Abstract
Activation of the macrophage cell line RAW 264.7 with lipopolysaccharide (LPS) transiently activates protein kinase C ζ (PKCζ) and Jun N-terminal kinase (JNK) through a phosphoinositide-3-kinase (PI3-kinase)-dependent pathway. Incubation of LPS-treated cells with the cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2) promoted a sustained activation of PKCζ and JNK and inhibited IκB kinase (IKK) and NF-κB activity. Accordingly, 15dPGJ2 induced an imbalance between JNK and IKK activities by increasing the former signaling pathway and inhibiting the latter signaling pathway. Under these conditions, apoptosis was significantly enhanced; this response was very dependent on PKCζ and JNK activation. The effect of 15dPGJ2 on PKCζ activity observed in LPS-activated macrophages was not dependent on a direct action of this prostaglandin on the enzyme but was due to the activation of a step upstream of PI3-kinase. Moreover, LPS promoted the redistribution of activated PKCζ from the cytosol to the nucleus, a process that was enhanced by treatment of the cells with 15dPGJ2 that favored a persistent and broader distribution of PKCζ in the nucleus. These results indicate that 15dPGJ2 and other cyclopentenone prostaglandins, through the sustained activation of PKCζ, might contribute significantly to the process of resolution of inflammation by promoting apoptosis of activated macrophages.
Macrophage activation is an essential step in the innate immune response and constitutes the first line of defense against bacterial, fungal, and viral infection. In addition to the action as phagocytic and antigen-presenting cells, macrophages release a variety of inflammatory mediators that modulate the immune response and encounter the infectious process (1, 47). Due to potential autotoxicity, the production of these molecules should be transient, upon demand, and efficient negative signaling cascades are essential to prevent this toxicity. In recent years, mechanisms that control the resolution of inflammation, when macrophage activation is no longer required, have been studied.
Cyclopentenone prostaglandins (PGs) have been considered important contributors to the resolution of inflammation due to their role as anti-inflammatory mediators. PGA2, PGA1, and PGJ2 are derived from arachidonic acid metabolism, whereas 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2) is formed by dehydration and subsequent modification of PGD2 (66). 15dPGJ2 is abundantly produced by mononuclear cells and has been proposed as an important immunoregulatory lipid mediator for a number of reasons. First, it can be detected at the nanomolar range in the resolution phase in a model of acute inflammation in rats (15, 29, 55). Second, 15dPGJ2 can repress the expression of most genes related to the onset of the inflammatory response in macrophages, such as nitric oxide synthase 2 (NOS-2), cyclooxygenase 2, tumor necrosis factor alpha (TNF-α), and gelatinase B (11, 37, 59, 60, 67). Third, this lipid metabolite promotes apoptosis in different cell types mainly through an increase in reactive oxygen species production (35, 46). In 1995, 15dPGJ2 was reported to be a high-affinity ligand for peroxysomal proliferator-activated receptor γ (PPAR-γ), and many of its effects have been attributed to the activation of this nuclear receptor (8, 25, 26). However, there is increasing evidence that 15dPGJ2 has many PPAR-γ-independent effects (30). Indeed, the PGJ and PGA series have a characteristic α,β-unsaturated carbonyl group that can modify accessible cysteine residues via Michael addition reactions (61, 66). This is the case for 15dPGJ2-mediated inhibition of IκB kinase 2 (IKK2) activity and the binding of nuclear factor κB (NF-κB) to specific DNA sequences reinforcing the view of 15dPGJ2 as a nuclear receptor-independent anti-inflammatory molecule (13, 61, 62, 67). Activation of NF-κB has been described as a key survival step through the expression of antiapoptotic genes of the Bcl-2 family and various members of the IAP family, which explains its capacity to inhibit caspase activation (3, 6, 75). In addition to this, it has been shown in cells treated with proinflammatory cytokines that inhibition of NF-κB leads to a sustained activation of Jun N-terminal kinase (JNK) that is involved in the apoptotic response through the expression of proapoptotic genes (21, 44, 71). However, in myeloid cells and in fibroblasts lacking NF-κB activity, the sustained activation of JNK in response to proinflammatory cytokines appears to exert an antiapoptotic effect, reinforcing the relevance of the cell type in the cross talk between pathways that regulate the survival and cell death balance (58).
Protein kinase C (PKC) was first identified as a phospholipid-dependent serine/threonine kinase (52), and members of the PKC family play crucial roles in controlling a broad array of cellular functions (48). Targeted disruption of several PKC genes has revealed an important contribution for some isotypes in the regulation of specific functions in cells of the immune system. Remarkably, macrophages from PKCɛ knockout mice show an attenuated inflammatory response after lipopolysaccharide (LPS) challenge (12); PKCθ plays an important role in NF-κB activation in mature lymphocytes as deduced from mice lacking this gene (68), and PKCζ mutant mice display impairment in NF-κB activation in response to TNF-α (45). In particular, PKCζ has been reported to be involved in LPS signaling cascades in macrophages (56). For this reason, we decided to analyze the effects of cyclopentenone PGs on the activity and biological role of this enzyme (57). Here we show that in LPS-stimulated macrophages, 15dPGJ2 inhibits NF-κB but further activates PKCζ because of an increase in the activity of the phosphoinositide-3-kinase (PI3-kinase)/phosphoinositide-dependent protein kinase 1 (PDK-1) pathway (18, 57). This increase in PKCζ activity induced by 15dPGJ2 enhances the activities of JNK and extracellular regulated protein kinase (ERK), producing an imbalance between JNK/mitogen-activated protein kinase (MAPK) and NF-κB pathways that results in an intensification of apoptosis that contributes to the resolution of inflammation.
MATERIALS AND METHODS
Chemicals.
Reagents were from Sigma (St. Louis, Mo.), Roche (Barcelona, Germany), and Merck (Darmstadt, Germany). Antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.), New England Biolabs (Beverly, Mass.), and Upstate Biotech (Lake Placid, N.Y.). PGs and PKC and JNK inhibitors were from Calbiochem (San Diego, Calif.). Serum and media were from Biowhittaker (Walkersville, Md.). Anti-PDK-1 antibody (Ab) was a generous gift from D. R. Alessi (Dundee, United Kingdom). Hemagglutinin (HA)-tagged atypical PKCs that were dominant negative (DN) and GFP-JNK1 DN and GFP-JNK2 DN plasmids were a generous gift from J. Moscat and A. Porras (Madrid, Spain), respectively. Biotinylated 15dPGJ2 was a gift from D. Pérez-Sala (Madrid, Spain) (13).
Cell culture.
RAW 264.7 cells were subcultured at 6 × 104 to 8 × 104/cm2 in RPMI 1640 medium supplemented with 2 mM glutamine, 10% fetal calf serum (FCS), and antibiotics (penicillin, streptomycin, and gentamicin [50 μg/ml each]). After 2 days in culture, the medium was replaced by RPMI 1640 medium containing 1% FCS, and cells were used in the next 24 h. PGs, rosiglitazone, a pharmacological agonist of PPAR-γ, and inhibitors of protein kinases were added 20 min prior to activation with LPS.
Preparation of plasmids and transfection of cells.
The DH5αF' strain of Escherichia coli was transformed with the plasmids PKCα DN, PKCɛ DN, HA-PKCζ DN, HA-PKCλ/ι, GFP-PKCζ, PKCζ-E410, PKCζ-A410, GFP-JNK1 DN, GFP-JNK2 DN, and p110CAAX that have been described previously (18, 24, 43, 65, 73). After bacterial growth, the plasmids were purified using EndoFree Qiagen columns (Hilden, Germany) (11). For transfection, subconfluent RAW 264.7 cells were transfected for 6 h with FuGENE 6 and 1 μg of DNA per dish (2.5-cm diameter), except for the HA-tagged PKC isotypes (200 ng, unless stated otherwise), following the instructions of the supplier (Roche), and kept overnight with 2 ml of RPMI 1640 medium plus 10% FCS. When transfected, cells were selected by cell sorting, a green fluorescent protein (GFP) plasmid (in a 1:4 DNA ratio) was cotransfected. After the cells were detached with ice-cold phosphate-buffered saline (PBS), the GFP-positive population was collected in a cell sorter (5 × 105 to 7 × 105 cells), seeded at 2 × 105 cells per cm2, and stimulated as indicated in the figures. The AP-1-dependent luciferase reporter plasmid (2 μg/ml; AP-1-LUC) or the promoterless luciferase reporter vector (p-LUC) was cotransfected with 0.3 μg of the β-galactosidase reporter vector per ml under control of the cytomegalovirus promoter (pCMV-β-gal; Clontech, Palo Alto, Calif.) that was used as a control in transfection experiments. Luciferase and β-galactosidase activities were measured using a commercial kit (Promega).
Preparation of cytosolic and nuclear extracts.
The macrophage layers (1.5 × 106 cells) were washed with ice-cold PBS, and cells were collected by centrifugation (11, 74). The cell pellets were homogenized in 100 μl of buffer A (10 mM HEPES [pH 7.9], 1 mM EDTA, 1 mM EGTA, 100 mM KCl, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg of aprotinin per ml, 10 μg of leupeptin per ml, 2 μg of Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK] per ml, 5 mM NaF, 1 mM NaVO4, 10 mM Na2MoO4). After 10 min at 4°C, Nonidet P-40 was added (0.5%, vol/vol), and the tubes were gently vortexed for 15 s. Nuclei were collected by centrifugation at 8,000 × g for 15 min, and the supernatants were stored at −80°C (cytosolic extracts). To obtain nuclear protein extracts, the pellets were resuspended in 50-μl portions of buffer A supplemented with 20% glycerol and 0.4 M KCl, gently shaken for 30 min at 4°C, and centrifuged at 13,000 × g for 15 min. The supernatant was stored at −80°C (nuclear extracts). Protein content was assayed using the detergent-compatible Bio-Rad protein reagent.
EMSAs.
The sequence 5′TGCTAGGGGGATTTTCCCTCTCTCTGT3′, corresponding to the distal NF-κB binding site (nucleotides −978 to −952) of the murine nitric oxide synthase 2 (NOS-2) promoter (77) was annealed with the complementary sequence and end labeled with Klenow enzyme fragment in the presence of 50 μCi of [α-32P]dCTP and the other three unlabeled deoxynucleoside triphosphates in a final volume of 50 μl (11). The DNA probe (5 × 104 dpm per assay) was incubated for 15 min at 4°C in a solution containing 3 μg of nuclear protein, 2 μg of poly(dI-dC), 5% glycerol, 1 mM EDTA, 100 mM KCl, 5 mM MgCl2, 1 mM DTT, and 10 mM Tris-HCl (pH 7.8) in a final volume of 20 μl. The DNA-protein complexes were separated on native 6% polyacrylamide gels in 0.5% Tris-borate-EDTA buffer (64). Supershift assays were performed after incubation (1 h at 4°C) of the nuclear extracts with 2 μg of Ab against the c-Rel proteins p50, c-Rel, and p65, followed by electrophoretic mobility shift assays (EMSAs) (not shown).
Characterization of proteins by Western blotting.
Cytosolic protein extracts were separated by size by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE). The gels were blotted onto a Hybond-P membrane (Amersham) and incubated with the following Abs: rabbit antiserum directed against P-T410-PKCζ (PKCζ with the T410 residue phosphorylated) (previously described [43, 57]); anti-PKCζ (an atypical PKC [aPKC] [sc-216], recognizing PKCζ and PKCλ/ι, and the Ab specific for PKCζ [sc-7262]); anti-PKCλ/ι (sc-11399), anti-IκBα (sc-371) and anti-IKK-1 (sc-7182) from Santa Cruz Biotechnology; anti-P-c-Jun and anti-p85α (PI3-kinase subunit) from Upstate Biotech; anti-P-S32-IκBα, anti-P-MAPKs and anti-MAPKs (New England Biolabs); and sheep anti-PDK-1 antiserum, a gift from D. R. Alessi (4). The blots were submitted to sequential reprobing with Abs after treatment with 100 mM β-mercaptoethanol and 2% SDS in Tris-buffered saline, and heated at 60°C for 30 min. The blots were revealed by enhanced chemiluminescence following the manufacturer's instructions (Amersham).
Confocal microscopy.
RAW 264.7 cells were grown on coverslips and incubated for 30 min with the indicated stimuli. After the coverslips were washed twice with ice-cold PBS, the cells were fixed for 2 min with methanol at −20°C, blocked for 30 min at room temperature with 3% bovine serum albumin, and incubated for 30 min with anti-P-T410-PKCζ Ab diluted 1:200. After the coverslips were washed with PBS, the cells were incubated with anti-rabbit immunoglobulin G (IgG) Ab (diluted 1:500) conjugated with Cy3 (Amersham). The cells were visualized using an MRC-1024 confocal microscope (Bio-Rad), and the fluorescence was measured using Laser-sharp software (Bio-Rad). Cells expressing GFP-PKCζ were directly visualized in the confocal microscope.
Measurement of PKCζ, IKK, and JNK activities.
Cells (107) were homogenized in 1 ml of buffer A and centrifuged for 10 min in a microcentrifuge. The supernatant was precleared, and PKCζ and IKK were immunoprecipitated with 1 μg of specific Ab. After the immunoprecipitate was washed with 4 ml of buffer A, the pellet was resuspended in kinase buffer (20 mM HEPES [pH 7.4], 0.1 mM EDTA, 100 mM NaCl, 1 mM DTT, 0.5 mM PMSF, 2 μg of aprotinin per ml, 10 μg of leupeptin per ml, 2 μg of TLCK per ml, 5 mM NaF, 1 mM NaVO4, 10 mM Na2MoO4, 10 nM okadaic acid). Kinase activity was assayed in 100 μl of kinase buffer containing 100 ng of immunoprecipitated protein and 50 μM [γ-32P]ATP (0.5 μCi), using myelin basic protein (MBP) as the substrate for PKCζ and PKCλ/ι and using glutathione S-transferase (GST)-IκBα as the substrate for IKK. The reactions were stopped by adding 1 ml of ice-cold buffer A supplemented with 5 mM EDTA (11). JNK activity was measured in cell extracts after pulldown of JNKs with GST-c-Jun (amino acids 1 to 79) following a protocol described elsewhere (73). The kinase assay was performed in the presence of 20 μM ATP (0.3 μCi of [γ-32P]ATP), and the phosphorylation of c-Jun was determined after SDS-PAGE and autoradiography.
Assay of PI3-kinase activity.
PI3-kinase activity was measured in the supernatants from cell extracts in buffer A after immunoprecipitation with anti-p85α Ab following the instructions of the supplier (Upstate Biotech). The activity of PI3-kinase present in the resuspended immunoprecipitate was determined using phosphatidylinositol (20 μg) and [γ-32P]ATP (22). After thin-layer chromatography, the amount of phosphorylated lipids (PIP) was evaluated using a FUJI BAS1000 detector.
Measurement of DEVDase activity.
Cell extracts were prepared in buffer A containing 0.5% Nonidet P-40. After centrifugation for 10 min in an Eppendorf centrifuge, DEVDase activity (corresponding mainly to caspases 3 and 7) was determined in the supernatant using N-acetyl-DEVD-7-amino-4-trifluoromethylcoumarin as the substrate and monitoring the fluorescence of the reaction product according to the instructions of the supplier (Calbiochem). The linearity of the caspase assay was determined over a 30-min reaction period (35).
Measurement of apoptosis by FACS analysis.
Propidium iodide staining of apoptotic cells was performed following a previous protocol (34-36). Cells were resuspended in PBS and analyzed in a FACScan cytometer (Becton & Dickinson) equipped with a 25-mW argon laser. The percentage of apoptotic cells was assessed using a dot plot of the forward scatter against the propidium iodide fluorescence. Cell sorting and analysis of DNA integrity of viable and apoptotic populations were performed to confirm the gating criteria (35). The percentage of apoptotic cells after transfection with PKC plasmids was determined by including a pGFP vector at a 1:4 ratio and gating cells positive for GFP expression. When cells were transfected with GFP-JNK1 DN and GFP-JNK2 DN, a similar protocol was followed to evaluate apoptosis.
Data analysis.
The number of experiments analyzed is indicated in the figures. Statistical differences (P < 0.05) between mean values were determined by one-way analysis of the variance followed by Student's t test. In experiments using X-ray films (Hyperfilm), different exposure times were employed to avoid saturation of the bands.
RESULTS
15dPGJ2 enhances PKCζ activation and inhibits IKK in macrophages challenged with LPS.
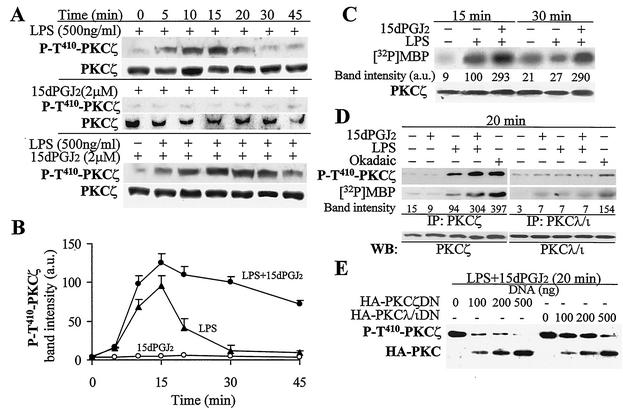
Treatment of RAW 264.7 cells with LPS resulted in the transient activation of PKCζ as deduced by the phosphorylation of the T410 residue (18, 57) using a specific Ab (maximal effect observed at 15 min) (Fig. 1A and B). This activation can also be monitored by the phosphorylation of MBP in an in vitro assay using the immunoprecipitated enzyme (Fig. 1C). Incubation of cells with the cyclopentenone PG 15dPGJ2 increased the time span of LPS-dependent activation of PKCζ for more than 30 min (Fig. 1A to C). The LPS-induced phosphorylation of the T410 activation loop, a domain conserved in aPKCs, was mainly due to PKCζ modification, since it was observed only after immunoprecipitation of PKCζ, but not when the cell extract was immunoprecipitated with a selective anti-PKCλ/ι Ab. Similar results were obtained when the enzyme activity was measured using MBP as the substrate (Fig. 1D). However, when cells were treated with 100 nM okadaic acid, a condition that favors the phosphorylation of T-410 by inhibiting protein phosphatase 2A activity, the anti-P-T410 PKC Ab recognized the phosphorylation of the activation loop in both aPKCs at the time that an increase in the catalytic activity was observed. In addition to this, when cells were transfected with tagged DN forms of PKCζ and PKCλ/ι, the phosphorylation of T-410 was inhibited, making PKCζ a more-efficient inhibitor than PKCλ/ι (Fig. 1E). Indeed, because both aPKCs have significant homology in the regulatory domains (24), overexpression of these DN forms abolished the phosphorylation of T410 in the activation loop, regardless of the aPKC isotype.
FIG. 1.
15dPGJ2 increased PKCζ activation in response to LPS challenge of macrophages. RAW 264.7 cells were incubated for 20 min with 2 μM 15dPGJ2 or 100 nM okadaic acid before stimulation with 500 ng of LPS per ml as indicated above the blots. (A) The phosphorylation in T410 of PKCζ was analyzed by Western blotting. (B) Quantitative analysis of the band intensities (in arbitrary units [a.u.]) of P-T410-PKCζ (mean ± standard deviation; n = 4). (C) PKCζ was immunoprecipitated from cells treated for 15 and 30 min as indicated over the blot, and the activity was determined using MBP as the substrate. (D) The isotype specificity of the LPS-dependent phosphorylation in T410 was assessed after immunoprecipitation (IP) of PKCζ and PKCλ/ι with specific Abs and followed by Western blotting (WB) using the anti-P-T410-PKCζ Ab and the corresponding isotype-specific Abs; an aliquot of the immunoprecipitated aPKCs was used to determine its catalytic activity using MBP as the substrate. (E) Cells expressing HA-tagged PKCζ DN or PKCλ/ι DN were incubated for 20 min with LPS and 15dPGJ2, and the phosphorylation of the T410 motif of aPKCs was determined by Western blotting. The blots show the results of one representative experiment (of four experiments).
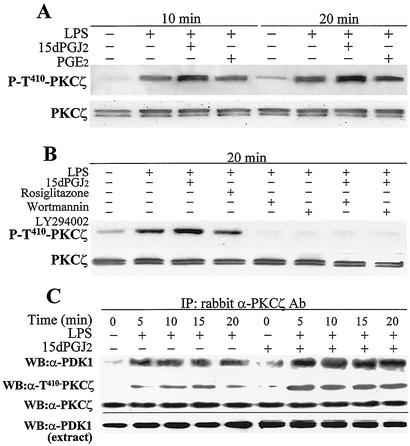
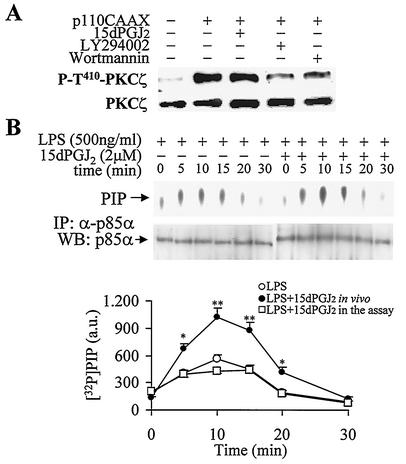
To evaluate the mechanisms by which 15dPGJ2 enhanced the LPS-dependent activation of PKCζ, macrophages were treated with this PG and with the noncylopentenone PGE2. As Fig. 2A shows, PGE2 was unable to increase the LPS-dependent phosphorylation at the T410 site of PKCζ. The specific pharmacological PPAR-γ agonist rosiglitazone also failed to increase PKCζ activation and was used to differentiate the effects dependent on the cyclopentenone motif of 15dPGJ2 from those mediated through PPAR-γ (Fig. 2B). In addition to this, the inhibitors of PI3-kinase wortmannin and LY294002 eliminated the LPS-dependent phosphorylation of T410 PKCζ regardless of the treatment with 15dPGJ2 (Fig. 2B). Moreover, when PKCζ was immunoprecipitated with a specific Ab, an enhancement in the band intensity of PDK-1 present in this fraction was observed in cells treated with 15dPGJ2, which suggests an association between PDK-1 and the activation of PKCζ (Fig. 2C). To further determine the contribution of PI3-kinase to the activation of PKCζ and the potentiation by 15dPGJ2, RAW 264.7 cells were transfected with p110CAAX plasmid to express constitutive PI3-kinase activity, and the phosphorylation of PKCζ in T410 was analyzed. As Fig. 3A shows, after transfection with p110CAAX, PKCζ was phosphorylated. This was not affected by 15dPGJ2 treatment but was significantly inhibited by wortmannin or LY294002, which suggests that PI3-kinase is necessary for PKCζ activation in LPS-treated macrophages. To corroborate this point, the activity of PI3-kinase was measured in these cells. Incubation of macrophages with 15dPGJ2 increased p85-associated PI3-kinase activity; this was not a direct effect, since the activity of the PI3-kinase assayed in vitro was not influenced by the addition of 15dPGJ2 in the assay (Fig. 3B).
FIG. 2.
Effects of PGs on PKCζ phosphorylation and elimination of the activation by PI3-kinase inhibitors. (A and B) Macrophages were preincubated for 20 min with the PGs, the PPAR-γ agonist rosiglitazone (2 μM), and the inhibitors of PI3-kinase wortmannin (200 nM) and LY294002 (20 μM) followed by activation with 500 ng of LPS per ml. Cell extracts were prepared, and the phosphorylation of T410-PKCζ was determined by Western blotting. (C) Association of PDK-1 with activated PKCζ was analyzed after immunoprecipitation (IP) of cell extracts with anti-PKCζ Ab followed by Western blotting (WB) using specific anti-PDK-1 Ab (α-PDK1) and anti-P-T410-PKCζ Ab (α-T410-PKCζ). Anti-PKCζ and anti-PDK-1 Abs were used to evaluate the PKCζ loads in the lanes of the Western blots and the amounts of PDK-1 in the cell extracts. Results are from one representative experiment (of three experiments).
FIG. 3.
15dPGJ2 increased PKCζ activation through a PI3-kinase-dependent process. RAW 264.7 cells were transfected for 6 h with the p110CAAX plasmid and maintained in culture for 14 h. (A) The phosphorylation at T410 of PKCζ was determined after 30 min of incubation with 2 μM 15dPGJ2, 200 nM wortmannin, and 20 μM LY294002. The activity of PI3-kinase (in arbitrary units [a.u.]) was measured by the synthesis of PIP in immunocomplexes from cells treated for the indicated times with 15dPGJ2 and LPS. (B) Immunoprecipitated (IP) PI3-kinase from LPS-treated cells was incubated for 10 min with 2 μM 15dPGJ2 to evaluate the direct effect of this PG on enzyme activity. WB, Western blot; α-p85α, anti-p85α Ab. Results are from one representative experiment (of three experiments) for panel A or are the means ± standard deviations for three experiments for panel B. Values that are significantly different from the value for the corresponding condition in the absence of 15dPGJ2 are indicated (P < 0.05 [*] and P < 0.01 [**]).
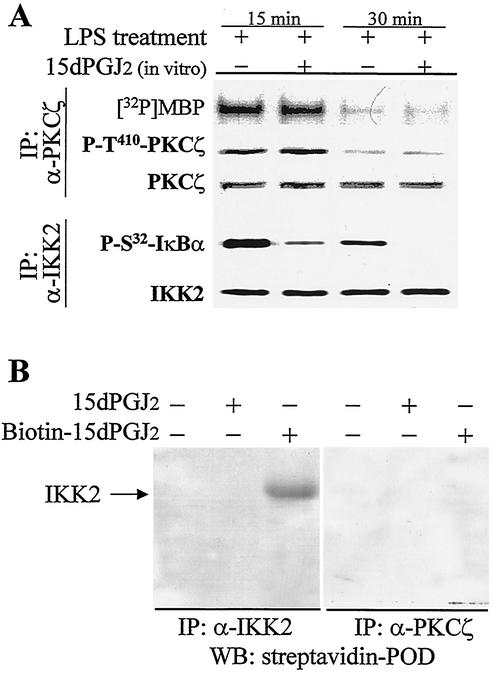
The activity of PKCζ is not directly affected by 15dPGJ2.
To evaluate the possibility of a direct effect of 15dPGJ2 on PKCζ phosphorylation and activity, cell extracts from LPS-activated macrophages were prepared and assayed in vitro in the presence of the PG. As Fig. 4A shows, samples obtained at 15 min exhibited enhanced activity (with MBP as the substrate) and an increase in T410 PKCζ phosphorylation. However, the presence of 15dPGJ2 in the assay did not modify either parameter. Extracts prepared after 30 min of LPS treatment, when activation of PKCζ is lost, failed to show an increase in activity after incubation with this PG. When these samples were analyzed after immunoprecipitation of the IKK complex, 15dPGJ2 inhibited IKK activity when GST-IκBα was the substrate. In addition, a biotinylated 15dPGJ2 derivative was unable to alkylate PKCζ, whereas a clear biotinylated IKK2 band was observed as a control (Fig. 4B).
FIG. 4.
15dPGJ2 does not activate PKCζ directly. (A) RAW 264.7 cells were treated with LPS (500 ng/ml) for 15 and 30 min. PKCζ and the IKK complex were immunoprecipitated, and the corresponding kinase activities were assayed in vitro in the absence (−) or presence (+) of 2 μM 15dPGJ2 using MBP (for PKCζ) or GST-IκBα (for IKK) as the substrate. The levels of P-T410-PKCζ and P-S32-IκBα were determined by Western blotting. Results are from one representative experiment (of three experiments) for panel A. (B) To evaluate the ability of 15dPGJ2 to alkylate IKK2 and PKCζ, macrophages were challenged for 15 min with LPS and the extract was treated for 10 min with 15dPGJ2 or biotinylated-15dPGJ2. PKCζ and IKK2 were immunoprecipitated (IP) with specific Abs (anti-PKCζ [α-PKCζ] and anti-IKK2 ]α-IKK2]), and the corresponding Western blots (WB) were revealed with streptavidin-peroxidase (POD).
15dPGJ2 increases MAPK activity through a mechanism dependent on PKCζ.
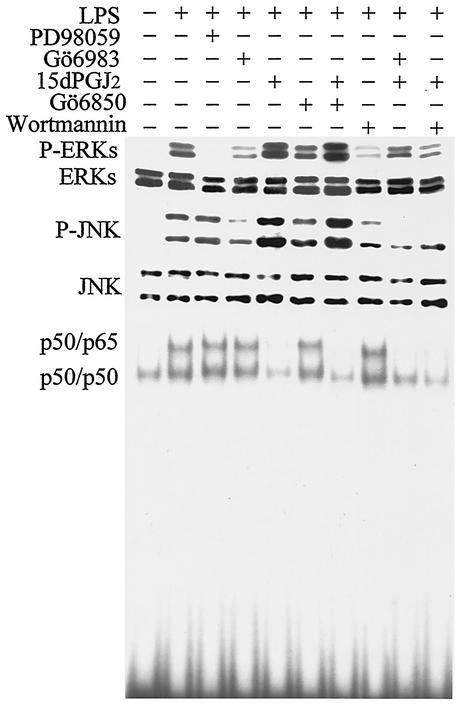
To identify targets of the sustained PKCζ activation in macrophages treated with LPS and 15dPGJ2, the levels of p42 and p44 phosphorylated ERKs (P-ERKs) and p46 and p54 P-JNK were analyzed. As Fig. 5 shows, treatment of cells with PD98059 inhibited ERK phosphorylation as expected. Significant inhibition was observed in cells treated with Gö6983, an inhibitor of classical PKCs (cPKCs) and aPKCs, but not with Gö6850, which inhibits cPKCs, but not aPKCs (54). Incubation of cells with 15dPGJ2 notably increased the intensity of the ERK phosphorylation, and Gö6983 and wortmannin, but not the cPKC inhibitor Gö6850, abolished this effect. In the case of JNK, an increase in the phosphorylation state was observed after incubation of the cells with 15dPGJ2, and again, this was prevented by Gö6983 and wortmannin, but not by Gö6850. Interestingly, when the activation of NF-κB was measured under these conditions, both PKC inhibitors were unable to modify the binding activity, at least at this sampling time (30 min) and at 60 min (not shown). As expected from previous work (11, 62), because of the inhibition of IKK2 by 15dPGJ2, NF-κB binding was abolished by this PG. Interestingly, wortmannin, which inhibits PKCζ activation, enhanced NF-κB binding as described previously (22). These data suggest that 15dPGJ2 inhibits NF-κB activity at the time that it potentiates ERK and JNK stimulation through a PKCζ-dependent mechanism.
FIG. 5.
PKCζ-dependent activation of ERK and JNK in response to 15dPGJ2 in macrophages challenged with LPS. Cells were preincubated (20 min) with 2 μM 15dPGJ2 and treated for 30 min with 200 ng of LPS per ml. The phosphorylation levels of p42 and p44 ERKs and p46 and p54 P-JNKs were determined by Western blotting. The activation of NF-κB was determined by EMSAs using the distal κB motif of the murine NOS-2 promoter. The nature of the retained complexes was determined by supershift assays (not shown). The inhibitors PD98059 (50 μM), Gö6983 (200 nM), Gö6850 (1 μM), and wortmannin (200 nM) were added 20 min prior to LPS. Results are from one representative blot (of four blots).
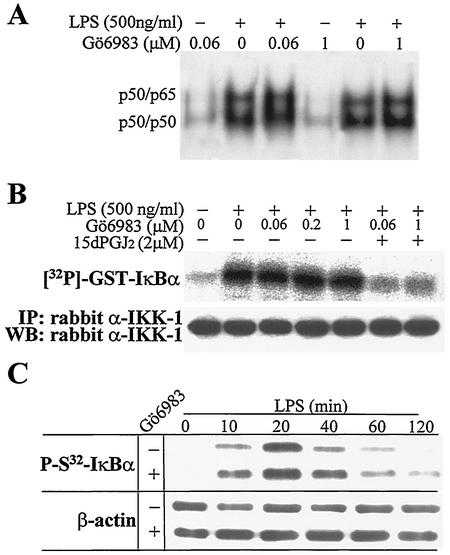
To analyze in more detail the effect of endogenous PKCζ on NF-κB activation in macrophages, a series of experiments were performed in view of the results from other groups (45) and the above data. Taking advantage of the observation that Gö6983 inhibits cPKCs at low concentrations (Ki = 6 to 10 nM) and aPKCs at higher concentrations (Ki = 60 nM) (54, 72, 76), we evaluated the effect of this inhibitor on NF-κB activity after 60 min of incubation of RAW 264.7 cells with LPS by EMSAs (see Discussion). As Fig. 6A shows, the binding of nuclear proteins to a κB motif was unaffected by this inhibitor. Moreover, when the activity of IKK from cells treated with LPS, Gö6983, and 15dPGJ2 was immunoprecipitated and assayed in vitro using GST-IκBα as the substrate, incubation with Gö6983 was unable to modulate IKK activity (cells treated from 0.06 to 1 μM), whereas 15dPGJ2 abolished this activity (Fig. 6B). In agreement with the previous data, the time course of IκBα phosphorylation in S32 measured using a specific antibody was also unaltered by incubation of cells with Gö6983 (Fig. 6C).
FIG. 6.
NF-κB activity was inhibited by 15dPGJ2 in macrophages treated with LPS. (A) RAW 264.7 cells were preincubated for 20 min with 15dPGJ2 or the indicated concentrations of Gö6983. The NF-κB binding was determined by EMSAs using protein nuclear extracts from cells activated for 60 min with LPS. (B) IKK activity was measured after immunoprecipitation (IP) of the IKK complex from cells treated for 20 min with the indicated molecules, using GST-IκBα as the substrate. WB, Western blotting; α-IKK-1, anti-IKK1. (C) The levels of P-S32-IκBα and β-actin were determined by Western blotting using specific Abs. Results are from one representative experiment (of three experiments).
15dPGJ2 favors the accumulation of activated PKCζ in the nucleus.
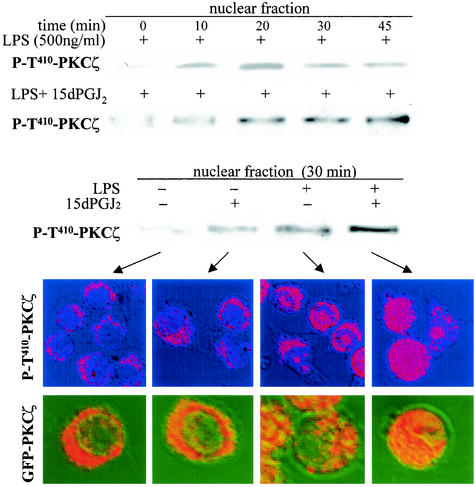
Previous work described a translocation of PKCζ from the cytosol to the nucleus upon cell activation (41, 51). Since the anti-P-T410 Ab preferentially recognizes the PKCζ activation loop after LPS stimulation, we analyzed the subcellular distribution of the active isoenzyme in macrophages treated with LPS. As Fig. 7 shows, a time-dependent nuclear accumulation of P-T410-PKCζ was observed and this process was notably enhanced in cells treated with 15dPGJ2. Using confocal microscopy to visualize the redistribution of P-T410-PKCζ, the fluorescence corresponding to the activated protein was present in the nucleus 30 min after LPS challenge. This fluorescence exhibited an accumulation in specific nuclear domains, probably excluding the nucleoli. When activated cells were treated with 15dPGJ2, this nuclear distribution was more even and of higher intensity. In addition to the use of this Ab, cells were transfected with a GFP-PKCζ expression vector, and the results of the distribution of the fluorescence in the nucleus were quite similar to those obtained with the anti-P-T410-PKCζ Ab, indicating that the active protein accumulates transiently in the nucleus and that this process is enhanced by treatment with 15dPGJ2.
FIG. 7.
15dPGJ2 increased PKCζ translocation to the nucleus in LPS-activated macrophages. Cells were incubated for the indicated times with LPS and 15dPGJ2 (2 μM). The levels of P-T410-PKCζ in the nucleus were determined by Western blotting using protein nuclear extracts and by confocal microscopy in fixed cells using a Cy3-labeled secondary Ab. Cells transfected with GFP-PKCζ were also used to visualize the subcellular distribution of the enzyme after cell challenge with LPS and 15dPGJ2.
PKCζ is involved in the imbalance between JNK activation and NF-κB inhibition promoted by 15dPGJ2.
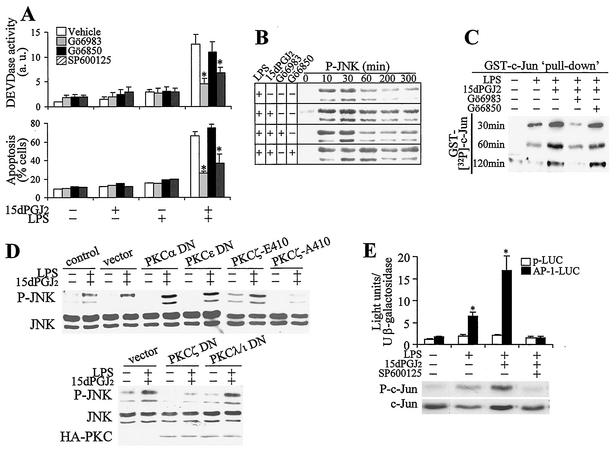
One phenomenological observation in LPS-activated macrophages treated with 15dPGJ2 was a marked increase in apoptosis observed at 24 h. To investigate the contribution of PKCζ to this process, a pharmacological approach was used in which RAW 264.7 cells were incubated with LPS, 15dPGJ2, and Gö6983 (an inhibitor of cPKCs and aPKCs), Gö6850 (an inhibitor of cPKCs, but not aPKCs), and SP600125 (an inhibitor of JNKs [7, 32]). The specificity of these inhibitors was confirmed in in vitro assays (not shown). As Fig. 8A shows, caspase 3 activity (measured as DEVDase) increased notably in cells treated with LPS and 15dPGJ2. This caspase activation was prevented when the cells were incubated with Gö6983, but not with Gö6850, suggesting that an aPKC was involved in the process. Moreover, SP600125 also protected cells from LPS- and 15dPGJ2-induced activation of DEVDase, indicating that JNK was involved in caspase activation. In agreement with these data, when the percentage of apoptotic cells was determined after 24 h of culture, a good correlation with the activation of caspase 3 was observed. In view of the description of a sustained activation of JNK as a mechanism contributing to apoptosis (21, 71), we determined the levels of P-JNK under these conditions. As Fig. 8B shows, treatment of cells with LPS and 15dPGJ2 promoted a sustained activation of JNK. This response decreased in the presence of Gö6983, but not when the cPKC inhibitor Gö6850 was used. Moreover, the kinetic differences in JNK activation were confirmed in pulldown experiments using GST—Jun as the substrate (Fig. 8C). To gain insight into the mechanism of PKCζ-dependent JNK activation in response to LPS and 15dPGJ2 challenge of macrophages, cells were transfected with distinct PKC constructs. Expression of the DN forms of PKCα and PKCɛ as representatives of the cPKC and new PKC families did not inhibit JNK phosphorylation (Fig. 8D). Transfection with PKCζ-A410, which corresponds to a DN form of the T-410 activation loop, impaired JNK activation compared with the vector or with the more active PKCζ-E410 form, which mimics the presence of an active phosphorylated residue (Fig. 8D). Moreover, controlled transfection with the PKCζ DN also impaired JNK phosphorylation, whereas transfection with PKCλ/ι did not significantly influence the phosphorylation of JNK. These constructs have a mutation in the catalytic domain, resulting in complete elimination of the endogenous activity and have been described previously (24). The specificity of SP600125 in JNK inhibition and the differences in activation in response to LPS and 15dPGJ2 were evaluated in cells transfected with an AP-1-LUC reporter vector. As Fig. 8E shows, SP600125 inhibited the expression of the luciferase gene, and the activity of this reporter was significantly higher in cells treated with LPS and 15dPGJ2. Moreover, the measurement of the phospho-c-Jun levels at 1 h after stimulation confirmed the ability of SP600125 to inhibit JNK activity in vivo.
FIG. 8.
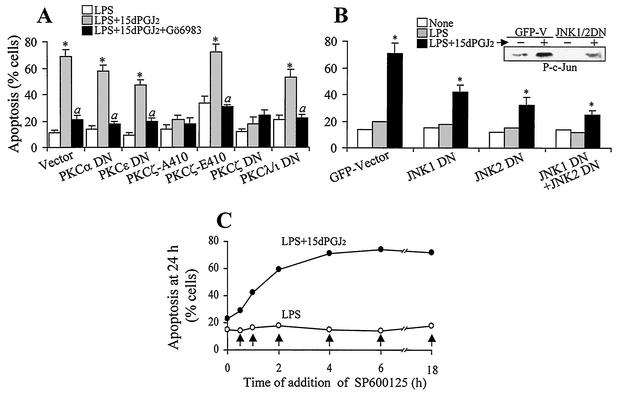
Stimulation of PKCζ by 15dPGJ2 enhanced apoptosis in activated macrophages through a JNK-dependent pathway. (A) RAW 264.7 cells were pretreated (20 min) with 2 μM 15dPGJ2, 200 nM Gö6983, 1 μM Gö6850, or 20 μM SP600125 and activated with 500 ng of LPS per ml. DEVDase activity (in arbitrary units [a.u.]) and the percentage of apoptotic cells were determined after 6 and 24 h of treatment, respectively. (B) The activation of JNK was determined by Western blotting using an Ab that recognized the p46 and p54 P-JNKs. (C) The activity of JNK was determined after pulldown assay of the kinase from extracts of cells incubated with LPS and 15dPGJ2 for the times indicated and was assayed in vitro using GST-c-Jun (amino acids 1 to 79) as the substrate. (D) The effect of the expression of DN forms of PKCα, PKCɛ, and the T410 mutated PKCζ-E410 and PKCζ-A410 or the catalytically inactive PKCζ DN and PKCλ/ι DN (200 ng of DNA; see Fig. 1E) on JNK phosphorylation was determined after GFP-cotransfected cells were sorted. GFP-adherent cells were treated for 60 min with LPS and 15dPGJ2, and the levels of P-JNK were determined by Western blotting. (E) Cells transfected with an AP-1-LUC vector or the promoterless luciferase vector p-LUC (2 μg/ml) and cotransfected with 0.3 μg of the pCMV-β-gal plasmid per ml were treated for 6 h with LPS, 15dPGJ2, and 20 μM SP600125, and the luciferase activity was measured and expressed with respect to β-galactosidase. The phosphorylation of c-Jun was determined at 1 h in cell lysates. Results are the means ± standard deviations from three experiments. In panels A and E, values that were significantly different from those for the vehicle or p-LUC condition or a representative blot (n = 3) are indicated (P < 0.01 [*]).
The contributions of PKCζ and JNK to apoptosis in cells incubated with 15dPGJ2 were determined in cells cotransfected with a pGFP plasmid and a vector encoding a constitutively active PKCζ (PKCζ-E410) or a DN form of a PKC in a 1:4 DNA ratio (18, 43) or with the GFP-JNK1 DN and GFP-JNK2 DN vectors (73). As Fig. 9A shows, cells expressing the catalytically inactive PKCζ-A410 form or the PKCζ DN were significantly resistant to 15dPGJ2-induced apoptosis. However, cells expressing PKCζ-E410 exhibited a higher basal apoptosis that was enhanced by 15dPGJ2 and in part prevented when PKC activity was inhibited with Gö6983. Cells expressing DN forms of PKCα, PKCɛ, and PKCλ/ι exhibited an important apoptotic rate that was prevented by incubation with Gö6983. Moreover, cells expressing the DN forms of JNK1 and JNK2 also exhibited protection against LPS- and 15dPGJ2-dependent apoptosis, particularly when both forms of JNK were coexpressed (Fig. 9B). In view of the relevance of JNK activation in this apoptotic response, macrophages were treated with LPS and 15dPGJ2, and the JNK inhibitor SP600125 was added at various times from 20 min prior to activation to 18 h after challenge (Fig. 9C). Protection from apoptosis was observed during the first 2 h after treatment with LPS and 15dPGJ2, which suggests that JNK-dependent apoptotic signaling occurs during this period.
FIG. 9.
Contribution of PKCζ and JNK to apoptosis in activated macrophages treated with 15dPGJ2. (A and B) Cells cotransfected with a GFP vector and the indicated PKC plasmids (A) or with GFP-JNK1 DN and GFP-JNK2 DN (B) were treated with the indicated stimuli, and the percentage of apoptotic cells was determined at 24 h by flow cytometry after gating for the GFP-positive cells. Results are means ± standard deviations from three experiments. Values that were significantly different from those for the LPS condition (P < 0.01 [*]) and for the corresponding LPS plus 15dPGJ2 condition (P < 0.01 [a]) are indicated. The insert in panel B shows the P-c-Jun levels 1 h after activation. (C) The time-dependent effect of the JNK inhibitor SP600125 (20 μM) on the induction of apoptosis by LPS and 15dPGJ2 was analyzed. The arrows indicate the time of SP600125 addition after activation. The 0-h point corresponds to the incubation with the inhibitor 20 min prior to activation. Apoptosis was measured at 24 h.
DISCUSSION
We have analyzed the effect of the cyclopentenone 15dPGJ2 on early signaling in LPS-activated macrophages. Under these conditions, one striking feature was the marked stimulation of apoptosis, a response absent in RAW 264.7 cells treated only with LPS. Indeed, this PG has been described as an efficient promoter of activation-dependent apoptosis in various cell types (9, 17, 19, 33, 35, 40, 46). In contrast to these observations, 15dPGJ2 potentiates cell growth in colorectal cancer cell lines, which reflects the relevance of the cell type in the biological response mediated by this PG, probably as a result of the relative contributions of PPAR-γ engagement and direct protein modification via Michael addition reactions (8, 16, 28, 37, 66, 67). Also, in human T cells and in THP-1 macrophages, 15dPGJ2 induces interleukin 8 (IL-8) production through an NF-κB- and MAPK-dependent mechanism suggesting that this PG may play different roles in the immune system (27, 33).
Looking for targets of this cyclopentenone PG, we observed that 15dPGJ2 prolonged the activation of PKCζ in LPS-treated RAW 264.7 cells. Interestingly, PKCζ was not activated directly by this PG and this enzyme was not a substrate for cyclopentenone PG modification, pointing to early LPS signaling steps as targets mediating the sustained activation of PKCζ by 15dPGJ2. Moreover, this was a very specific response of PKCζ, not shared by PKCλ/ι, and on further analysis, it involved in part steps upstream of PI3-kinase activation.
PKCζ has been implicated in the control of many cell functions, including viability in various cell types (42, 48, 52), so we investigated whether this was the case in macrophages. Our data show that PKCζ plays a relevant role in the sustained activation of JNK that follows 15dPGJ2 treatment of LPS-stimulated macrophages. Previous work demonstrated that JNK is transiently activated in macrophages treated with LPS (31, 56, 57). The signaling involves the Toll-like receptors 4 and 2 that activate NF-κB through the MyD88-IRAK-TRAF-6-IKK pathway (2, 38). Moreover, it has been reported that LPS activates JNK through a pathway that is mainly dependent on PI3-kinase and PKCζ (57). We confirmed these results in RAW 264.7 cells and showed that the activation of PKCζ in response to LPS was strictly dependent on PI3-kinase activation. In Bac-1 macrophages, where this pathway has been described in detail, this activation of PKCζ stimulates phosphatidylcholine-dependent phospholipase C and acidic sphingomyelinase activities, which constitute the downstream steps responsible for JNK activation (57). In addition to this, it has been described in alveolar macrophages and in other cell types that PKCζ is involved in the activation of ERKs (49, 63). Moreover, in vascular smooth muscle cells, PPAR-γ ligands have been implicated in the activation of ERKs in a PI3-kinase-dependent manner. However, in that report higher concentrations of thiazolidinediones and 15dPGJ2 were necessary to activate the MAPK pathway (69).
In LPS-stimulated macrophages, the balance between cell activation and cell death is controlled by a strict regulation of several intracellular signaling cascades. LPS activates multiple protein kinases such as IKK, p38, p44/p42 ERKs, JNK, PI3-kinase and PKB/Akt that constitute key steps in determining the final macrophage fate (70). It has been extensively reported that activation of PI3-kinase triggers an antiapoptotic signaling cascade that involves the sequential activation of PDK1-Akt-mTOR. However, the relevance of Akt during the inflammatory response has not been fully elucidated and is a subject of intense research (53). In this regard, we were unable to detect Akt activation after LPS stimulation in RAW 264.7 cells, using the commercially available anti-phospho-Akt antibodies. Indeed, an absence of Akt phosphorylation after TNF-α stimulation has been reported (20), whereas other researchers have found that Akt is a part of the signaling pathway activated after gram-positive bacterial infection (2). Thus, it is not completely understood whether Akt is contributing to the control of cell viability in the inflammatory response to LPS.
Sustained activation of JNK has been associated with apoptotic signaling, specifically when the NF-κB pathway is inhibited (3, 6, 21, 71), and more recently, it has been reported that JNK promotes apoptosis through a Bax-dependent mechanism (44); however, it appears that JNK activity can also protect from apoptosis in other cases (58), reflecting the complexity of the cross-regulation between the JNK and NF-κB pathways. Because IKK is inhibited in the presence of 15dPGJ2 (11, 61, 62), the imbalance between IKK and JNK pathways might contribute to the observed apoptotic response. Indeed, when LPS-dependent NF-κB activation was inhibited with SN50, the percentage of apoptotic cells increased only up to 35% (not shown), whereas the apoptosis triggered by 15dPGJ2 reached ca. 70% of the cells under similar conditions, suggesting other specific effects mediated by this PG. A schematic representation of this signaling is shown in Fig. 10. Using pharmacological (with more or less specific inhibitors of PKCζ and JNK), biochemical and genetic approaches and the correlation between the functional studies on caspase 3 activation and apoptosis on the one hand and the LPS/15dPGJ2-dependent inhibition of NF-κB and sustained activation of JNK in these cells on the other hand, our data suggest that PKCζ is an important step in the control of apoptosis in RAW 264.7 macrophages under conditions of resolution of inflammation. PKCζ has been implicated in the regulation of the survival or apoptosis switch through engagement of different targets (10, 39, 41, 50). The interaction of the regulatory domain of PKCζ and other aPKCs with prostate apoptosis response 4 protein (Par-4) has been shown to impair apoptosis (5, 23), whereas the interaction with p62 has been suggested to recruit PKCζ to the NF-κB complex and to impair apoptosis (14); however, the contribution of both proteins to apoptosis in RAW 264.7 cells remains to be established. In this regard, we have used several strategies to evaluate the ability of PKCζ to modulate NF-κB and JNK activation in these cells. Although aPKCs exhibit a conserved homology in the catalytic domain, the regulatory regions are different, and our data suggest that PKCζ is the main isotype of this family up-regulated by 15dPGJ2. Moreover, using Gö6983, the broad inhibitor of cPKCs and aPKCs, at concentrations higher than 100 nM were required to observe the inhibitory effects on JNK, whereas it was ineffective at concentrations in the range of the Ki value for cPKCs (20 nM [not shown]). It should be noted that kinetic parameters for kinase inhibitors are mainly deduced from in vitro studies in which the concentration of substrates, in particular ATP, are much lower than in the cell. However, this caution is overcome by other reports in which concentration-dependent effects, in the range of those used in this work, have been reported (54, 72, 76). In addition to this, DN forms of the activation loop of PKCζ (T410A) or the catalytic domain (HA-PKCζ DN), but not the DN forms of PKCα, PKCɛ and PKCλ/ι, prevented JNK activation and apoptosis. Moreover, pharmacological inhibition of JNK with SP600125 or transfection of cells with DN forms of JNK1 and JNK2 also inhibited apoptosis, suggesting that JNK accounts for most of the apoptotic response promoted by 15dPGJ2 in LPS-treated RAW 264.7 cells. An ancillary conclusion from the analysis with inhibitors is that PKCζ appears not to be essential for the activation of NF-κB under conditions of LPS challenge of macrophages. In agreement with these data, it has been shown that in animals lacking PKCζ NF-κB activation persists, although this depends on the cell type and stimulus used (42, 45). However, overexpression of PKCζ (whether constitutively active or the native form) promotes NF-κB activation in macrophages through mechanisms not yet established (unpublished work).
FIG. 10.
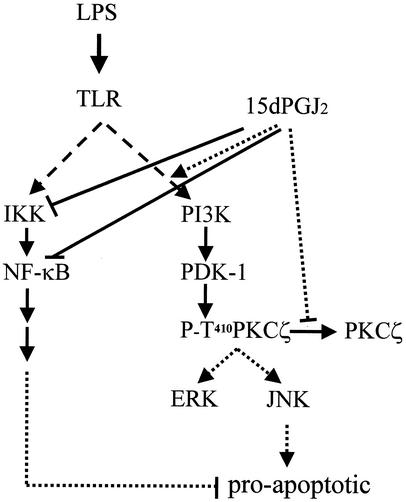
Schematic diagram of 15dPGJ2-dependent imbalance between MAPK and NF-κB pathways in activated macrophages. LPS activation of macrophages involves the engagement of several pathways that, in the absence of NO synthesis, maintain cell viability. Treatment with 15dPGJ2 promotes an imbalance between NF-κB (which is inhibited) and JNK (which is overactivated), favoring an increase in apoptotic death. PKCζ plays an important role in the enhancement of JNK and ERK activation under these conditions. TLR, Toll-like receptors.
In addition to the data on the effects of 15dPGJ2 on the JNK and NF-κB pathways, we observed a persistent accumulation of P-T410-PKCζ in the nucleus of activated cells, although the biological role of this translocation remains to be established. In agreement with this observation, a parallel distribution of GFP-PKCζ confirmed the specificity of the translocation observed in these cells. Activation-dependent nuclear localization of PKCζ has been described in other cell types, such as B lymphocytes and PC12 cells treated with nerve growth factor (41, 51), although the role of this process remains to be established in these cells.
Previous work indicated that 15dPGJ2 acted to increase synthesis of reactive oxygen and nitrogen species, in particular peroxynitrite, in macrophages treated with LPS and gamma interferon (IFN-γ) (35). However, treating RAW 264.7 cells with LPS, at least at concentrations below 500 ng/ml, is not sufficient to induce NOS-2, and indeed, we were unable to observe significant synthesis of NO and peroxynitrite under these conditions. However, in the presence of IFN-γ, a coordinate response exists between the distal κB and GAS/ISRE/IRF-1 sites of the murine NOS-2 promoter, allowing the expression of the NOS-2 gene (78) (this is not the case in elicited peritoneal macrophages, which express NOS-2 after LPS challenge even at the low doses used in this work). Therefore, the apoptotic effects observed in LPS-activated RAW 264.7 cells cannot be attributed to the synthesis of NO or peroxynitrite but rather appear to be dependent on the activation of PKCζ. Interestingly, the pattern of PKCζ activation observed in RAW 264.7 cells in response to 15dPGJ2 challenge was also observed in peritoneal macrophages from wild-type and NOS-2-deficient mice, indicating that this is a characteristic response of activated macrophages.
In conclusion, our data show that in the presence of 15dPGJ2, PKCζ and JNK remain activated for longer periods of time, whereas the signaling through IKK/NF-κB is blocked. As a result of this dual action of the PG (mediated through PKCζ), the apoptotic process is enhanced. Taken together, these data outline a novel 15dPGJ2-dependent apoptotic pathway in macrophages and suggest that this pathway may contribute to the resolution of inflammation when cyclopentenone PGs accumulate.
Acknowledgments
We thank Dario R. Alessi for anti-PDK1 antibody, Dolores Perez-Sala for biotinylated15dPGJ2, Jorge Moscat for aPKC DN plasmids, and Almudena Porras for JNK reagents. We also thank Peter Tontonoz for critically reading the manuscript.
This work was supported in part by grants SAF2002-00783 from Comisión Interministerial de Ciencia y Tecnología and 08.3/0010/00 from Comunidad de Madrid.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, A. S. 2001. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J. Clin. Investig. 107:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balendran, A., A. Casamayor, M. Deak, A. Paterson, P. Gaffney, R. Currie, C. P. Downes, and D. R. Alessi. 1999. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9:393-404. [DOI] [PubMed] [Google Scholar]
- 5.Barradas, M., A. Monjas, M. T. Diaz-Meco, M. Serrano, and J. Moscat. 1999. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 18:6362-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 7.Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardo, A., G. Levi, and L. Minghetti. 2000. Role of the peroxisome proliferator-activated receptor-γ (PPAR-γ) and its natural ligand 15-deoxy-Δ12, 14-prostaglandin J2 in the regulation of microglial functions. Eur. J. Neurosci. 12:2215-2223. [DOI] [PubMed] [Google Scholar]
- 9.Bishop-Bailey, D., and T. Hla. 1999. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Δ12, 14-prostaglandin J2. J. Biol. Chem. 274:17042-17048. [DOI] [PubMed] [Google Scholar]
- 10.Canman, C. E., and M. B. Kastan. 1996. Signal transduction. Three paths to stress relief. Nature 384:213-214. [DOI] [PubMed] [Google Scholar]
- 11.Castrillo, A., M. J. Diaz-Guerra, S. Hortelano, P. Martin-Sanz, and L. Bosca. 2000. Inhibition of IκB kinase and IκB phosphorylation by 15-deoxy-Δ12,14-prostaglandin J2 in activated murine macrophages. Mol. Cell. Biol. 20:1692-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castrillo, A., D. J. Pennington, F. Otto, P. J. Parker, M. J. Owen, and L. Bosca. 2001. Protein kinase Cɛ is required for macrophage activation and defense against bacterial infection. J. Exp. Med. 194:1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cernuda-Morollon, E., E. Pineda-Molina, F. J. Canada, and D. Perez-Sala. 2001. 15-Deoxy-Δ12,14-prostaglandin J2 inhibition of NF-κB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 276:35530-35536. [DOI] [PubMed] [Google Scholar]
- 14.Chang, S., J. H. Kim, and J. Shin. 2002. p62 forms a ternary complex with PKCζ and PAR-4 and antagonizes PAR-4-induced PKCζ inhibition. FEBS Lett. 510:57-61. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Y., J. D. Morrow, and L. J. Roberts. 1999. Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J. Biol. Chem. 274:10863-10868. [DOI] [PubMed] [Google Scholar]
- 16.Chinery, R., R. J. Coffey, R. Graves-Deal, S. C. Kirkland, S. C. Sanchez, W. E. Zackert, J. A. Oates, and J. D. Morrow. 1999. Prostaglandin J2 and 15-deoxy-Δ12,14-prostaglandin J2 induce proliferation of cyclooxygenase-depleted colorectal cancer cells. Cancer Res. 59:2739-2746. [PubMed] [Google Scholar]
- 17.Chinetti, G., S. Griglio, M. Antonucci, I. P. Torra, P. Delerive, Z. Majd, J. C. Fruchart, J. Chapman, J. Najib, and B. Staels. 1998. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 273:25573-25580. [DOI] [PubMed] [Google Scholar]
- 18.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase C ζ by PI 3-kinase and PDK-1. Curr. Biol. 8:1069-1077. [DOI] [PubMed] [Google Scholar]
- 19.Clay, C. E., G. I. Atsumi, K. P. High, and F. H. Chilton. 2001. Early de novo gene expression is required for 15-deoxy-Δ12,14-prostaglandin J2-induced apoptosis in breast cancer cells. J. Biol. Chem. 276:47131-47135. [DOI] [PubMed] [Google Scholar]
- 20.Delhase, M., N. Li, and M. Karin. 2000. Kinase regulation in inflammatory response. Nature 406:367-368. [DOI] [PubMed] [Google Scholar]
- 21.De Smaele, E., F. Zazzeroni, S. Papa, D. U. Nguyen, R. Jin, J. Jones, R. Cong, and G. Franzoso. 2001. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414:308-313. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Guerra, M. J., A. Castrillo, P. Martin-Sanz, and L. Bosca. 1999. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J. Immunol. 162:6184-6190. [PubMed] [Google Scholar]
- 23.Diaz-Meco, M. T., M. M. Municio, S. Frutos, P. Sanchez, J. Lozano, L. Sanz, and J. Moscat. 1996. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell 86:777-786. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Meco, M. T., M. M. Municio, P. Sanchez, J. Lozano, and J. Moscat. 1996. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ι and stimulates its kinase activity in vitro and in vivo. Mol. Cell. Biol. 16:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman, B. M., J. Chen, and R. M. Evans. 1996. The peroxisome proliferator-activated receptors: ligands and activators. Ann. N. Y. Acad. Sci. 804:266-275. [DOI] [PubMed] [Google Scholar]
- 26.Forman, B. M., P. Tontonoz, J. Chen, R. P. Brun, B. M. Spiegelman, and R. M. Evans. 1995. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83:803-812. [DOI] [PubMed] [Google Scholar]
- 27.Fu, Y., N. Luo, and M. F. Lopes-Virella. 2002. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis 160:11-20. [DOI] [PubMed] [Google Scholar]
- 28.Galli, A., D. Crabb, D. Price, E. Ceni, R. Salzano, C. Surrenti, and A. Casini. 2000. Peroxisome proliferator-activated receptor γ transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology 31:101-108. [DOI] [PubMed] [Google Scholar]
- 29.Gilroy, D. W., P. R. Colville-Nash, D. Willis, J. Chivers, M. J. Paul-Clark, and D. A. Willoughby. 1999. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5:698-701. [DOI] [PubMed] [Google Scholar]
- 30.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 31.Hambleton, J., S. L. Weinstein, L. Lem, and A. L. DeFranco. 1996. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 93:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han, Z., D. L. Boyle, L. Chang, B. Bennett, M. Karin, L. Yang, A. M. Manning, and G. S. Firestein. 2001. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 108:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris, S. G., and R. P. Phipps. 2002. Prostaglandin D2, its metabolite 15-d-PGJ2, and peroxisome proliferator activated receptor-γ agonists induce apoptosis in transformed, but not normal, human T lineage cells. Immunology 105:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hortelano, S., A. M. Alvarez, and L. Bosca. 1999. Nitric oxide induces tyrosine nitration and release of cytochrome c preceding an increase of mitochondrial transmembrane potential in macrophages. FASEB J. 13:2311-2317. [DOI] [PubMed] [Google Scholar]
- 35.Hortelano, S., A. Castrillo, A. M. Alvarez, and L. Bosca. 2000. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J. Immunol. 165:6525-6531. [DOI] [PubMed] [Google Scholar]
- 36.Hortelano, S., A. López-Collazo, and L. Boscá. 1999. Protective effect of cyclosporin A and FK506 from nitric oxide-dependent apoptosis in activated macrophages. Br. J. Pharmacol. 126:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang, C., A. T. Ting, and B. Seed. 1998. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82-86. [DOI] [PubMed] [Google Scholar]
- 38.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 39.Kavurma, M. M., F. S. Santiago, E. Bonfoco, and L. M. Khachigian. 2001. Sp1 phosphorylation regulates apoptosis via extracellular FasL-Fas engagement. J. Biol. Chem. 276:4964-4971. [DOI] [PubMed] [Google Scholar]
- 40.Kawahito, Y., M. Kondo, Y. Tsubouchi, A. Hashiramoto, D. Bishop-Bailey, K. Inoue, M. Kohno, R. Yamada, T. Hla, and H. Sano. 2000. 15-deoxy-Δ12,14-PGJ2 induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J. Clin. Investig. 106:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kronfeld, I., G. Kazimirsky, E. W. Gelfand, and C. Brodie. 2002. NGF rescues human B lymphocytes from anti-IgM induced apoptosis by activation of PKCζ. Eur. J. Immunol. 32:136-143. [DOI] [PubMed] [Google Scholar]
- 42.Lallena, M. J., M. T. Diaz-Meco, G. Bren, C. V. Paya, and J. Moscat. 1999. Activation of IκB kinase β by protein kinase C isoforms. Mol. Cell. Biol. 19:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 44.Lei, K., A. Nimnual, W. X. Zong, N. J. Kennedy, R. A. Flavell, C. B. Thompson, D. Bar-Sagi, and R. J. Davis. 2002. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol. Cell. Biol. 22:4929-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leitges, M., L. Sanz, P. Martin, A. Duran, U. Braun, J. F. Garcia, F. Camacho, M. T. Diaz-Meco, P. D. Rennert, and J. Moscat. 2001. Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol. Cell 8:771-780. [DOI] [PubMed] [Google Scholar]
- 46.Li, L., J. Tao, J. Davaille, C. Feral, A. Mallat, J. Rieusset, H. Vidal, and S. Lotersztajn. 2001. 15-Deoxy-Δ12,14-prostaglandin J2 induces apoptosis of human hepatic myofibroblasts. A pathway involving oxidative stress independently of peroxisome-proliferator-activated receptors. J. Biol. Chem. 276:38152-38158. [DOI] [PubMed] [Google Scholar]
- 47.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 48.Mellor, H., and P. J. Parker. 1998. The extended protein kinase C superfamily. Biochem. J. 332:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monick, M. M., A. B. Carter, G. Gudmundsson, R. Mallampalli, L. S. Powers, and G. W. Hunninghake. 1999. A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide-stimulated human alveolar macrophages. J. Immunol. 162:3005-3012. [PubMed] [Google Scholar]
- 50.Murray, N. R., and A. P. Fields. 1997. Atypical protein kinase C ι protects human leukemia cells against drug-induced apoptosis. J. Biol. Chem. 272:27521-27524. [DOI] [PubMed] [Google Scholar]
- 51.Neri, L. M., A. M. Martelli, P. Borgatti, M. L. Colamussi, M. Marchisio, and S. Capitani. 1999. Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) triphosphate synthesis precede PKC-ζ translocation to the nucleus of NGF-treated PC12 cells. FASEB J. 13:2299-2310. [PubMed] [Google Scholar]
- 52.Nishizuka, Y. 1995. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 9:484-496. [PubMed] [Google Scholar]
- 53.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82-85. [DOI] [PubMed] [Google Scholar]
- 54.Parker, P. J. 1999. Inhibition of protein kinase C: do we, can we, and should we? Pharmacol. Ther. 82:263-267. [DOI] [PubMed] [Google Scholar]
- 55.Petrova, T. V., K. T. Akama, and L. J. Van Eldik. 1999. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Δ12,14-prostaglandin J2. Proc. Natl. Acad. Sci. USA 96:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Procyk, K. J., M. R. Rippo, R. Testi, F. Hoffmann, P. J. Parker, and M. Baccarini. 1999. Distinct mechanisms target stress and extracellular signal-activated kinase 1 and Jun N-terminal kinase during infection of macrophages with Salmonella. J. Immunol. 163:4924-4930. [PubMed] [Google Scholar]
- 57.Procyk, K. J., M. R. Rippo, R. Testi, F. Hofmann, P. J. Parker, and M. Baccarini. 2000. Lipopolysaccharide induces jun N-terminal kinase activation in macrophages by a novel Cdc42/Rac-independent pathway involving sequential activation of protein kinase C ζ and phosphatidylcholine-dependent phospholipase C. Blood 96:2592-2598. [PubMed] [Google Scholar]
- 58.Reuther-Madrid, J. Y., D. Kashatus, S. Chen, X. Li, J. Westwick, R. J. Davis, H. S. Earp, C. Y. Wang, and J. A. Baldwin, Jr. 2002. The p65/RelA subunit of NF-κB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol. Cell. Biol. 22:8175-8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricote, M., J. T. Huang, J. S. Welch, and C. K. Glass. 1999. The peroxisome proliferator-activated receptor (PPARγ) as a regulator of monocyte/macrophage function. J. Leukoc. Biol. 66:733-739. [DOI] [PubMed] [Google Scholar]
- 60.Ricote, M., A. C. Li, T. M. Willson, C. J. Kelly, and C. K. Glass. 1998. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391:79-82. [DOI] [PubMed] [Google Scholar]
- 61.Rossi, A., G. Elia, and M. G. Santoro. 1997. Inhibition of nuclear factor κB by prostaglandin A1: an effect associated with heat shock transcription factor activation. Proc. Natl. Acad. Sci. USA 94:746-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi, A., P. Kapahi, G. Natoli, T. Takahashi, Y. Chen, M. Karin, and M. G. Santoro. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403:103-108. [DOI] [PubMed] [Google Scholar]
- 63.Schonwasser, D. C., R. M. Marais, C. J. Marshall, and P. J. Parker. 1998. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 18:790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with “mini-extracts,” prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Standaert, M. L., G. Bandyopadhyay, Y. Kanoh, M. P. Sajan, and R. V. Farese. 2001. Insulin and PIP3 activate PKC-ζ by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40:249-255. [DOI] [PubMed] [Google Scholar]
- 66.Straus, D. S., and C. K. Glass. 2001. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med. Res. Rev. 21:185-210. [DOI] [PubMed] [Google Scholar]
- 67.Straus, D. S., G. Pascual, M. Li, J. S. Welch, M. Ricote, C. H. Hsiang, L. L. Sengchanthalangsy, G. Ghosh, and C. K. Glass. 2000. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc. Natl. Acad. Sci. USA 97:4844-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 69.Takeda, K., T. Ichiki, T. Tokunou, N. Iino, and A. Takeshita. 2001. 15-Deoxy-Δ12,14-prostaglandin J2 and thiazolidinediones activate the MEK/ERK pathway through phosphatidylinositol 3-kinase in vascular smooth muscle cells. J. Biol. Chem. 276:48950-48955. [DOI] [PubMed] [Google Scholar]
- 70.Takeuchi, O., and S. Akira. 2001. Toll-like receptors: their physiological role and signal transduction system. Int. Immunopharmacol. 1:625-635. [DOI] [PubMed] [Google Scholar]
- 71.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 72.Toullec, D., P. Pianetti, H. Coste, P. Bellevergue, T. Grand-Perret, M. Ajakane, V. Baudet, P. Boissin, E. Boursier, and F. Loriolle. 1991. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266:15771-15781. [PubMed] [Google Scholar]
- 73.Valladares, A., A. M. Alvarez, J. J. Ventura, C. Roncero, M. Benito, and A. Porras. 2000. p38 mitogen-activated protein kinase mediates tumor necrosis factor-α-induced apoptosis in rat fetal brown adipocytes. Endocrinology 141:4383-4395. [DOI] [PubMed] [Google Scholar]
- 74.Velasco, M., M. J. Diaz-Guerra, P. Martin-Sanz, A. Alvarez, and L. Bosca. 1997. Rapid up-regulation of IκBβ and abrogation of NF-κB activity in peritoneal macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 272:23025-23030. [DOI] [PubMed] [Google Scholar]
- 75.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 76.Wang, D., X. Yu, and P. Brecher. 1998. Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J. Biol. Chem. 273:33027-33034. [DOI] [PubMed] [Google Scholar]
- 77.Xie, Q. W., H. J. Cho, J. Calaycay, R. A. Mumford, K. M. Swiderek, T. D. Lee, A. Ding, T. Troso, and C. Nathan. 1992. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256:225-228. [DOI] [PubMed] [Google Scholar]
- 78.Xie, Q. W., R. Whisnant, and C. Nathan. 1993. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J. Exp. Med. 177:1779-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]