Abstract
Secretion of bile salts into the duodenum was studied in eight normal subjects, in 10 patients with cirrhosis, and in two cholecystectomized subjects. Duodenal juice was aspirated continuously through a double-lumen tube during an unstimulated period, after an intravenous injection of pancreozymin/cholecystokinin, and during a continuous intravenous infusion of secretin given at a rate of 3 units per kilogram body weight per hour. Precautions were taken to try to ensure quantitative recovery during the studies, and recovery of an infused nonabsorbable marker was greater than 80% in all subjects.
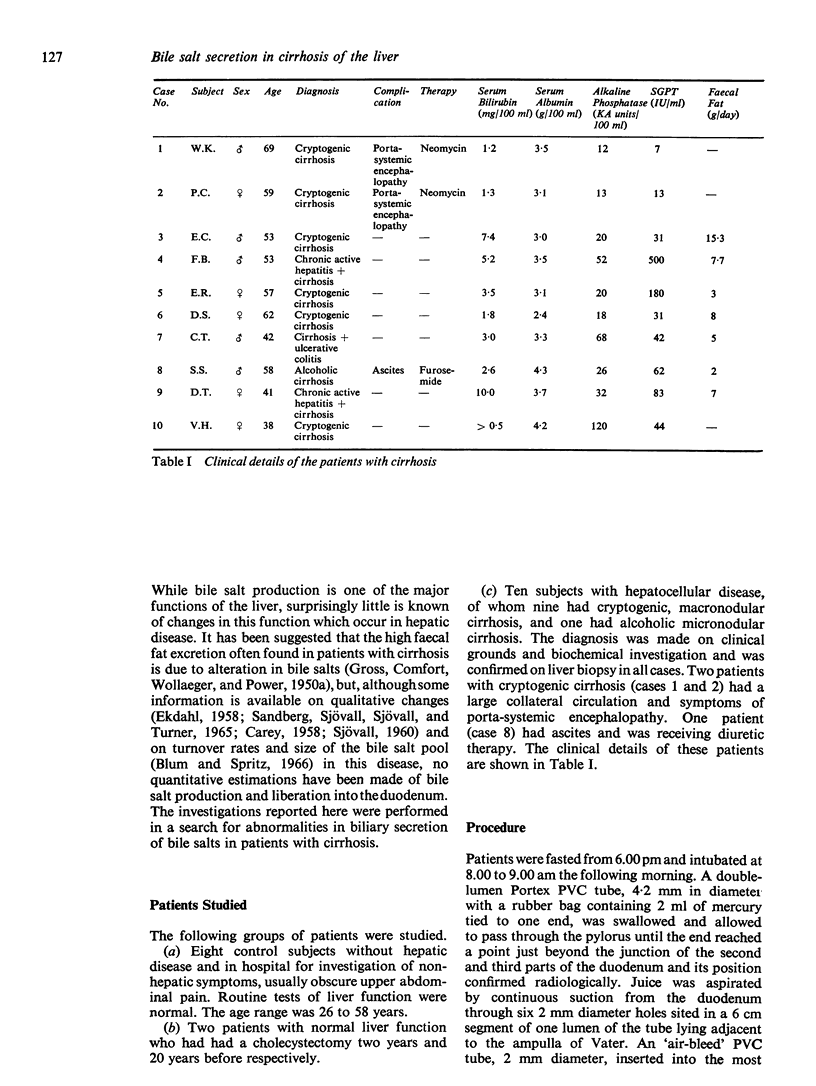
Secretin induced a flow of a greater volume of juice in the cirrhotic patients than in the normal group (49 to 57 ml per 10 minutes compared with 28 to 49 ml per 10 minutes). This change may have resulted from a higher effective dose of secretin if it is assumed that the cirrhotic liver fails to catabolize secretin.
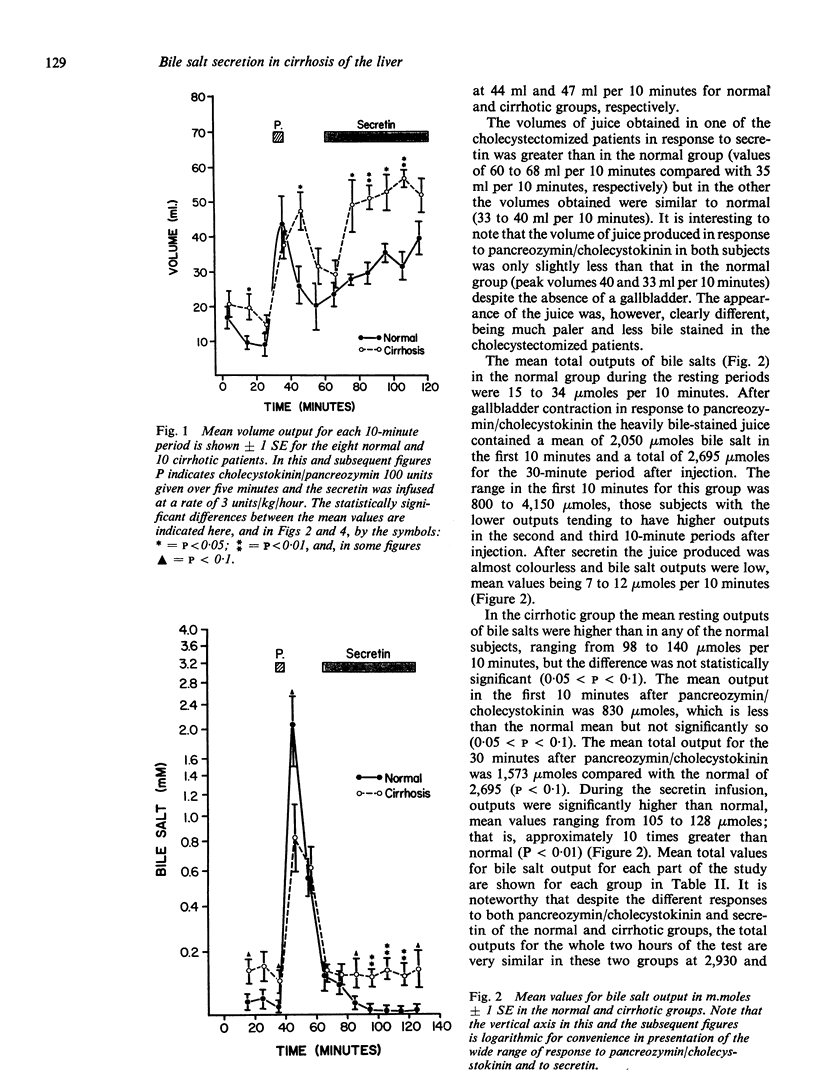
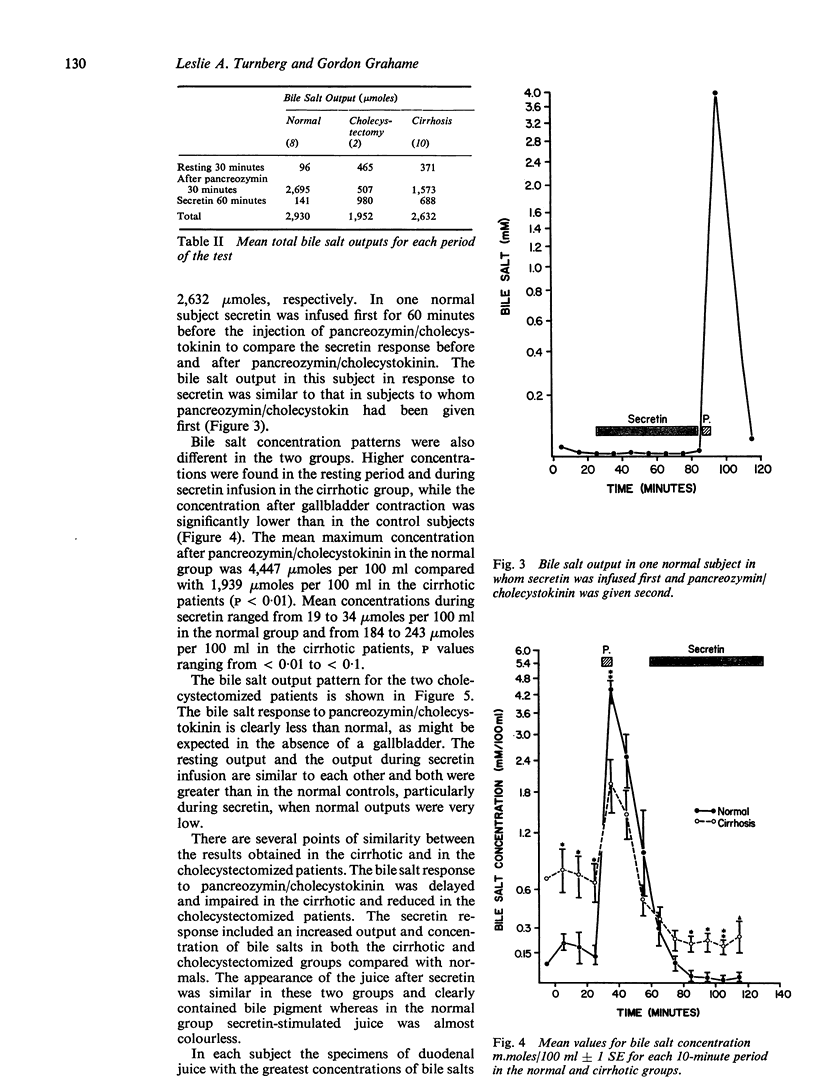
The bile acid response to pancreozymin/cholecystokinin followed by secretin in the cirrhotic subjects resembled that seen in patients after cholecystectomy in whom pancreozymin/cholecystokinin induces only a slight increase in bile salt output but in whom the output of bile salts during rest and secretin stimulation is markedly greater than normal. This response in cirrhosis is probably best interpreted as due to impaired function of the gallbladder. The total amount of bile salt liberated over the two hours of the test in the cirrhotic patients was similar to normal The concentration of bile salt after pancreozymin/cholecystokinin was less than in normal subjects, but similar to that in cholecystectomized patients. It is unlikely therefore that deficient output or concentration of bile salt can be held responsible for steatorrhea in cirrhosis.
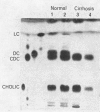
There was a marked decrease in the deoxycholate conjugates and a reduction in the glycine: taurine ratio in the bile of cirrhotic patients. The former change may reflect a change in bacterial flora and the latter a defect in hepatic conjugating mechanisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARAONA E., ORREGO H., FERNANDEZ O., AMENABAR E., MALDONADO E., TAG F., SALINAS A. Absorptive function of the small intestine in liver cirrhosis. Am J Dig Dis. 1962 Apr;7:318–330. doi: 10.1007/BF02231855. [DOI] [PubMed] [Google Scholar]
- Blum M., Spritz N. The metabolism of intravenously injected isotopic cholic acid in Laennec's cirrhosis. J Clin Invest. 1966 Feb;45(2):187–193. doi: 10.1172/JCI105331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY J. B., Jr The serum trihydroxy-dihydroxy bile acid ratio in liver and biliary tract disease. J Clin Invest. 1958 Nov;37(11):1494–1503. doi: 10.1172/JCI103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNIFF C. L., DOLAN M. A., LEEVY C. M. Cholecystography in portal cirrhosis without jaundice. Gastroenterology. 1953 Dec;25(4):557–564. [PubMed] [Google Scholar]
- Carey J. B., Jr, Wilson I. D., Zaki F. G., Hanson R. F. The metabolism of bile acids with special reference to liver injury. Medicine (Baltimore) 1966 Nov;45(6):461–470. doi: 10.1097/00005792-196645060-00009. [DOI] [PubMed] [Google Scholar]
- DREILING D. A. Studies of pancreatic function. III. The use of the secretin test in the diagnosis of patients with the post-cholecystectomy syndrome. Gastroenterology. 1950 Sep;16(1):162–171. [PubMed] [Google Scholar]
- EKDAHL P. H. On the conjugation and formation of bile acids in the human liver. VI. On the conjugation of cholic acid -2414C in human liver homogenates in various diseases with special reference to patients with jaundice; bile acids and steroids 66. Acta Chir Scand. 1958;115(3):208–226. [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J. B., COMFORT M. W., WOLLAEGER E. E., POWER M. H. External pancreatic function in primary parenchymatous hepatic disease as measured by analysis of duodenal contents before and after stimulation with secretin. Gastroenterology. 1950 Sep;16(1):151–161. [PubMed] [Google Scholar]
- GROSS J. B., COMFORT M. W., WOLLAEGER E. E., POWER M. H. Total solids, fat and nitrogen in the feces: V. A study of patients with primary parenchymatous hepatic disease. Gastroenterology. 1950 Sep;16(1):140–150. [PubMed] [Google Scholar]
- IWATA T., YAMASAKI K. ENZYMATIC DETERMINATION AND THIN-LAYER CHROMATOGRAPHY OF BILE ACIDS IN BLOOD. J Biochem. 1964 Nov;56:424–431. doi: 10.1093/oxfordjournals.jbchem.a128013. [DOI] [PubMed] [Google Scholar]
- SANDBERG D. H., SJOEVALL J., SJOEVALL K., TURNER D. A. MEASUREMENT OF HUMAN SERUM BILE ACIDS BY GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1965 Apr;6:182–192. [PubMed] [Google Scholar]
- Turnberg L. A., Anthony-Mote A. The quantitative determination of bile salts in bile using thin-layer chromatography and 3 alpha-hydroxysteroid dehydrogenase. Clin Chim Acta. 1969 May;24(2):253–259. doi: 10.1016/0009-8981(69)90321-0. [DOI] [PubMed] [Google Scholar]