Abstract
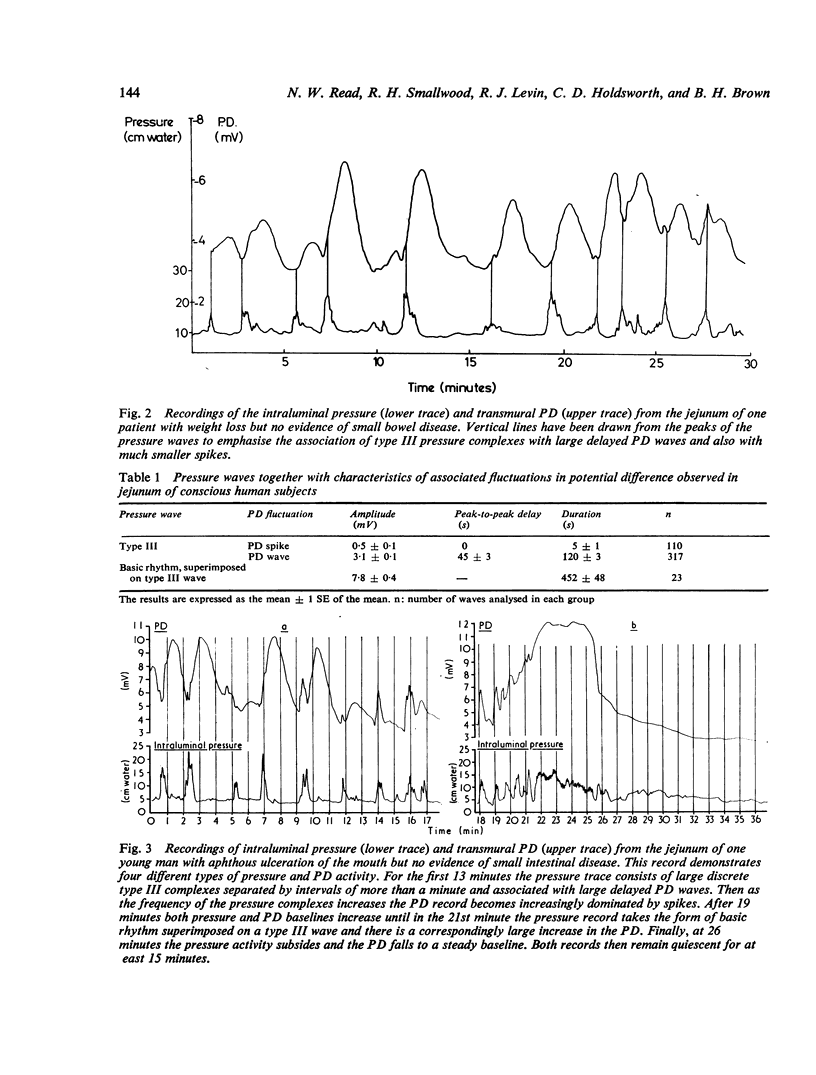
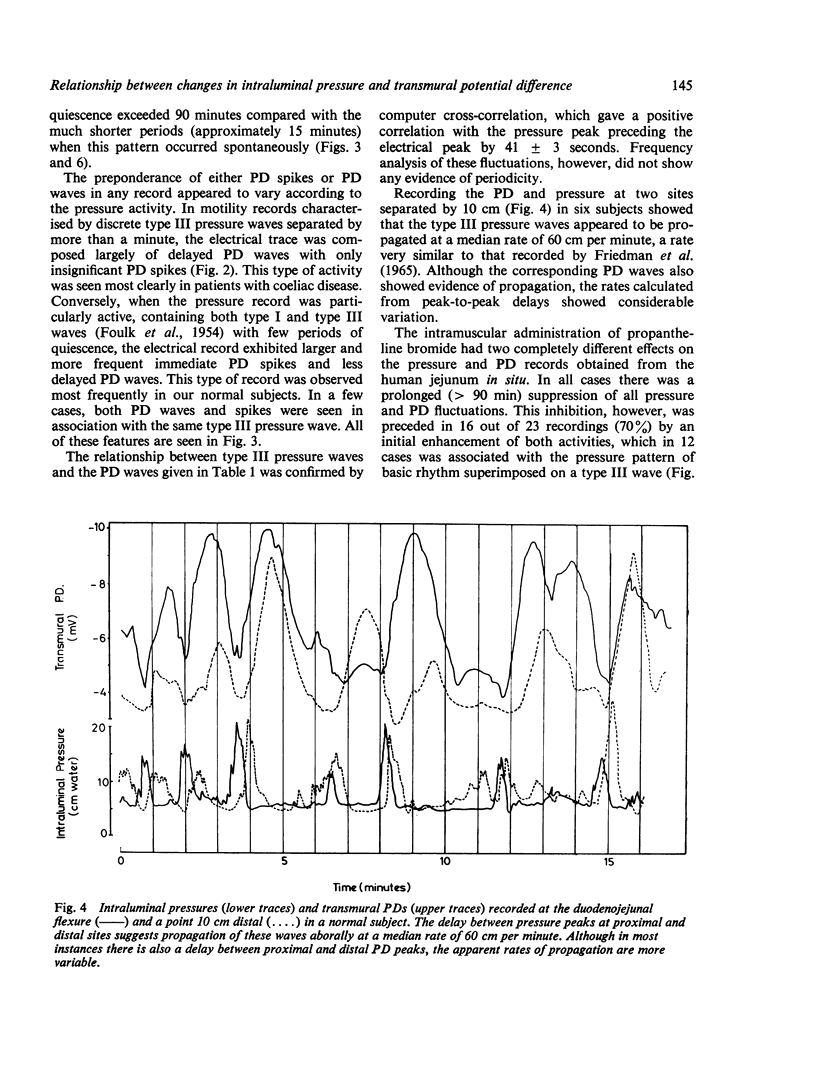
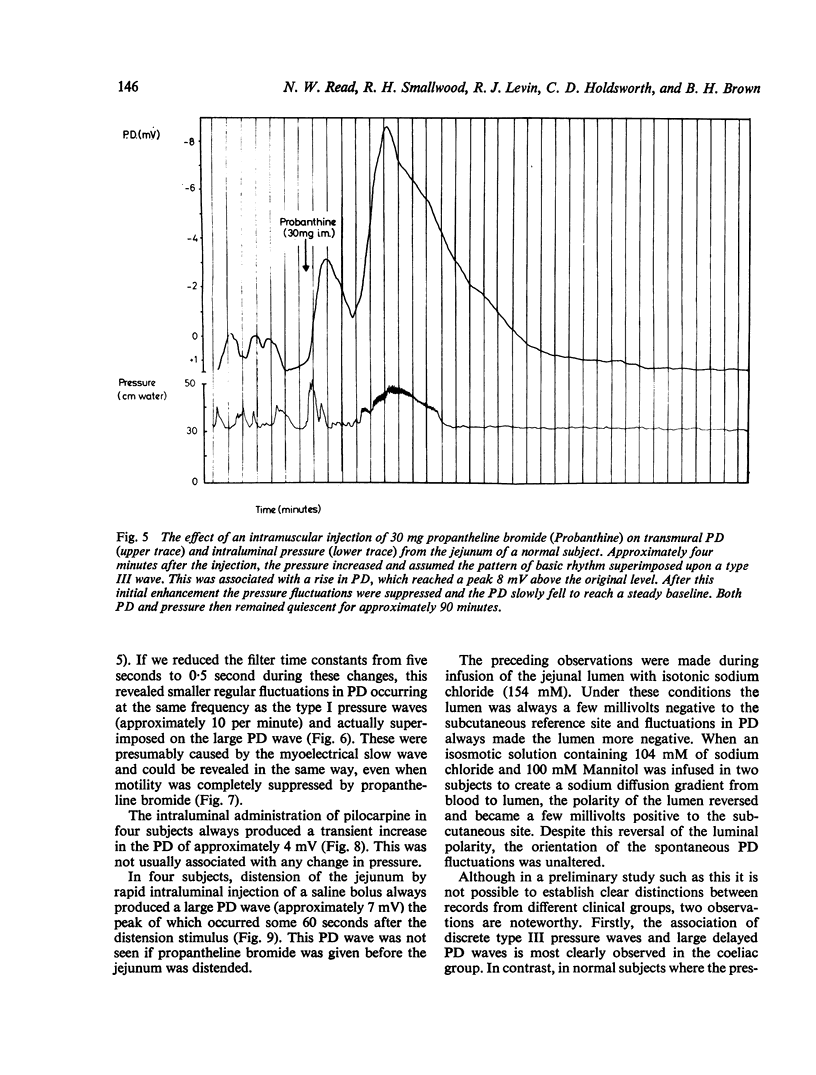
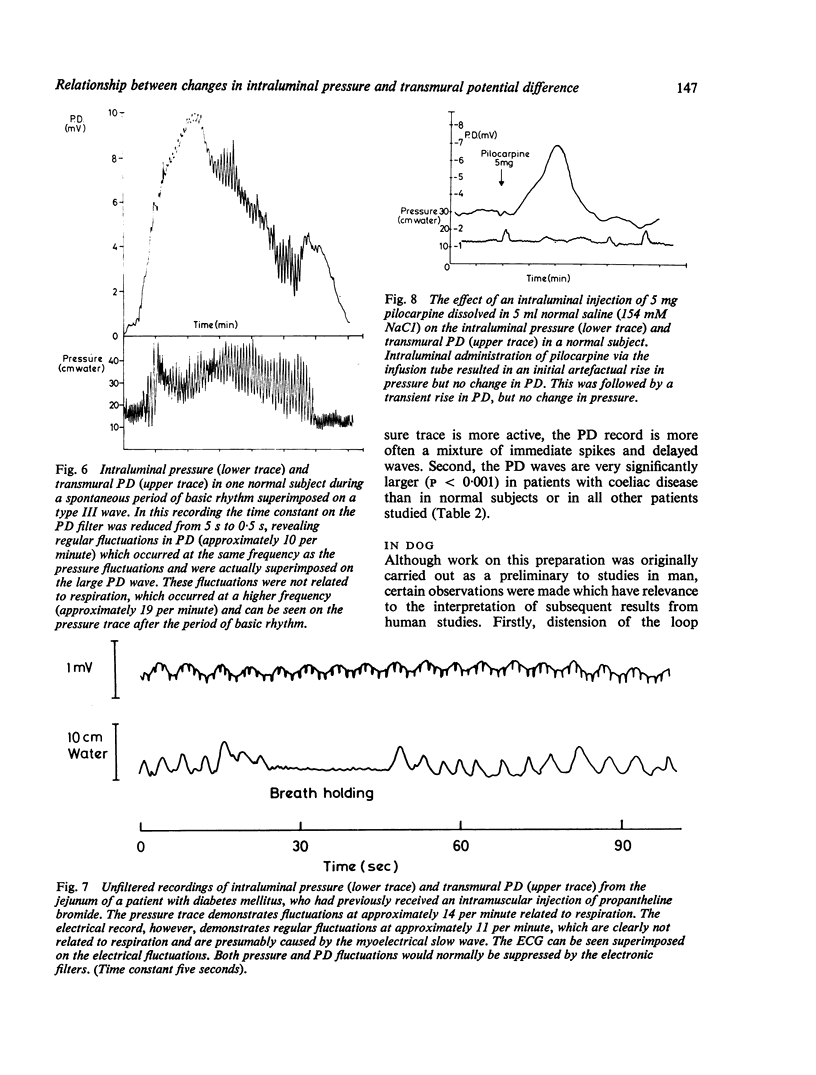
Recordings of transmural potential difference (PD) across the jejunum of conscious man in situ are characterised by spontaneous fluctuations of up to 10 mV. In 25 of 31 subjects (comprising seven normal controls and 24 patients under investigation for malabsorption, six of whom had coeliac disease) we observed a clear association between these fluctuations and changes in intraluminal pressure recorded at the same site. The most frequent PD changes were associated with type III pressure waves. These consisted predominantly of large waver (3-1 +/- 0-1 mV; mean +/- SEM, n = 317) which reached maximal amplitude approximately 45 seconds after the pressure peak and had a duration of 120 +/- 3 s, but also included less frequent spikes (0-5 +/- 0-1 mV; n = 110) concurrent with the pressure wave with a duration of 5 +/- 1 s. Although by recording at two sites in the jejunum 10 cm apart we were able to demonstrate that type III pressure waves appeared to be propagated aborally at a median rate of 60 cm per minute, the apparent rates of propagation of the corresponding PD waves were much more variable. The largest PD changes (7-8 +/- 0-4 mV; n = 19), lasting several minutes, were found in association with runs of type I waves (basic rhythm) superimposed on a type III wave. Both pressure and PD activities were suppressed by intramuscular propantheline bromide. Intraluminal pilocarpine caused a transient rise in PD not always accompanied by a change in pressure. Distention of the jejunum by rapid injection of a bolus of isotonic sodium chloride produced a delayed rise in the PD which could be prevented by prior administration of propantheline bromide. Experiments using Thirty-Vella loops of proximal jejunum in conscious dogs confirmed the effect of jejunal distension on the PD and also demonstrated that spontaneous retching is preceded by an increase in the PD. Consideration of these results in conjunction with data from other workers suggests the hypothesis that the larger spontaneous fluctuations in transmural PD in the jejunum of conscious man are caused by changes in electrogenic secretion associated with intestinal motility and mediated by cholinergic mechanisms. The possible association of increased secretory activity with motility may have functions of lubrication as well as diluting and mixing the chyme for easier digestion and absorption.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown B. H., Holdsworth C. D., Levin R. J., Read N. W., Smallwood R. H. Proceedings: The relationship between intestinal motility and fluctuations in transmural potential difference in the human jejunum. J Physiol. 1976 Jul;259(1):29P–30P. [PubMed] [Google Scholar]
- CROSBY W. H., KUGLER H. W. Intraluminal biopsy of the small intestine; the intestinal biopsy capsule. Am J Dig Dis. 1957 May;2(5):236–241. doi: 10.1007/BF02231100. [DOI] [PubMed] [Google Scholar]
- FOULK W. T., CODE C. F., MORLOCK C. G., BARGEN J. A. A study of the motility patterns and the basic rhythm in the duodenum and upper part of the jejunum of human beings. Gastroenterology. 1954 Apr;26(4):601–611. [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Carter N. W. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968 Apr;47(4):884–900. doi: 10.1172/JCI105781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. A. The nervous pathways of intestinal reflexes associated with nausea and vomiting. J Physiol. 1947 Mar 15;106(1):95–103. [PMC free article] [PubMed] [Google Scholar]
- Hardcastle P. T., Eggenton J. The effect of acetylcholine on the electrical activity of intestinal epithelial cells. Biochim Biophys Acta. 1973 Feb 27;298(1):95–100. doi: 10.1016/0005-2736(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Kazić T., Varagić V. M. Effect of increased intraluminal pressure on the release of acetylcholine from the isolated guinea-pig ileum. Br J Pharmacol Chemother. 1968 Jan;32(1):185–192. doi: 10.1111/j.1476-5381.1968.tb00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuchansky C., Huet P. M., Mary J. Y., Rambaud J. C., Bernier J. J. Effects of cholecystokinin and metoclopramide on jejunal movements of water and electrolytes and on transit time of luminal fluid in man. Eur J Clin Invest. 1972 Mar;2(3):160–175. doi: 10.1111/j.1365-2362.1972.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Moore W. L., Jr, Bieberdorf F. A., Morawski S. G., Finkelstein R. A., Fordtran J. S. Ion transport during cholera-induced ileal secretion in the dog. J Clin Invest. 1971 Feb;50(2):312–318. doi: 10.1172/JCI106496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W., Holdsworth C. D., Levin R. J. Electrical measurement of intestinal absorption of glucose in man. Lancet. 1974 Sep 14;2(7881):624–627. doi: 10.1016/s0140-6736(74)91946-1. [DOI] [PubMed] [Google Scholar]
- Read N. W., Levin R. J., Holdsworth C. D. Electrogenic glucose absorption in untreated and treated coeliac disease. Gut. 1976 Jun;17(6):444–449. doi: 10.1136/gut.17.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. A., Salem S. N. Upper intestinal motility in ulcerative colitis, idiopathic steatorrhoea, and the irritable colon syndrome. Gut. 1965 Aug;6(4):325–337. doi: 10.1136/gut.6.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachar D. B., Taylor J. O., Saha J. R., Phillips R. A. Intestinal transmural electric potential and its response to glucose in acute and convalescent cholera. Gastroenterology. 1969 Mar;56(3):512–521. [PubMed] [Google Scholar]
- TIDBALL C. S. Active chloride transport during intestinal secretion. Am J Physiol. 1961 Feb;200:309–312. doi: 10.1152/ajplegacy.1961.200.2.309. [DOI] [PubMed] [Google Scholar]
- Wingate D. L., Hayward M. G., Johnson C. M., Marczewski A. G., Petty R. G., Wilson E. J. Physiological changes in human transjejunal potential difference. Scand J Gastroenterol. 1973;8(6):473–478. [PubMed] [Google Scholar]
- Wingate D., Green R., Symes J., Pilot M. Interpretation of fluctuation of transmural potential difference across the proximal small intestine. Gut. 1974 Jul;15(7):515–520. doi: 10.1136/gut.15.7.515. [DOI] [PMC free article] [PubMed] [Google Scholar]


