and LT9048 markedly reduced the induction of STAT-dependent transcription by GPCR agonists (Fig. 7E). The results with the bacterial toxins are summarized in Table 1. To confirm the involvement of Rac and Cdc42, we cotransfected the STAT-dependent reporter together with dominant-negative mutants of Rac1 and Cdc42 in vascular SMC. Expression of Rac1N17 or Cdc42N17 completely blocked the stimulatory effect of GPCR agonists on STAT-dependent transcription (Fig. 7F). However, the finding that both Rac1 and Cdc42 interfering mutants block STAT activity should be interpreted with caution, since the guanine nucleotide exchange factors that are inactivated by these mutants may regulate both Rac and Cdc42 isoforms (34, 64).
FIG. 7.
Role of Rac and Cdc42 in Jak2 activation and induction of STAT-dependent transcription by GPCRs. (A) Quiescent vascular SMC were pretreated or not for 4 h with C. sordellii LT82 (5 μg/ml) or LT9048 (5 μg/ml), and then stimulated with thrombin or Ang II for 3 min. The activation of Jak2 was monitored by immunoblotting of lysate proteins with a phospho-specific antibody. (B to D) Quiescent cells were pretreated or not for 4 h with LT82 or LT9048 and then stimulated with Ang II or thrombin for the indicated times. The activation and serine phosphorylation of STAT1 (B) and STAT3 (C and D) were monitored by immunoblotting with phospho-specific antibodies. (E) Vascular SMC were transfected with pGL-2xIFP53GAS-luc reporter plasmid. Serum-starved cells were pretreated or not for 4 h with LT82 or LT9048, and then stimulated with Ang II or thrombin for 24 h. The activity of luciferase was measured and normalized to β-galactosidase. (F) Vascular SMC were transfected with the pGL-2xIFP53GAS-luc reporter together with 500 ng of pRK5-MycRac1N17 or pRK5-MycCdc42N17. Serum-starved cells were stimulated with Ang II or thrombin, and luciferase activity was measured. Luciferase data are presented as increase over unstimulated control and represent the means of triplicate determinations (error bars, standard error). (A to F) Results are representative of three independent experiments. (G) Phase-contrast micrographs showing the effects of LT82 and LT9048 on the morphology of vascular SMC. (H) Quiescent cells were pretreated or not with LT82 or LT9048 and then stimulated with IFN-γ for 15 min. The activation of Jak1 and STAT1 was monitored as described above. (I) Vascular SMC were transfected with the GAS reporter, and treated or not with LT82 or LT9048. Serum-starved cells were then stimulated with IFN-γ for 6 h, and luciferase activity was measured. (J) Vascular SMC were transfected with the GAS reporter together with 500 ng of pRK5-MycRac1N17 or pRK5-Cdc42N17. Serum-starved cells were stimulated with IFN-γ for 6 h, and luciferase activity was measured. Results are representative of three experiments.
TABLE 1.
Substrate selectivity of different bacterial toxins and their effects on activation of Jaks and STATs by GPCRs
| Toxin | Inhibitiona of:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho | Rac | Cdc42 | Ras | Ral | Rap | Jak2 (YY1007/1008) | STAT1 (Y701) | STAT1 (S727) | STAT3 (Y705) | STAT3 (S727) | STAT transcriptional activity | |
| C. difficile toxin B | ++ | ++ | ++ | − | − | − | ++ | ++ | + | ++ | − | ++ |
| C. sordellii LT82 | − | ++ | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| C. sordellii LT9048 | − | ++ | ++ | ++ | +/− | + | ++ | ++ | ++ | ++ | ++ | ++ |
| C. botulinum C3 transferase | ++ | − | − | − | − | − | − | ND | ND | ND | ND | ++ |
| Iota Ib | − | − | − | − | − | − | − | ND | ND | ND | ND | − |
| Iota Ib + Ia | − | − | − | − | − | − | − | ND | ND | ND | ND | − |
| Ib + Ia-C3 | ++ | − | − | − | − | − | − | − | − | − | − | ++ |
Symbols: ++, strong inhibition; +, moderate inhibition; −, no inhibitory effect; +/−, weak inhibition; ND, not determined.
As observed with C. difficile toxin B, the LTs induced profound morphological changes in vascular SMC (Fig. 7G). However, incubation with the toxins had no effect on PDGF-BB-induced tyrosine phosphorylation of the PDGF receptor and of other downstream effectors (data not shown). Furthermore, the LTs did not interfere with the stimulatory effect of IFN-γ on Jak1 and STAT1 tyrosine phosphorylation (Fig. 7H) or STAT-dependent transcription (Fig. 7I), confirming their specificity. Similarly, overexpression of Rac1N17 and Cdc42N17 failed to inhibit IFN-γ-induced STAT-driven transcription (Fig. 7J). We conclude from these results that Rac (and possibly Cdc42) is required for activation of Jaks and induction of STAT-dependent transcription by GPCRs.
Activation of Jak2 and STAT-dependent transcription by Rac and Rho.
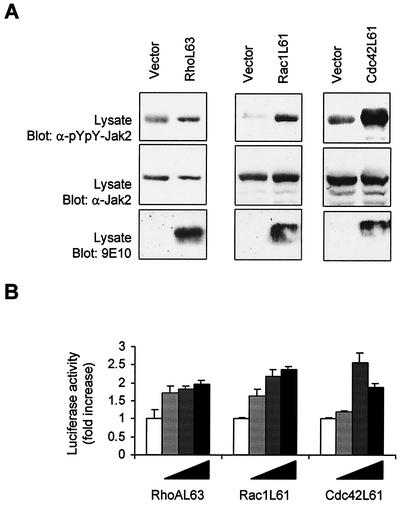
The results presented above indicate that Rho GTPases are essential components of the pathway linking GPCRs to the activation of Jaks and STATs. We next wished to determine whether activation of Rho family members is sufficient to modulate Jak/STAT signaling. For these experiments, COS-7 cells were cotransfected with Jak2 and expression vectors encoding activated forms of RhoA, Rac1, or Cdc42, and the activating phosphorylation of Jak2 was analyzed by immunoblotting. Expression of active RhoAL63 had little effect on Jak2 phosphorylation, whereas the activated Rac1L61 and Cdc42L61 proteins markedly increased Jak2 activity (Fig. 8A). We also evaluated the effect of Rho GTPases on STAT activity in vascular SMC. Expression of increasing amounts of all three activated GTPases was found to significantly induce STAT-dependent transcription in serum-starved cells (Fig. 8B).
FIG. 8.
Activation of Jak2 and STAT-dependent transcription by Rho GTPases. (A) COS-7 cells were transfected with pEF-BOS-Jak2 (250 ng) together with 250 ng of pRK5-MycRhoAL63 (left panel), pRK5-MycRac1L61 (middle panel), or pRK5-MycCdc42L61 (right panel). The cells were serum starved for 24 h and the activation of Jak2 was monitored by immunoblotting of total lysate proteins with a phospho-specific antibody to activating tyrosine residues. Expression level of Jak2 and Rho GTPases was analyzed by immunoblotting with anti-Jak2 and anti-Myc antibodies, respectively. (B) Vascular SMC were transfected with the pGL-2xIFP53GAS-luc reporter together with increasing amounts (from 50 to 400 ng) of pRK5-MycRhoAL63, pRK5-MycRac1L61 or pRK5-MycCdc42L61. The cells were serum starved for 18 h and luciferase activity was measured. Results are representative of three independent experiments.
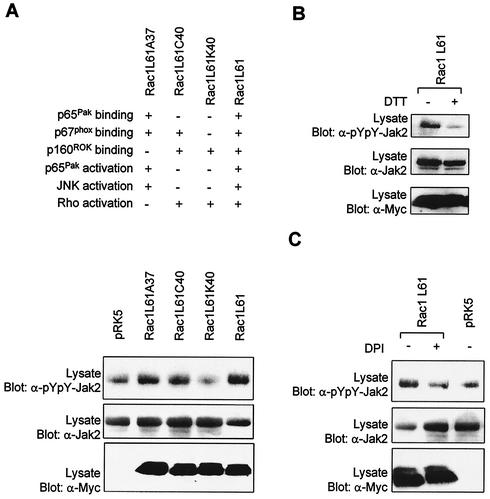
To further dissect the molecular mechanisms underlying the action of Rac on the Jak/STAT pathway, we analyzed the effect of effector loop mutants of Rac1 on the activity of Jak2. Introduction of the Y40C mutation into the effector loop of Rac disrupts its interaction with CRIB-containing proteins, such as Pak and WASP, but does not interfere with binding to NADPH oxidase subunit p67phox or ROK. The F37A mutation blocks ROK interaction, while the Y40K mutation inhibits the interaction of Rac with Pak and p67phox but not with ROK (39) (Fig. 9A). Expression of Y40C- or F37A-substituted Rac1L61 in COS-7 cells significantly enhanced Jak2 tyrosine phosphorylation (Fig. 9A). In contrast, the Y40K mutant, which has lost interaction with p67phox, was no longer able to activate Jak2. Consistent with the idea that Rac mediates GPCR-induced production of ROS, which in turn activate Jaks, we found that incubation of transfected COS-7 cells with DTT (Fig. 9B) or DPI (Fig. 9C) prevented Jak2 activation by Rac1Q61L. These results provide additional support for the idea that Rac regulates the Jak/STAT pathway through activation of the NADPH oxidase and production of ROS.
FIG. 9.
Effect of Rac1 effector loop mutants on Jak2 activity. (A) COS-7 cells were transfected with pEF-BOS-Jak2 (100 ng) together with 250 ng of the indicated mutants of Rac1. The cells were serum starved for 24 h, and the activation of Jak2 was monitored by immunoblotting with a phospho-specific antibody. Expression levels of Jak2 and Rac were analyzed by immunoblotting with anti-Jak2 and anti-Myc antibodies, respectively. (B and C) Same as panel A, except that COS-7 cells were treated for 1 h with DTT (30 mM) or DPI (10 μM) prior to harvesting. Results are representative of three independent experiments.
Sustained activation of STAT3 by GPCRs requires synthesis of an autocrine factor.
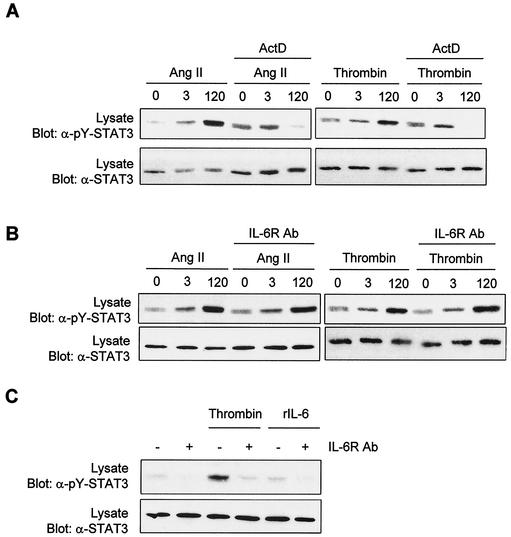
It has been recently suggested that Rac1 induces STAT3 activation by an indirect mechanism involving the autocrine production and action of IL-6 (17). In their study, the authors also observed that Rac1-mediated activation of STAT3 is sensitive to SOCS3 (suppressor of cytokine signaling 3), a known inhibitor of gp130 signaling (46). In the present study, we found that GPCR agonists activate STAT3 in a biphasic manner. The first peak of activation is weak and transient and correlates with the activation of Jaks by GPCRs. In contrast, the second peak of activation is stronger and sustained, suggesting a possible requirement for the synthesis of an autocrine factor. To test this hypothesis, we first examined the effect of the transcriptional inhibitor ActD on GPCR-induced STAT3 tyrosine phosphorylation. Pretreatment of vascular SMC with ActD completely abolished the late peak of STAT3 activation, but not the first peak (Fig. 10A). This result is consistent with the idea that long-term activation of STAT3 is dependent on synthesis of a novel ligand and/or receptor.
FIG. 10.
Evidence that late-phase activation of STAT3 is mediated by an autocrine factor. (A) Vascular SMC were treated or not with ActD (5 μg/ml) for 60 min and then stimulated with Ang II or thrombin for the times indicated. Activation of STAT3 was monitored by immunoblotting of lysate proteins with a phospho-specific antibody. (B) Quiescent vascular SMC were incubated for 60 min in the absence or presence of a neutralizing antibody to IL-6 receptor, prior to stimulation with Ang II or thrombin. Activation of STAT3 was monitored as described above. (C) Growth-arrested HeLa cells were incubated or not with the IL-6 receptor antibody and then stimulated with thrombin for 2 h. Activation of STAT3 was monitored as above. Results are representative of three experiments.
Thrombin and Ang II were previously reported to induce IL-6 expression in vascular SMC (27, 55, 63). To verify the possibility that IL-6 mediates the long-term activation of STAT3 through an autocrine loop, we tested the effect of a neutralizing antibody to the human IL-6 receptor. The antibody did not affect stimulation of STAT3 tyrosine phosphorylation by either Ang II or thrombin (Fig. 10B). However, these findings should be interpreted with caution, since the neutralizing antibody may not recognize the rat IL-6 receptor. IL-6 also failed to activate STAT3 in vascular SMC, indicating that the concentration of receptor is limiting (data not shown). We next asked whether the IL-6 receptor neutralizing antibody could inhibit thrombin-induced STAT3 activation in a human cell line. The antibody effectively abolished the activation of STAT3 by thrombin in HeLa cells (Fig. 10C). However, IL-6 only weakly activated STAT3 in these cells. Together, our findings strongly suggest that late and sustained activation of STAT3 by GPCRs is dependent on the autocrine production of a ligand by a Rac-dependent mechanism. IL-6 appears to be a likely candidate.
DISCUSSION
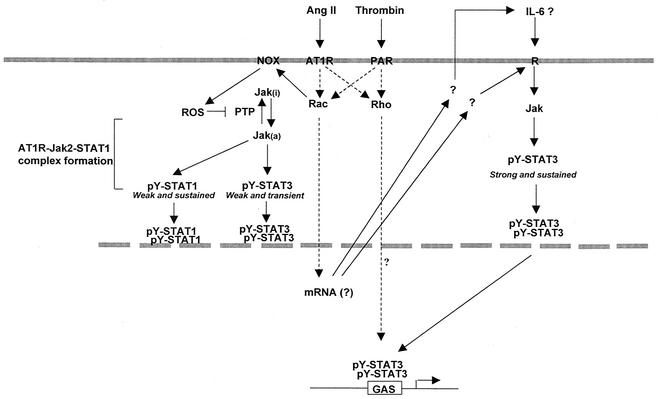
As with cytokine receptors, agonist stimulation of GPCRs results in tyrosine phosphorylation and activation of Jak and STAT family members. To date, several different GPCRs have been reported to activate the Jak/STAT pathway (7, 21, 33, 41-43, 52, 53, 65, 70). However, the mechanism by which GPCRs activate this signaling pathway remains largely unknown. Here, we demonstrate for the first time that the small GTPases Rho and Rac are required for transcriptional activation of STATs by GPCRs. Previous studies have shown that Rho is activated by a wide variety of GPCR agonists, including Ang II and thrombin in vascular SMC (57, 71). Although much less is known about the regulation of Rac and Cdc42 by this receptor family, agonists like formylmethionyl-Leu-Phe or sphingosine-1-phosphate have been shown to activate Rac and Cdc42 in their target cells (6, 48). In this study, we observed that Ang II and thrombin increase GTP loading of Rac, but not Cdc42, in vascular SMC. Inhibition of Rac function with toxin B and LTs, or by expression of dominant-negative Rac1 protein, was found to suppress the activating phosphorylation of Jaks and STATs and the induction of STAT-dependent transcription in response to GPCRs. Consistent with findings by others (17, 61), expression of activated Rac1 was sufficient to enhance the phosphorylation of Jaks and the transcriptional activity of STATs. We also showed that blocking the function of endogenous Rho with C3 transferase or dominant-negative RhoA prevents the transcriptional activation of STATs by GPCR agonists, without interfering with Jak activity or STAT phosphorylation. Collectively, our results lead to a working model in which the small GTPases Rac and Rho act in concert to mediate the regulatory effects of GPCRs on STAT-dependent gene expression (Fig. 11). While activated Rac1 and RhoA can signal independently, both are necessary for maximal transcriptional activation of STATs.
FIG. 11.
Proposed model for the role of Rho GTPases in the regulation of Jak activation and STAT-dependent transcription in response to GPCR engagement.
In addition to its well-described action on the actin cytoskeleton, Rac is also a known regulator of the NADPH oxidase, a multisubunit enzyme complex of phagocytic cells that catalyses the production of superoxide (5, 8, 9). Rac interacts with cytochrome b to regulate the initial transfer of electrons from NADPH to flavin adenine dinucleotide and binds to the p67phox subunit to induce the subsequent electron transfer to molecular oxygen (15). Similar NADPH oxidase-like enzymes have been found in a variety of cells of mesodermal origin, including vascular SMC, and Rac has been implicated in the generation of ROS by growth factors and cytokines in these cells (5, 62). The results presented here suggest the existence of a linear signaling pathway, Rac-NADPH oxidase-ROS, that links GPCRs to Jak activation in vascular SMC (Fig. 11). This model is supported by the following observations. (i) GPCR agonists activate Rac in vascular SMC, and Rac is both necessary and sufficient for Jak activation. (ii) Treatment with antioxidants or with the NADPH oxidase inhibitor DPI inhibits Jak activation by GPCR agonists and also by activated Rac1. (iii) Exposure to H2O2 activates the three Jak isoforms expressed in vascular SMC and bypasses the requirement for Rac activity. (iv) The effector loop mutation Y40K in the activated form of Rac1 (Rac1L61-Y40K), which prevents the interaction of Rac with p67phox, is also unable to activate Jak2. It is noteworthy that many GPCRs known to activate the Jak/STAT pathway have also been shown to induce the formation of ROS; in addition to the receptors for Ang II and thrombin, these include the platelet-activating factor, bradykinin, α1-adrenergic, and endothelin-1 receptors (22, 24, 47, 66). The pathway described here appears specific to GPCR signaling, since activation of Jak1 by the cytokine IFN-γ was not influenced by inhibition of Rac or the NADPH oxidase.
The exact mechanism by which ROS production leads to activating phosphorylation of Jaks remains to be clarified, but one likely possibility is through inhibition of tyrosine phosphatase activity. Early reports have shown that treatment of cells with oxidants or SH-alkylating agents induces rapid tyrosine phosphorylation of numerous receptor tyrosine kinases, by preventing their dephosphorylation by tyrosine phosphatases (36). This occurs by reversible oxidation of a redox-sensitive cysteine residue present in the active site of these enzymes (10, 11, 14, 40). Interestingly, among the tyrosine phosphatases shown to be inactivated by oxidants are PTP-1B (40) and SHP-1 (11), two enzymes that were also found to negatively regulate Jak/STAT signaling (31, 45). Thus, the generation of ROS may transiently inactivate tyrosine phosphatases and switch the equilibrium towards autophosphorylation of Jak tyrosine kinases. In support of this idea, we observed that addition of vanadate rapidly increases activating tyrosine phosphorylation of Jak family members in vascular SMC.
Our study also revealed that Rho is required for maximal transcriptional activation of STATs by GPCRs. This action of Rho is specific, since incubation of cells with Iota-C3 toxin or expression of RhoAN19 failed to inhibit STAT transcriptional activation induced by the cytokine IFN-γ. However, Rho activity is dispensable for Jak activation and for activating tyrosine phosphorylation or Ser727 phosphorylation of STAT1/3 by GPCR agonists. We also observed that a dominant-negative RhoA mutant does not block STAT-dependent transcription induced by activated Rac1L61, indicating that RhoA is not downstream of Rac (data not shown). Additional studies are clearly warranted to establish the precise mechanism by which Rho regulates STAT transcriptional activity.
Another important finding of this study was the observation that GPCR agonists stimulate tyrosine phosphorylation of STAT3 in a biphasic manner. A rapid but transient peak of tyrosine phosphorylation is followed by a much stronger and sustained phase of activation. The first peak correlates well with the transient activation of Jaks by GPCRs. Both the early and late activation phases of STAT3 are dependent on Rac (and possibly Cdc42) activity. The rapid activation of STAT3 by Ang II was previously reported to be sensitive to inhibition of NADPH oxidase (55). In contrast, we found that late activation of STAT3 is not significantly inhibited by DPI in vascular SMC (data not shown). These results suggest that long-term activation of STAT3 may involve an effector of Rac distinct from the NADPH oxidase complex. We also provide evidence of the involvement of an autocrine factor in mediating the late effects of GPCRs on STAT3. One likely candidate for this factor is the cytokine IL-6. Previous work has shown that Rac1 induces STAT3 activation through the production and autocrine action of IL-6 in HeLa cells (17). In agreement with these findings, a neutralizing IL-6 receptor antibody abolished the stimulatory effect of thrombin on late activation of STAT3 in HeLa cells. The IL-6 receptor antibody failed to inhibit GPCR-mediated STAT3 activation in vascular SMC, but this may be explained by the fact that the antibody is raised against the human form. We did not detect any effect of IL-6 on STAT3 in vascular SMC, indicating that expression of the receptor is probably limiting. Indeed, the mRNAs for the IL-6 receptor and gp130 subunits were not detected in vascular SMC (35, 44). However, it is still possible that GPCR activation stimulates the production of both IL-6 and its receptor. Consistent with this idea, Faruqi et al. showed that expression of RacV12 induces the expression of both IL-6 and IL-6 receptor in HeLa cells (17).
FIG. 7—Continued.
Acknowledgments
We thank A. Koromilas, N. Lamarche, R. Treisman, and D. Wojchowski for reagents. We also thank A. Veillette for critical reading of the manuscript and discussion.
This work was supported by a grant to S.M. from the Canadian Institutes for Health Research (CIHR; MOP-14650). S.P. and P.C. are the recipients of studentships from the Heart and Stroke Foundation of Canada and the CIHR, respectively.
REFERENCES
- 1.Aktories, K., G. Schmidt, and I. Just. 2000. Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 381:421-426. [DOI] [PubMed] [Google Scholar]
- 2.Ali, M. S., P. P. Sayeski, and K. E. Bernstein. 2000. Jak2 acts as both a STAT1 kinase and as a molecular bridge linking STAT1 to the angiotensin II AT1 receptor. J. Biol. Chem. 275:15586-15593. [DOI] [PubMed] [Google Scholar]
- 3.Ali, M. S., P. P. Sayeski, L. B. Dirksen, D. J. Hayzer, M. B. Marrero, and K. E. Bernstein. 1997. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J. Biol. Chem. 272:23382-23388. [DOI] [PubMed] [Google Scholar]
- 4.Ali, M. S., P. P. Sayeski, A. Safavi, M. Lyles, and K. E. Bernstein. 1998. Janus kinase 2 (Jak2) must be catalytically active to associate with the AT1 receptor in response to angiotensin II. Biochem. Biophys. Res. Commun. 249:672-677. [DOI] [PubMed] [Google Scholar]
- 5.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 6.Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. [DOI] [PubMed] [Google Scholar]
- 7.Bhat, G. J., T. J. Thekkumkara, W. G. Thomas, K. M. Conrad, and K. M. Baker. 1994. Angiotensin II stimulates sis-inducing factor-like DNA binding activity. Evidence that the AT1A receptor activates transcription factor-Stat91 and/or a related protein. J. Biol. Chem. 269:31443-31449. [PubMed] [Google Scholar]
- 8.Bishop, A. L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 9.Bokoch, G. M. 1994. Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr. Opin. Cell Biol. 6:212-218. [DOI] [PubMed] [Google Scholar]
- 10.Caselli, A., R. Marzocchini, G. Camici, G. Manao, G. Moneti, G. Pieraccini, and G. Ramponi. 1998. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J. Biol. Chem. 273:32554-32560. [DOI] [PubMed] [Google Scholar]
- 11.Cunnick, J. M., J. F. Dorsey, L. Mei, and J. Wu. 1998. Reversible regulation of SHP-1 tyrosine phosphatase activity by oxidation. Biochem. Mol. Biol. Int. 45:887-894. [DOI] [PubMed] [Google Scholar]
- 12.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 13.Decker, T., and P. Kovarik. 2000. Serine phosphorylation of STATs. Oncogene 19:2628-2637. [DOI] [PubMed] [Google Scholar]
- 14.Denu, J. M., and K. G. Tanner. 1998. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37:5633-5642. [DOI] [PubMed] [Google Scholar]
- 15.Diebold, B. A., and G. M. Bokoch. 2001. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2:211-215. [DOI] [PubMed] [Google Scholar]
- 16.El Hadj, N. B., M. R. Popoff, J. C. Marvaud, B. Payrastre, P. Boquet, and B. Geny. 1999. G-protein-stimulated phospholipase D activity is inhibited by lethal toxin from Clostridium sordellii in HL-60 cells. J. Biol. Chem. 274:14021-14031. [DOI] [PubMed] [Google Scholar]
- 17.Faruqi, T. R., D. Gomez, X. R. Bustelo, D. Bar-Sagi, and N. C. Reich. 2001. Rac1 mediates STAT3 activation by autocrine IL-6. Proc. Natl. Acad. Sci. USA 98:9014-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkel, T. 1998. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 10:248-253. [DOI] [PubMed] [Google Scholar]
- 20.Gauzzi, M. C., L. Velazquez, R. McKendry, K. E. Mogensen, M. Fellous, and S. Pellegrini. 1996. Interferon-alpha-dependent activation of Tyk2 requires phosphorylation of positive regulatory tyrosines by another kinase. J. Biol. Chem. 271:20494-20500. [DOI] [PubMed] [Google Scholar]
- 21.Giasson, E., M. J. Servant, and S. Meloche. 1997. Cyclic AMP-mediated inhibition of angiotensin II-induced protein synthesis is associated with suppression of tyrosine phosphorylation signaling in vascular smooth muscle cells. J. Biol. Chem. 272:26879-26886. [DOI] [PubMed] [Google Scholar]
- 22.Goldman, R., S. Moshonov, and U. Zor. 1999. Calcium-dependent PAF-stimulated generation of reactive oxygen species in a human keratinocyte cell line. Biochim. Biophys. Acta 1438:349-358. [DOI] [PubMed] [Google Scholar]
- 23.Gouilleux, F., C. Pallard, I. Dusanter-Fourt, H. Wakao, L. A. Haldosen, G. Norstedt, D. Levy, and B. Groner. 1995. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 14:2005-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene, E. L., V. Velarde, and A. A. Jaffa. 2000. Role of reactive oxygen species in bradykinin-induced mitogen-activated protein kinase and c-fos induction in vascular cells. Hypertension 35:942-947. [DOI] [PubMed] [Google Scholar]
- 25.Griendling, K. K., C. A. Minieri, J. D. Ollerenshaw, and R. W. Alexander. 1994. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 74:1141-1148. [DOI] [PubMed] [Google Scholar]
- 26.Gupta, S., H. Yan, L. H. Wong, S. Ralph, J. Krolewski, and C. Schindler. 1996. The SH2 domains of Stat1 and Stat2 mediate multiple interactions in the transduction of IFN-alpha signals. EMBO J. 15:1075-1084. [PMC free article] [PubMed] [Google Scholar]
- 27.Han, Y., M. S. Runge, and A. R. Brasier. 1999. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ. Res. 84:695-703. [DOI] [PubMed] [Google Scholar]
- 28.Heim, M. H., I. M. Kerr, G. R. Stark, and J. E. Darnell, Jr. 1995. Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science 267:1347-1349. [DOI] [PubMed] [Google Scholar]
- 29.Hill, C. S., J. Wynne, and R. Treisman. 1995. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81:1159-1170. [DOI] [PubMed] [Google Scholar]
- 30.Ihle, J. N. 1995. Cytokine receptor signalling. Nature 377:591-594. [DOI] [PubMed] [Google Scholar]
- 31.Jiao, H., K. Berrada, W. Yang, M. Tabrizi, L. C. Platanias, and T. Yi. 1996. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol. Cell. Biol. 16:6985-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jove, R. 2000. STAT signaling. Oncogene 19:2466-2656. [DOI] [PubMed] [Google Scholar]
- 33.Ju, H., V. J. Venema, H. Liang, M. B. Harris, R. Zou, and R. C. Venema. 2000. Bradykinin activates the Janus-activated kinase/signal transducers and activators of transcription (JAK/STAT) pathway in vascular endothelial cells: localization of JAK/STAT signalling proteins in plasmalemmal caveolae. Biochem. J. 351:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjoller, L., and A. Hall. 1999. Signaling to Rho GTPases. Exp. Cell Res. 253:166-179. [DOI] [PubMed] [Google Scholar]
- 35.Klouche, M., S. Bhakdi, M. Hemmes, and S. Rose-John. 1999. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J. Immunol. 163:4583-4589. [PubMed] [Google Scholar]
- 36.Knebel, A., H. J. Rahmsdorf, A. Ullrich, and P. Herrlich. 1996. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15:5314-5325. [PMC free article] [PubMed] [Google Scholar]
- 37.Kodama, H., K. Fukuda, J. Pan, S. Makino, M. Sano, T. Takahashi, S. Hori, and S. Ogawa. 1998. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ. Res. 82:244-250. [DOI] [PubMed] [Google Scholar]
- 38.Kovarik, P., M. Mangold, K. Ramsauer, H. Heidari, R. Steinborn, A. Zotter, D. E. Levy, M. Muller, and T. Decker. 2001. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. EMBO J. 20:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 40.Lee, S. R., K. S. Kwon, S. R. Kim, and S. G. Rhee. 1998. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 273:15366-15372. [DOI] [PubMed] [Google Scholar]
- 41.Lukashova, V., C. Asselin, J. J. Krolewski, M. Rola-Pleszczynski, and J. Stankova. 2001. G-protein-independent activation of Tyk2 by the platelet-activating factor receptor. J. Biol. Chem. 276:24113-24121. [DOI] [PubMed] [Google Scholar]
- 42.Marrero, M. B., B. Schieffer, W. G. Paxton, L. Heerdt, B. C. Berk, P. Delafontaine, and K. E. Bernstein. 1995. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature 375:247-250. [DOI] [PubMed] [Google Scholar]
- 43.Mellado, M., J. M. Rodriguez-Frade, A. Aragay, G. del Real, A. M. Martin, A. J. Vila-Coro, A. Serrano, F. Mayor, Jr., and A. Martinez. 1998. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 161:805-813. [PubMed] [Google Scholar]
- 44.Mullberg, J., T. Geib, T. Jostock, S. H. Hoischen, P. Vollmer, N. Voltz, D. Heinz, P. R. Galle, M. Klouche, and S. Rose-John. 2000. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J. Immunol. 164:4672-4677. [DOI] [PubMed] [Google Scholar]
- 45.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein tyrosine phosphatase 1B. J. Biol. Chem. 276:47771-47774. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson, S. E., T. A. Willson, A. Farley, R. Starr, J. G. Zhang, M. Baca, W. S. Alexander, D. Metcalf, D. J. Hilton, and N. A. Nicola. 1999. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 18:375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishio, E., and Y. Watanabe. 1997. The involvement of reactive oxygen species and arachidonic acid in alpha 1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br. J. Pharmacol. 121:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paik, J. H., S. Chae, M. J. Lee, S. Thangada, and T. Hla. 2001. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vb. J. Biol. Chem. 276:11830-11837. [DOI] [PubMed] [Google Scholar]
- 49.Patterson, C., J. Ruef, N. R. Madamanchi, P. Barry-Lane, Z. Hu, C. Horaist, C. A. Ballinger, A. R. Brasier, C. Bode, and M. S. Runge. 1999. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J. Biol. Chem. 274:19814-19822. [DOI] [PubMed] [Google Scholar]
- 50.Popoff, M. R. 1987. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect. Immun. 55:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richard, J. F., L. Petit, M. Gibert, J. C. Marvaud, C. Bouchaud, and M. R. Popoff. 1999. Bacterial toxins modifying the actin cytoskeleton. Int. Microbiol. 2:185-194. [PubMed] [Google Scholar]
- 52.Rodriguez-Frade, J. M., A. J. Vila-Coro, A. Martin, M. Nieto, F. Sanchez-Madrid, A. E. Proudfoot, T. N. Wells, A. Martinez, and M. Mellado. 1999. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J. Cell Biol. 144:755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Linares, B., and S. P. Watson. 1994. Phosphorylation of JAK2 in thrombin-stimulated human platelets. FEBS Lett. 352:335-338. [DOI] [PubMed] [Google Scholar]
- 54.Royal, I., N. Lamarche-Vane, L. Lamorte, K. Kaibuchi, and M. Park. 2000. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 11:1709-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schieffer, B., M. Luchtefeld, S. Braun, A. Hilfiker, D. Hilfiker-Kleiner, and H. Drexler. 2000. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ. Res. 87:1195-1201. [DOI] [PubMed] [Google Scholar]
- 56.Schindler, C., and J. E. Darnell, Jr. 1995. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 64:621-651. [DOI] [PubMed] [Google Scholar]
- 57.Seasholtz, T. M., M. Majumdar, D. D. Kaplan, and J. H. Brown. 1999. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ. Res. 84:1186-1193. [DOI] [PubMed] [Google Scholar]
- 58.Servant, M. J., P. Coulombe, B. Turgeon, and S. Meloche. 2000. Differential regulation of p27(Kip1) expression by mitogenic and hypertrophic factors: involvement of transcriptional and posttranscriptional mechanisms. J. Cell Biol. 148:543-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shuai, K., C. M. Horvath, L. H. Huang, S. A. Qureshi, D. Cowburn, and J. E. Darnell, Jr. 1994. Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell 76:821-828. [DOI] [PubMed] [Google Scholar]
- 60.Simon, A. R., U. Rai, B. L. Fanburg, and B. H. Cochran. 1998. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 275:C1640-C1652. [DOI] [PubMed] [Google Scholar]
- 61.Simon, A. R., H. G. Vikis, S. Stewart, B. L. Fanburg, B. H. Cochran, and K. L. Guan. 2000. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 290:144-147. [DOI] [PubMed] [Google Scholar]
- 62.Sundaresan, M., Z. X. Yu, V. J. Ferrans, D. J. Sulciner, J. S. Gutkind, K. Irani, P. J. Goldschmidt-Clermont, and T. Finkel. 1996. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokunou, T., T. Ichiki, K. Takeda, Y. Funakoshi, N. Iino, H. Shimokawa, K. Egashira, and A. Takeshita. 2001. Thrombin induces interleukin-6 expression through the cAMP response element in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 21:1759-1763. [DOI] [PubMed] [Google Scholar]
- 64.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 65.Vila-Coro, A. J., J. M. Rodriguez-Frade, D. A. Martin, M. C. Moreno-Ortiz, A. Martinez, and M. Mellado. 1999. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 13:1699-1710. [PubMed] [Google Scholar]
- 66.Wedgwood, S., R. W. Dettman, and S. M. Black. 2001. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L1058-L1067. [DOI] [PubMed] [Google Scholar]
- 67.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]
- 68.Wick, K. R., and M. T. Berton. 2000. IL-4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol. Immunol. 37:641-652. [DOI] [PubMed] [Google Scholar]
- 69.Wong, A. H., J. E. Durbin, S. Li, T. E. Dever, T. Decker, and A. E. Koromilas. 2001. Enhanced antiviral and antiproliferative properties of a STAT1 mutant unable to interact with the protein kinase PKR. J. Biol. Chem. 276:13727-13737. [DOI] [PubMed] [Google Scholar]
- 70.Wong, M., S. Uddin, B. Majchrzak, T. Huynh, A. E. Proudfoot, L. C. Platanias, and E. N. Fish. 2001. Rantes activates Jak2 and Jak3 to regulate engagement of multiple signaling pathways in T cells. J. Biol. Chem. 276:11427-11431. [DOI] [PubMed] [Google Scholar]
- 71.Yamakawa, T., S. Tanaka, K. Numaguchi, Y. Yamakawa, E. D. Motley, S. Ichihara, and T. Inagami. 2000. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension 35:313-318. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto, K., F. W. Quelle, W. E. Thierfelder, B. L. Kreider, D. J. Gilbert, N. A. Jenkins, N. G. Copeland, O. Silvennoinen, and J. N. Ihle. 1994. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol. Cell Biol. 14:4342-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeh, T. C., and S. Pellegrini. 1999. The Janus kinase family of protein tyrosine kinases and their role in signaling. Cell Mol. Life Sci. 55:1523-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhuang, H., Z. Niu, T. C. He, S. V. Patel, and D. M. Wojchowski. 1995. Erythropoietin-dependent inhibition of apoptosis is supported by carboxyl-truncated receptor forms and blocked by dominant-negative forms of Jak2. J. Biol. Chem. 270:14500-14504. [DOI] [PubMed] [Google Scholar]