Abstract
Skeletal muscle and kidney enriched inositol phosphatase (SKIP) is an inositol polyphosphate 5-phosphatase that hydrolyzes phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] to downregulate intracellular levels. In this study, we show that SKIP inhibits phosphoinositide 3-kinase signaling in insulin-stimulated CHO cells. Ectopic expression of SKIP did not inhibit insulin-induced PI(3,4,5)P3 generation but did rapidly decrease insulin-induced intracellular PI(3,4,5)P3 levels compared with those in control cells. Further, insulin-induced phosphorylation of some downstream targets such as Akt and p70 S6 kinase was markedly inhibited by the ectopic expression of SKIP, whereas phosphorylation of mitogen-activated protein kinase was not. In contrast, downregulation of intracellular SKIP levels by antisense oligonucleotides dramatically enhanced Akt (protein kinase B) phosphorylation in response to insulin, suggesting that endogenous SKIP downregulates insulin signaling. SKIP also markedly inhibited GLUT4 translocation and membrane ruffle formation. We conclude that SKIP preferentially regulates glucose transport and actin cytoskeletal rearrangement among a variety of PI(3,4,5)P3 downstream events.
The binding of insulin to its receptor stimulates tyrosine autophosphorylation of the receptor, leading to the activation of its intrinsic tyrosine kinase. This kinase phosphorylates insulin receptor substrates (IRSs), Shc, and other proteins (21, 22, 28, 41, 49). Tyrosine phosphorylation of IRS-1 and Shc promotes their interaction with the regulatory subunit of phosphoinositide (PI) 3-kinase (52). PI 3-kinase is a heterodimer composed of a 110-kDa catalytic subunit and an 85-kDa regulatory subunit. Activated PI 3-kinase phosphorylates phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to generate phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] (15), which plays a crucial role in the biological responses induced by insulin (43).
One of the major roles of insulin is the activation of glucose uptake in muscle and adipose tissue through the translocation of the glucose transporter GLUT4 (8). PI 3-kinase is necessary for this process (9, 25). Akt (protein kinase B [PKB]) is a serine/threonine kinase located downstream of PI 3-kinase, and in its constitutively active form, Akt causes increased glucose uptake and GLUT4 translocation in 3T3-L1 adipocytes (18). For the activation of Akt, phosphorylation by Akt kinase phosphoinositide-dependent kinase (PDK) is also important (39, 40). In this case, binding of PI(3,4,5)P3 and PI(3,4)P2 to the pleckstrin homology domain of Akt, in addition to phosphorylation by PDK, is required for its full activation (6, 40). However, recent data suggest that PI(3,4,5)P3 is a more potent activator of Akt than is PI(3,4)P2 (17). Growth factors such as insulin and platelet-derived growth factor induce actin cytoskeletal rearrangement, leading to stress fiber breakdown and membrane ruffling (25, 26). Such membrane ruffling requires PI(3,4,5)P3 formation, which presumably causes the activation of Rac guanine nucleotide exchange factors such as Vav and Tiam1 through their pleckstrin homology domains (7, 35). The regulation of cell cycle progression also depends on PI 3-kinase activity (5, 11, 14).
Phosphoinositide levels are regulated by the actions of phosphoinositide kinases and phosphatases. Each phosphoinositide exerts pleiotropic effects (13, 20, 34). A number of phosphoinositide phosphatases have been reported to be involved in insulin-induced biological responses. The recently identified phosphatase pharbin, also called inositol polyphosphate phosphatase IV, inhibits insulin-like growth factor-1-induced Akt activation and leads to apoptotic cell death (17). The phosphatases SHIP and PTEN have also been shown to negatively regulate glucose transport in adipocytes (29, 45, 47). SHIP2 is expressed abundantly in insulin-sensitive cells, and its loss leads to increased sensitivity to insulin. Mice heterozygous for the SHIP2 mutation show increased glucose tolerance and insulin sensitivity (4). Thus, dephosphorylation of PI(3,4,5)P3 by these phosphatases may be an important way to regulate the biological effects of insulin.
In this study, we determined whether a skeletal muscle and kidney enriched inositol phosphatase (SKIP) is one of the potent negative regulators of PI 3-kinase in insulin signaling. Expression of SKIP inhibited GLUT4 translocation and membrane ruffle formation in CHO cells. SKIP also suppressed insulin-induced glucose incorporation and glycogen synthesis in L6 myocytes, whereas phosphatase-negative mutant SKIP did not. Translocation of SKIP protein from the Golgi apparatus to the plasma membrane by insulin was also observed. This translocation may be the basis for the insulin-induced inhibition of the PI 3-kinase signaling pathway.
MATERIALS AND METHODS
Materials.
Bovine pancreas insulin was purchased from Sigma Chemical Co. (St. Louis, Mo.). Antibodies against c-Myc, IRS-1, insulin receptor β (IRβ), ERK1 and ERK2, phospho-ERK1, phospho-ERK 2, and PTEN were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-green fluorescent protein (GFP) antibody was supplied by Clontech Laboratories (Palo Alto, Calif.). Anti-p70 S6 kinase, phospho-p70 S6 kinase, phospho-Akt (Ser473), anti-phospho Akt (Thr308), and anti-Akt antibodies were purchased from Cell Signaling Technology (Beverly, Mass.), and anti-phosphotyrosine monoclonal antibody (4G10) was purchased from Upstate Biotechnology (Lake Placid, N.Y.). Anti-SKIP antibody was generated as described previously (12). Inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] and inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4] were purchased from Sigma Chemical Co., and phosphoinositides were purchased from CellSignals Inc. (Lexington, Ky.). [3H]thymidine, [32P]orthophosphate, and [γ-32P]ATP were purchased from Perkin Elmer Life Sciences (Harbor City, Calif.).
Expression constructs.
A phosphatase-negative mutant of SKIP (SKIP-Δphos) was generated by deletion of the DNA sequences that code for amino acids conserved among 5-phosphatases (DLIIWFGDMNERIE, amino acids 185 to 198, and EKKRKPAWTDRILWRLK, amino acids 260 to 276). GFP-conjugated wild-type SKIP (GFP-SKIP-WT) and a phosphatase-negative mutant (GFP-SKIP-Δphos) were generated by introduction of the relevant cDNAs into the pEGFP-C1 mammalian expression vector (Clontech).
Cell culture.
HEK-293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) in an atmosphere of 5% CO2 at 37°C. Chinese hamster ovary (CHO) cells were cultured in Ham's F-12 medium with 10% FBS under the same conditions. CHO-K1 cells stably transfected with insulin receptor (CHO-IR cells) or GLUT4 glucose transporter were kindly provided by Tomoichiro Asano (University of Tokyo, Tokyo, Japan) and were cultured under the same conditions as those for CHO cells. L6 myoblasts were grown and passaged in DMEM containing 10% FBS. Confluent cells were differentiated in DMEM containing 2% FBS. After the cells were fully differentiated into myotubes, they were used for experiments.
Production of recombinant adenoviruses in mammalian cells.
Recombinant adenoviruses expressing GFP, wild-type SKIP, or the phosphatase-negative mutant SKIP (Ad-GFP, Ad-SKIP-WT, and Ad-SKIP-Δphos, respectively) were generated as described previously (10). Wild-type or phosphatase-negative SKIP cDNA tagged with c-Myc was introduced into the pShuttle-CMV vector. Recombinant adenoviral plasmids were generated by homologous recombination in Escherichia coli. These plasmids were linearized by digestion with PacI and transfected into HEK-293 cells. After 10 days, the supernatants were collected and were used as virus solutions.
Immunoprecipitation and Western blotting.
CHO cells and CHO cells expressing GLUT4 (5.0 × 104 cells each) were infected with Ad-GFP, Ad-SKIP-WT, and Ad-SKIP-Δphos for 24 h to achieve 100% expression. Infected cells were incubated for 24 h in 10% FBS and then serum starved for 16 h and stimulated with insulin (100 nM) for 5 to 30 min. The cells were harvested and lysed with lysis buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 1% Triton X-100, 2 mM EDTA) containing 1 mM phenylmethylsulfonyl fluoride and 1 mM sodium orthovanadate. The cell lysates were centrifuged at 10,000 × g for 1 min at 4°C to remove the insoluble fractions. For Western blot analysis, 10 μg of cell lysates was denatured and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were transferred to nitrocellulose membrane (Atto, Tokyo, Japan) and immunoblotted with the indicated antibodies. For immunoprecipitation, the antibodies were incubated with the lysates for 1 h at 4°C prior to the addition of protein A beads (Pharmacia, Peapack, N.J.) and then further incubated for 1 h. Beads were washed three times with phosphate-buffered saline (PBS) containing 1% Nonidet P-40, then three times with wash buffer (100 mM Tris-HCl [pH 7.5], 500 mM LiCl), and finally twice with assay buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 mM NaCl). The immunoprecipitated material was used for the assay.
PI 3-kinase assay.
In vitro phosphorylation of PI was carried out as follows. CHO cells were infected with adenovirus expressing SKIP or its mutant for 24 h. Cells were serum starved for 4 h and incubated in the presence or absence of insulin (100 nM) for 10 min. Cells were washed once with PBS and lysed with lysis buffer (20 mM Tris-HCl [pH 7.5], 1 mM MgCl2, 1 mM CaCl2, 0.2 M NaCl, 10% glycerol, 1% Nonidet P-40). Lysates were immunoprecipitated with anti-IRS-1 antibodies as described above. The immune complexes were resuspended in 100 μl of kinase buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA) and incubated with [γ-32P]ATP (3,000 Ci/mmol) for 10 min at 37°C. The reaction was terminated with the addition of 100 μl of 1 N HCl. The lipids were extracted with chloroform-methanol (2:1, vol/vol) and applied to a thin-layer chromatography plate (Merck, Darmstadt, Germany). The plates were developed in chloroform-methanol-H2O-ammonium (60:47:11.6:2, vol/vol/vol/vol), dried, and visualized by autoradiography with X-ray film (Kodak, Rochester, N.Y.).
HPLC analysis and quantification of phosphoinositides by [32P] labeling.
CHO cells were cultured in orthophosphate-free DMEM and radiolabeled with [32P]orthophosphate for 2 h. After the cells were stimulated with insulin (100 nM) and washed three times with ice-cold PBS, the phosphoinositides were extracted by the addition of 1.5 ml of methanol followed by the same volume of chloroform-methanol (1:2, vol/vol). After brief sonication, the same volume of chloroform and also 1 N HCl were added. The organic phase was pooled and dried under nitrogen gas. The phosphoinositides were deacylated with methylamine as described previously (37). Samples were dissolved in 10 mM (NH4)2PO4, pH 3.8, and separated by high-performance liquid chromatography (HPLC) on a Partisphere strong anion exchange column (Whatman, Clifton, N.J.). The radioactivity was quantified with a Flow Scintillation analyzer (Packard, Meridian, Calif.).
Measurement of calcium influx.
The calcium indicator fura-2 AM was used to monitor intracellular calcium changes. CHO-IR cells were harvested and suspended in calcium assay buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM KCl, 10 mM glucose) and then loaded with 1 μM fura-2 AM. For intracellular calcium measurements, cells were resuspended in assay buffer at 37°C and fluorescence was monitored by the CAF-110 intracellular ion calculator (JASCO International, Tokyo, Japan) at an excitation wavelength of 340 nm and an emission wavelength of 380 nm. Experiments were calibrated by adding 0.1% Triton X-100 to set the maximum fluorescence value or 10 mM EGTA to establish the minimum fluorescence value.
Antisense oligonucleotides.
Antisense and sense oligonucleotides for SKIP as well as scrambled control sequences were synthesized and purified by Hokkaido System Science, Inc. (Sapporo, Japan). The 18-mer of human SKIP included the presumed initiation site for translation in both the sense and antisense directions. The sequences were as follows: antisense, 5′-CCG CGA GCT CAT GGC CGC-3′; sense, 5′-GCG GCC ATG AGC TCG CGG-3′; and scrambled, 5′-GCG AGC TCA TCT CAG CTT CC-3′. The oligonucleotides (5 μM) were transfected into CHO cells (5 × 105 cells) by using Lipofectamine reagent (Invitrogen Inc., Groningen, The Netherlands) according to the manufacturer's protocol. Cells were cultured in Ham's F-12 medium containing 10% fetal calf serum for 48 h and then switched to serum-deprived medium for 4 h. The cells were then stimulated with insulin (100 nM) and lysed as described above.
Immunofluorescence microscopy.
CHO cells (1.0 × 104 cells) were cultured on cover glass and stimulated with insulin as described above. Slides were washed with PBS and fixed with 3.7% formaldehyde in PBS for 15 min. Cells were washed and permeabilized with 0.2% Triton X-100 in PBS for 5 min and then washed again with PBS. To visualize the actin cytoskeleton, permeabilized cells were incubated with rhodamine-conjugated phalloidin for 1 h. The cells were analyzed by confocal immunofluorescence microscopy.
Immunofluorescence of SKIP and cell surface GLUT4.
CHO cells expressing Myc epitope-tagged GLUT4 (1.0 × 104 cells) were transfected with GFP-tagged constructs expressing wild-type SKIP or its phosphatase-negative mutant. Cells were seeded onto cover glass and fixed after stimulation with insulin as described above. Cells were incubated with anti-Myc monoclonal antibody before permeabilization to visualize the plasma membrane-bound GLUT4. They were then incubated with anti-SKIP polyclonal antibody. After being washed with PBS, the cells were incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G and tetramethyl rhodamine isocyanate-conjugated anti-rabbit immunoglobulin G and analyzed by immunofluorescence microscopy.
2-Deoxyglucose transport assay.
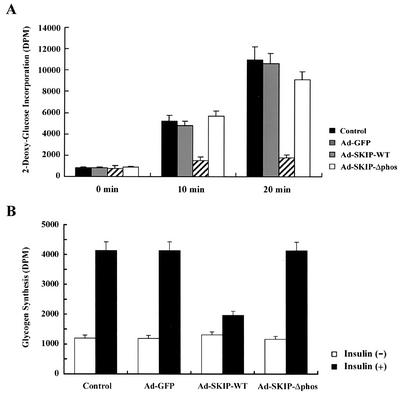
Differentiated L6 myotubes were infected with Ad-GFP, Ad-SKIP-WT, or Ad-SKIP-Δphos for 24 h. After being cultured in serum- and glucose-deprived conditions, cells were incubated in DMEM supplemented with or without 10 nM insulin for 30 min or 1 h at 37°C. Glucose uptake was determined after the addition of substrate (0.1 μCi of [2-3H]deoxyglucose and 1 μCi of l-[14C]glucose).
Glycogen synthesis assay.
Differentiated L6 myotubes were infected with Ad-GFP, Ad-SKIP-WT, or Ad-SKIP-Δphos for 24 h. After being cultured in serum- and glucose-deprived conditions, the medium was replaced with DMEM containing 5 mM glucose containing 1 μCi of l-[14C]glucose in the presence of 10 nM insulin for 1 h at 37°C. After incubation, the cells were washed three times with 50 mM phosphate buffer (pH 7.4) and solubilized with 30% KOH for 30 min at 37°C. Samples were boiled for 30 min with 4 mg of glycogen, and glycogen was precipitated by the addition of cold ethanol and incubation for 16 h at 4°C. Glucose incorporation into glycogen was measured by liquid scintillation counting.
RESULTS
SKIP is a PI(3,4,5)P3 phosphatase.
We have previously reported that SKIP is a type II inositol polyphosphate phosphatase, which hydrolyzes PI(4,5)P2, PI(3,4,5)P3, Ins(1,4,5)P3, and Ins(1,3,4,5)P4. We have also reported that SKIP possessed a sixfold higher phosphatase activity for PI(4,5)P2 than for Ins(1,4,5)P3 according to an assay with glutathione S-transferase-tagged recombinant SKIP protein. However, precise substrate specificities were not characterized. In the present study, we assessed the Km values for phosphoinositides and inositol phosphates with purified SKIP protein. SKIP exhibited particularly high activity for PI(3,4,5)P3 (0.89 μM). PI(4,5)P2 was also a good substrate, with a Km value of 1.80 μM, which was twofold higher than that of PI(3,4,5)P3. However, Ins(1,4,5)P3 and Ins(1,3,4,5)P4 were weakly hydrolyzed (Km values of 32.3 and 28.7 μM, respectively). Thus, we conclude that SKIP is a PI(3,4,5)P3 phosphatase, like SHIP and PTEN.
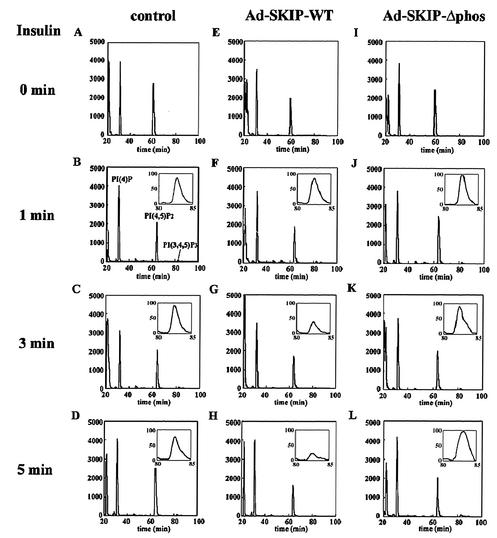
SHIP and PTEN are known to downregulate intracellular PI(3,4,5)P3 levels in response to growth factors and insulin. To determine whether SKIP also decreases PI(3,4,5)P3 levels, we generated recombinant adenoviruses. CHO cells were infected for 24 h with recombinant adenoviruses expressing GFP (control), wild-type SKIP, or phosphatase-negative mutant SKIP and were cultured for another 24 h (Fig. 1A and B). Specific expression was identified as a band at approximately 50 kDa by Western blotting incubated with anti-SKIP and anti-Myc antibodies (Fig. 1C). Endogenous SKIP was also identified. Recombinant SKIP proteins were expressed in a dose-dependent manner (Fig. 1C). Cells infected with these adenoviruses showed expression of SKIP proteins as much as fivefold higher than that in uninfected cells. To specify which phosphoinositides SKIP hydrolyzes in vivo, phosphoinositides isolated from control and SKIP-expressing CHO cells were analyzed by HPLC after deacylation. As shown in Fig. 2, PI(4,5)P2 levels in SKIP-expressing cells were reduced to 78.4% ± 2.1% (mean ± standard deviation) of the levels in control cells even in the absence of insulin stimulation, indicating that SKIP partially suppressed intracellular PI(4,5)P2 levels. However, the ratio of PI(4,5)P2 levels between control and SKIP-expressing cells did not change as a result of insulin treatment (Fig. 2E to H). PI(3,4,5)P3 was not detected in the absence of insulin treatment (Fig. 2A, E, and I). Insulin treatment elevated intracellular PI(3,4,5)P3 levels after 1 min. In cells expressing wild-type SKIP, this increase occurred in up to 95.1% of control cells at 1 min but in only 19.3% of control cells after 3 min (Fig. 2G and H). In contrast, expression of the phosphatase-negative mutant SKIP did not affect PI(3,4,5)P3 levels even after stimulation for 5 min (97.3% of control cells). These data indicate that SKIP hydrolyzes PI(3,4,5)P3, generated in response to insulin.
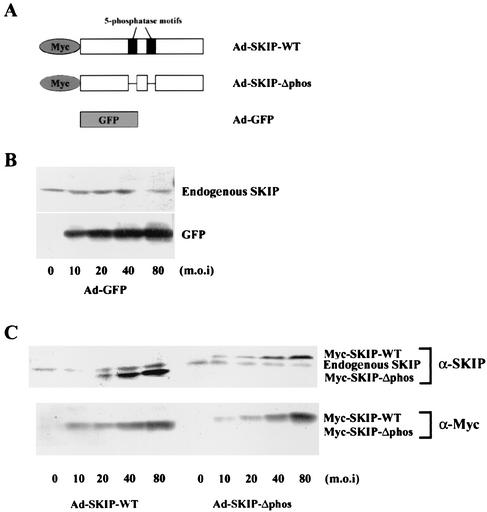
FIG. 1.
Western blot analysis of CHO cells expressing GFP and SKIP. (A) Schematic representation of GFP (Ad-GFP), Myc-tagged wild-type SKIP protein (Ad-SKIP-WT), and phosphatase-negative mutant SKIP (Ad-SKIP-Δphos), which lacks the catalytic motifs of the wild type. (B and C) CHO cells were either left uninfected (0) or infected with increasing concentrations of recombinant adenovirus expressing GFP (B) or wild-type SKIP or its phosphatase-negative mutant (C) for 24 h. Cell lysates were prepared 48 h later as described in Materials and Methods and were immunoblotted with anti-GFP (B), anti-Myc (α-Myc) (C), or anti-SKIP (α-SKIP) (B and C) antibodies. m.o.i, multiplicity of infection.
FIG. 2.
Effect of SKIP on intracellular phosphoinositide levels. Control cells (A to D) or CHO cells infected with Ad-SKIP-WT (E to H) or Ad-SKIP-Δphos (I to L) were labeled with [32P]orthophosphate for 2 h. Serum-deprived cells were either unstimulated (A, E, and I) or stimulated for 1 min (B, F, and J), 3 min (C, G, and K), or 5 min (D, H, and L) with insulin. Phospholipids were extracted, deacylated, and applied to a strong anion exchange column as described in Materials and Methods. The insets show the region of PI(3,4,5)P3 elution from the column. Profiles were normalized to the total counts in each run. Values on the left of all panels indicate disintegrations per minute (102).
SKIP does not affect upstream signaling of PI 3-kinase activation in response to insulin.
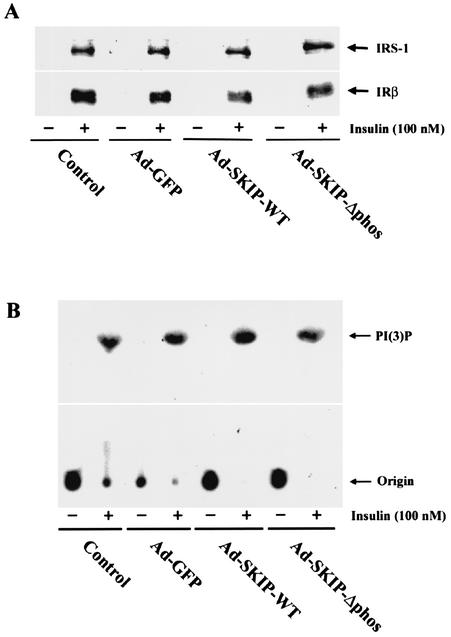
Insulin induces autophosphorylation of its receptor to activate its intrinsic tyrosine kinase, leading to phosphorylation of cellular substrates IRS-1 and Shc. Tyrosine phosphorylation of these proteins allows them to interact with PI 3-kinase. We examined the effect of ectopic expression of SKIP on insulin-mediated tyrosine phosphorylation of IRβ and IRS-1. The tyrosine phosphorylation levels of IRβ and IRS-1 were not affected by the expression of wild-type or mutant SKIP protein (Fig. 3A). We further examined PI 3-kinase activity in response to insulin. Insulin treatment led to a more than 20-fold increase in PI 3-kinase activity, which was unaffected by overexpression of SKIP or its phosphatase-negative mutant (Fig. 3B). This indicates that the phosphorylation of IRS-1 or PI 3-kinase activation by insulin treatment was not altered by SKIP expression.
FIG. 3.
Effect of SKIP on insulin-signaling proteins. (A) Insulin-stimulated tyrosine phosphorylation of IRS-1 and IRβ. CHO cells were infected with recombinant adenovirus and then stimulated with insulin (100 nM) for 10 min. Whole-cell lysates were immunoprecipitated with anti-phosphotyrosine antibodies and then blotted with anti-IRS-1 or anti-IRβ antibodies. (B) Effect of SKIP on insulin-stimulated PI 3-kinase activity in CHO cells. Adenovirus-infected cells were stimulated with insulin (100 nM) for 10 min. Cell lysates were immunoprecipitated with anti-IRS-1 antibodies and assayed for PI 3-kinase activity with PI as the substrate. The products were resolved by thin-layer chromatography and visualized by autoradiography.
Inhibitory effect of SKIP on insulin-induced Akt phosphorylation.
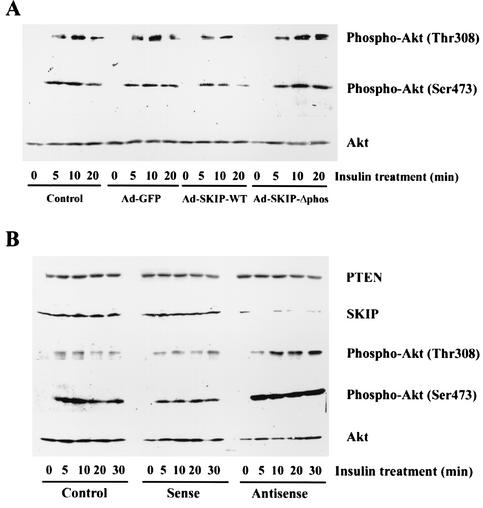
Akt/PKB is activated in response to insulin stimulation in a PI 3-kinase-dependent manner. This activation is mediated by phosphorylation at Thr-308 and Ser-473 of Akt, which is increased by the major PI 3-kinase product PI(3,4,5)P3 (1). Recently, Scheid et al. reported that PI(3,4)P2 and PI(3,4,5)P3 are required for Akt activation in SHIP1 knockout mice (36). They suggested that PI(3,4,5)P3 attracts Akt to the plasma membrane for phosphorylation at Thr-308 by PDK, whereas PI(3,4)P2 is necessary for phosphorylation of Akt at Ser-473 (36). Since SKIP rapidly hydrolyzed PI(3,4,5)P3 generated in response to insulin stimulation, we evaluated the effect of SKIP on phosphorylation at Thr-308 and Ser-473 of Akt by using phosphorylation site-specific antibodies to determine whether SKIP downregulates PI 3-kinase signaling pathways. Insulin treatment caused a marked increase in Akt phosphorylation after 5 min, and this phosphorylation peaked at 10 min. The expression of wild-type SKIP reduced the level of Ser-473 phosphorylation of Akt to 57.3% ± 2.4% of that in control cells at 10 min, and this inhibitory effect increased with incubation time. In contrast, expression of the phosphatase-negative mutant SKIP increased Ser-473 phosphorylation to 110.2% ± 5.4% of that in control cells (Fig. 4A). Phosphorylation at Thr-308 was also decreased in cells expressing wild-type SKIP. These results indicate that overexpression of SKIP negatively regulates Akt phosphorylation. To analyze the effects of endogenous SKIP on insulin-induced Akt phosphorylation in CHO cells, SKIP antisense oligonucleotides were transfected into these cells. As expected, antisense oligonucleotides dramatically reduced SKIP expression levels, whereas sense and control oligonucleotides did not (Fig. 4B). The expression levels of PTEN, another PI(3,4,5)P3 phosphatase, were not affected. In cells transfected with antisense oligonucleotides, a low level of Akt phosphorylation was identified even after serum starvation. However, in response to insulin, antisense treatment induced a very strong phosphorylation of Akt at Thr-308 and Ser-473 (Fig. 4B), an effect similar to that obtained by expression of constitutively active PI 3-kinase.
FIG. 4.
SKIP involvement in insulin-induced Akt phosphorylation in CHO cells. (A) SKIP inhibition of Akt phosphorylation in CHO cells. CHO cells were infected with Ad-GFP, Ad-SKIP-WT, or Ad-SKIP-Δphos for 24 h. Control cells were uninfected. Serum-deprived cells were stimulated with insulin (100 nM) for up to 20 min. Lysates were immunoblotted with phospho-specific Akt antibodies (upper and middle panels) or anti-Akt antibody (lower panel). The phospho-specific antibody only detects Akt phosphorylated at Thr-308 or Ser-473. The Western blots shown are representative of three independent experiments. (B) Akt activation by SKIP inhibition. CHO cells were transfected with antisense, sense, or control oligonucleotides for 48 h. Serum-starved cells were stimulated with insulin (100 nM) for up to 30 min. Cells were lysed, and equal amounts of lysate were blotted with anti-PTEN antibody, anti-SKIP antibody, phospho-specific Akt antibodies, or anti-Akt antibody.
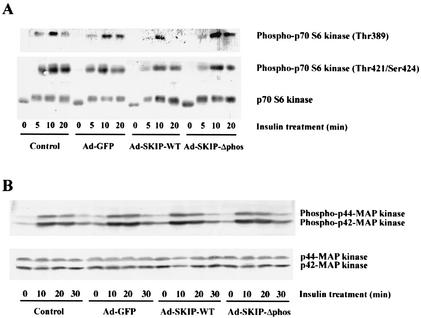
Effect of SKIP on p70 S6 kinase and MAP kinase.
p70 S6 kinase is a downstream target of PI 3-kinase and is phosphorylated on serine-threonine residues by insulin treatment (3). As shown in Fig. 5A, insulin induced the phosphorylation of p70 S6 kinase. A mobility shift assay showed that p70 S6 kinase phosphorylation was partially inhibited in SKIP-expressing cells after insulin stimulation for 20 min (Fig. 5A, bottom panel). Phosphorylation of Thr-389 in the catalytic domain by FRAP/mTOR, a downstream target of Akt, is critical for p70 S6 kinase activation, whereas phosphorylation of Thr-421 and Ser-424 via the mitogen-activated protein (MAP) kinase cascade is believed to upregulate activity (3, 27, 32). This phosphorylation of Thr-389 by insulin is PI 3-kinase dependent and is inhibited by wortmannin treatment (51). We identified a dramatic inhibition of Thr-389 phosphorylation by SKIP expression after insulin treatment for 20 min, whereas Thr-421 and Ser-424 phosphorylations were not significantly altered (Fig. 5A, top and middle panels). This inhibition of site-specific phosphorylation is consistent with a partial band shift as identified by the mobility shift assay. Thus, inhibition of p70 S6 kinase phosphorylation in response to SKIP expression appears to occur via hydrolysis of PI(3,4,5)P3 by SKIP and subsequent inhibition of Akt.
FIG. 5.
SKIP inhibition of p70 S6 kinase but not of MAP kinase. (A) Effect of SKIP on insulin-stimulated p70 S6 kinase activation. CHO cells that were uninfected (control) or infected with Ad-GFP, Ad-SKIP-WT, or Ad-SKIP-Δphos were incubated in the presence or absence of insulin for the indicated times. Cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with either phospho-specific p70 S6 kinase antibody, which detects p70 S6 kinase only when phosphorylated at Thr-389 (upper panel), or phospho-p70 S6 kinase antibody, which detects p70 S6 kinase phosphorylated at Thr-421 and Ser-424 (middle panel). The same lysates were blotted with anti-p70 S6 kinase antibody (lower panel). SKIP inhibited insulin-induced phosphorylation at Thr-389, whereas phosphorylation at Thr-421 and Ser-424 was only slightly inhibited. (B) Effect of SKIP on MAP kinase phosphorylation. CHO cells infected with adenovirus were either unstimulated or stimulated with insulin (100 nM) for 10 to 30 min. Cell lysates were immunoblotted with anti-phospho-p44 or anti-phospho-p42 MAP kinase (upper panel) or anti-MAP kinase antibody (lower panel). Neither wild-type SKIP nor its phosphatase-negative mutant inhibited MAP kinase activation after insulin stimulation for 30 min.
To examine the specificity of the effect of SKIP on the PI 3-kinase/Akt signaling pathway, we evaluated the effect of SKIP expression on insulin-induced MAP kinase (ERK1 and ERK2) activation. As shown in Fig. 5B, insulin increased the phosphorylations of p44 and p42 MAP kinases. It has been reported that the PI(3,4,5)P3 phosphatases SHIP and PTEN inhibit insulin-induced MAP kinase phosphorylation by interfering with the formation of the IRS-1/Grb2/Shc complex (46, 50). However, overexpression of SKIP did not affect MAP kinase activation even after 20 min of insulin treatment (Fig. 5B, upper panel). Thus, SKIP is likely to hydrolyze only PI(3,4,5)P3 generated by insulin stimulation and not affect IRS-1/Grb2/Shc complex formation or MAP kinase activation. These findings suggest that SKIP specifically downregulates the PI 3-kinase and Akt pathway by PI(3,4,5)P3 hydrolysis.
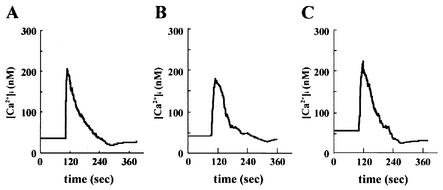
SKIP partially suppressed insulin-induced calcium mobilization.
As shown in Fig. 2, expression of SKIP enhanced hydrolysis of PI(3,4,5)P3 generated by insulin stimulation. However, intracellular levels of PI(4,5)P2, another substrate of SKIP, were slightly reduced in SKIP-expressing cells. To determine whether the decrease in basal intracellular PI(4,5)P2 levels caused by SKIP expression affects cellular events downstream of PI(4,5)P2, we examined insulin-induced calcium mobilization, which is mediated by Ins(1,4,5)P3, the product of PI(4,5)P2 hydrolysis by phospholipase C. As previously reported, in CHO cells, calcium mobilization was not induced by insulin. In contrast, in CHO-IR cells, insulin stimulation evoked cytoplasmic calcium mobilization (30). Thus, we used CHO-IR cells to examine Ca2+ mobilization by insulin. SKIP expression reduced insulin-mediated calcium mobilization to 76.8% of that of the control, whereas expression of the phosphatase-negative mutant resulted in calcium mobilization at 106.3% of that in control cells (Fig. 6B and C). This result indicates that the decrease in basal intracellular PI(4,5)P2 levels caused by SKIP partly influences calcium mobilization in CHO cells.
FIG. 6.
Effect of SKIP on insulin-induced calcium mobilization. CHO cells (A) and CHO cells expressing wild-type SKIP (B) or phosphatase-negative mutant SKIP (C) were loaded with fura-2 AM. Cells were stimulated with insulin (100 nM) 100 s into each experiment. The experiments were performed in triplicate, and the graph in each panel depicts a representative run.
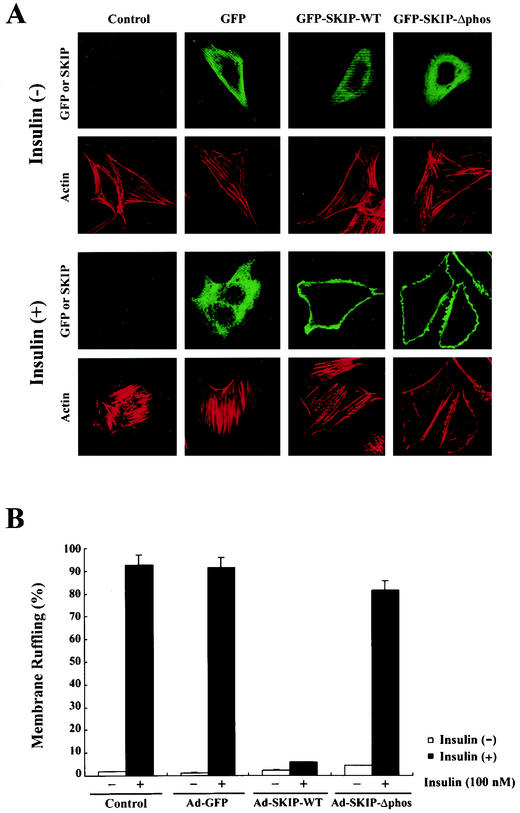
Effect of SKIP on insulin-induced membrane ruffle formation and GLUT4 translocation.
As previously reported, SKIP negatively regulates the actin cytoskeleton. Actin stress fibers are not present where SKIP is concentrated (12). Cortical actin cytoskeletal rearrangement, another biological effect induced by insulin, has been shown to be dependent on PI 3-kinase activity (19). Insulin stimulation causes the breakdown of actin stress fibers, which is followed by membrane ruffling. We assessed the effect of SKIP expression on insulin-induced membrane ruffling. As shown in Fig. 7B, less than 5% of control cells exhibited membrane ruffling under resting conditions but this increased to approximately 90% after insulin stimulation. SKIP expression had a tendency to decrease the number of actin stress fibers under resting conditions. Membrane ruffling was observed in 92.5% ± 4.6% of control cells and 91.3% ± 4.6% of GFP-expressing cells in response to insulin. Expression of SKIP significantly inhibited membrane ruffling (5.8% ± 0.4% of cells), whereas in phosphatase-negative mutant SKIP-expressing cells, membrane ruffles were induced in 81.5% ± 4.9% of cells (Fig. 7A and B), indicating that SKIP expression abolishes insulin-induced membrane ruffle formation.
FIG. 7.
Inhibition by SKIP of insulin-induced membrane ruffling in CHO cells. (A) Control CHO cells and those infected with adenovirus expressing GFP, wild-type SKIP, or phosphatase-negative mutant SKIP for 24 h were serum starved and stimulated with insulin (100 nM) for 10 min and stained with anti-Myc antibody (SKIP) or rhodamine-phalloidin (Actin). Membrane ruffle formation in SKIP-expressing cells was significantly inhibited. (B) CHO cells expressing GFP and SKIP were stimulated with insulin, and the numbers of cells exhibiting membrane ruffling were counted. Wild-type SKIP substantially inhibited insulin-induced membrane ruffling. Open bars represent the basal rate of membrane ruffle formation in the absence of insulin, whereas the solid bars represent values in the presence of insulin (100 nM). Results are means of five separate experiments.
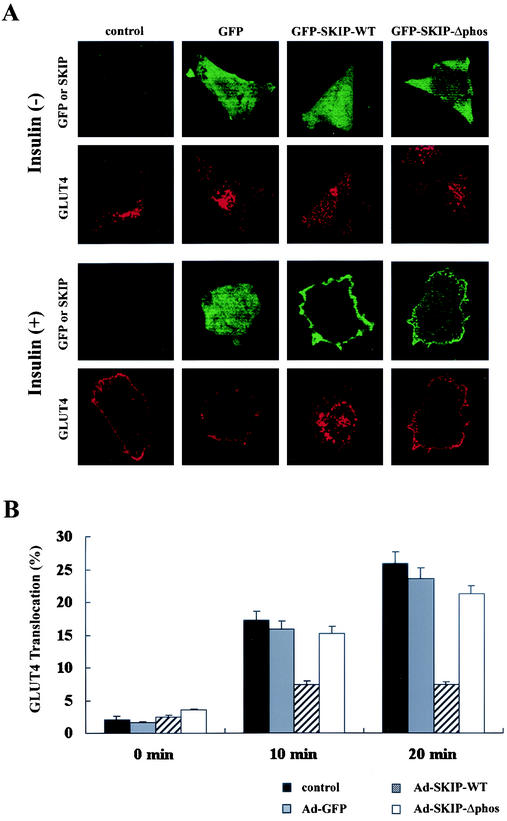
Insulin stimulates glucose uptake in skeletal muscle and adipose tissue by inducing the translocation of the insulin-responsive glucose transporter GLUT4 from intracellular stores to the plasma membrane (33). Under resting conditions, GLUT4 cycles between the plasma membrane and the intracellular compartment, but most of it resides within the intracellular compartment. Translocation of the glucose transporter is a major biological response to insulin, which requires PI 3-kinase activation (25, 38). We analyzed GLUT4 localization by transfection of GFP-tagged SKIP in CHO cells stably expressing Myc-tagged GLUT4. After serum deprivation, cells were stimulated with insulin. Membrane-bound GLUT4 was visualized with anti-Myc antibody, and immunoreactive cells were counted. In unstimulated cells, GLUT4 was localized predominantly around the nucleus, and insulin stimulation caused rapid translocation of GLUT4 to the plasma membrane (Fig. 8A, panels labeled “control”). GLUT4 translocation occurred in 25.8% ± 1.9% of control cells and 23.5% ± 1.7% of GFP-expressing cells after insulin stimulation for 20 min (Fig. 8B). In contrast, GLUT4 translocation occurred in only 7.4% ± 0.4% of cells expressing wild-type SKIP (Fig. 8A). Expression of the phosphatase-negative mutant did not affect insulin-induced GLUT4 translocation (21.2% ± 1.2% of cells), indicating that SKIP inhibits insulin-induced GLUT4 translocation (Fig. 8A and B).
FIG. 8.
Inhibition of GLUT4 translocation in SKIP-expressing cells. (A) CHO cells constitutively expressing GLUT4 were infected with adenovirus expressing wild-type SKIP or its phosphatase-negative mutant for 24 h. Cells were starved overnight and stimulated with insulin (100 nM) for 20 min. Cells were stained with anti-GLUT4 antibody and anti-SKIP antibody. The number of SKIP-positive cells exhibiting GLUT4 translocation was counted. SKIP expression inhibited insulin-induced GLUT4 translocation by approximately 75%. (B) CHO cells expressing GFP and SKIP were stimulated with insulin for 10 or 20 min, and the number of cells showing GLUT4 translocation was counted. Results are means of five separate experiments.
SKIP negatively regulates insulin-induced glucose uptake and glycogen synthesis in L6 myocytes.
We examined the effect of SKIP on insulin-induced glucose transport in L6 myocytes that express endogenous SKIP as well as GLUT4. Treatment with insulin resulted in an approximately 10-fold increase in glucose uptake after 20 min. Expression of exogenous SKIP inhibited glucose uptake by 89.3% ± 1.1%, whereas the phosphatase-negative mutant did not produce such inhibition (Fig. 9A). We further examined the effect of SKIP on insulin-induced glycogen synthesis. Insulin stimulation resulted in an approximately threefold increase in glucose incorporation into glycogen in control cells. Insulin-induced glycogen synthesis was inhibited by 75.9% ± 3.3% in response to SKIP expression (Fig. 9B). These results indicate that SKIP suppressed insulin-induced glucose transport in myocytes.
FIG. 9.
Inhibition by SKIP of insulin-induced glucose transport and glycogen synthesis in L6 myoblasts. (A) Insulin-induced glucose transport in adenovirus-infected cells. L6 myoblasts expressing GFP or SKIP were stimulated with insulin for 10 or 20 min. (B) Insulin-induced glucose incorporation into glycogen in transfected cells. Cells expressing GFP or SKIP were stimulated with insulin for 1 h. Results are means of three separate experiments.
Insulin induces SKIP translocation from the Golgi apparatus to the plasma membrane.
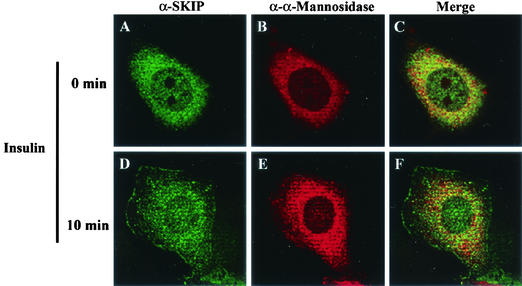
Immunofluorescence staining of CHO cells with anti-SKIP antibody showed that endogenous SKIP is mainly localized to an intracellular organelle, presumably the trans-Golgi reticulum because of colocalization with α-mannosidase under resting conditions (Fig. 10A to C). In response to insulin, some SKIP immunoreactivity translocated to the plasma membrane, similar to GLUT4 (Fig. 10D). SKIP translocation was also clearly identified in GFP-SKIP-expressing cells (Fig. 8A). This translocation may be responsible for a decrease in PI(3,4,5)P3 levels at the plasma membrane and would require a time lag after insulin stimulation in SKIP-expressing cells. These data suggest that SKIP specially attenuates the effects of PI(3,4,5)P3, which is newly synthesized at the plasma membrane after insulin treatment (Fig. 10D to F).
FIG. 10.
Insulin-induced translocation of SKIP from intracellular compartments to the plasma membrane. CHO cells were serum starved for 16 h and then stimulated with insulin for 10 min. Cells were fixed and immunostained with anti-SKIP antibody (α-SKIP) (A and D) or anti-α-mannosidase (α-α-Mannosidase), a trans-Golgi marker (B and E). The combined images are also shown (C and F). Localization of SKIP to the trans-Golgi reticulum under resting conditions and translocation to the plasma membrane upon insulin stimulation can be observed.
DISCUSSION
SKIP is a phosphoinositide 5-phosphatase.
SKIP is a recently identified inositol polyphosphate 5-phosphatase that is ubiquitously and abundantly expressed in skeletal muscle, kidney, and heart tissues (12). SKIP is a type II phosphatase and hydrolyzes the 5-phosphate of phosphoinositides and inositol phosphates. Previously, our group reported that SKIP hydrolyzes PI(4,5)P2 and PI(3,4,5)P3 in vitro (12). In the present study, we examined the substrate specificity in vivo and found that SKIP hydrolyzes PI(3,4,5)P3, which is synthesized by PI 3-kinase in response to insulin stimulation. PI 3-kinase is a mediator of several metabolic actions of insulin and is critical but not sufficient for glucose uptake (2). Activated PI 3-kinase generates PI(3,4,5)P3, a phosphoinositide that activates the downstream target Akt. The molecular events downstream of PI 3-kinase have been extensively studied, and some phosphoinositide phosphatases, particularly PI(3,4,5)P3 phosphatases such as PTEN, SHIP, and SHIP2, are considered to be negative regulators of insulin function (23). PTEN, which was identified as a tumor suppressor gene, dephosphorylates the 3-phosphate of PI(3,4,5)P3 (24). SHIP, however, is a PI(3,4,5)P3 5-phosphatase which is predominantly involved in insulin signaling. Vollenweider et al. have reported that SHIP inhibits insulin-induced GLUT4 translocation (45). Thus, SKIP, which is closer to SHIP rather than PTEN, is likely to be a phosphoinositide phosphatase and plays an inhibitory role in the insulin-mediated PI 3-kinase signaling pathway by hydrolyzing PI(3,4,5)P3. Expression of SKIP also downregulated PI(4,5)P2 levels and the downstream events induced by PI(4,5)P2, such as calcium mobilization. Activation of a calcium-permeable channel by the action of insulin, which is a unique feature in CHO-IR cells (30), is necessary for the regulation of cell growth. The role of PI(4,5)P2 in insulin-responsive tissues, such as liver and skeletal muscle, is still unknown. Thakker et al. reported that phospholipase C activity and an increase in cytoplasmic calcium in human diabetic liver might be postulated to induce insulin resistance (42). SKIP may play a role in the downregulation of PI(4,5)P2 levels to induce insulin resistance.
Involvement of SKIP in the downregulation of insulin signaling.
In this study, we examined the effect of SKIP on events downstream of insulin stimulation. We found that SKIP inhibits insulin-induced increases in intracellular PI(3,4,5)P3 levels, suggesting that it acts as a negative regulator in insulin signaling, similar to PTEN and SHIP. Since tyrosine phosphorylations of the insulin receptor and IRS-1 and PI 3-kinase activity were not affected, SKIP likely downregulates insulin signaling through reduction of PI(3,4,5)P3 levels. Interestingly, overexpression of SKIP did not affect PI(3,4,5)P3 levels after the first minute of insulin stimulation, whereas an approximately 80% decrease in PI(3,4,5)P3 levels was observed after 5 min, showing the time lag of SKIP action. PI(3,4,5)P3 levels peaked within 1 min after insulin treatment, and it was slowly dephosphorylated thereafter by phosphatases (49). The levels of PI(4,5)P2, another SKIP substrate, were decreased slightly in SKIP-expressing cells in the absence of insulin, but the rate of dephosphorylation in response to insulin was the same regardless of SKIP expression. Our results also showed that overexpression of SKIP, unlike that of its phosphatase-negative mutant, inhibited insulin-induced Akt phosphorylation in CHO cells. However, this inhibition was very weak until 5 min after insulin stimulation and then rose substantially by 10 min (up to 42.7%). These time lag effects appear to be caused by the requirement of SKIP to translocate from the intracellular compartment to the plasma membrane in response to insulin. As our group has described previously, SKIP is localized throughout the cell, with the exception of the cell periphery (12). In CHO cells, SKIP protein is expressed throughout the cells but is concentrated in the intracellular compartment, particularly in the trans-Golgi reticulum. Thus, this translocation appears to be important for the hydrolysis of PI(3,4,5)P3, because PI(3,4,5)P3 is formed at the plasma membrane in response to insulin. Under resting conditions, SKIP is localized to the trans-Golgi reticulum, away from the vicinity of its lipid substrates. Therefore, SKIP cannot access its substrates in the absence of insulin stimulation. In contrast to what is seen with SKIP, overexpression of PTEN or SHIP strongly inhibits Akt phosphorylation within 5 min after stimulation (30, 48). Generally, insulin-induced PI(3,4,5)P3 production peaks within 1 min and then Akt becomes maximally active by 5 min, maintaining this maximum level for up to 4 h (49). Rapid dephosphorylation of Akt by SKIP occurs approximately 5 min after insulin stimulation and is probably due to the rapid decrease in intracellular PI(3,4,5)P3 levels induced by the translocation of SKIP to the plasma membrane. In the present study, interference of SKIP expression by antisense oligonucleotides was found to lead to increased phosphorylation of Akt in response to insulin. This hyperactivation was maintained for more than 30 min. These results support the idea that endogenous SKIP participates in the downregulation of insulin signaling through attenuation of PI(3,4,5)P3 levels.
SKIP in membrane ruffle formation and glucose transport.
PTEN plays an important role in growth control; consequently, mutations in PTEN have been implicated in a variety of human cancers (23). In contrast, SHIP2, another PI(3,4,5)P3 phosphatase, is believed to regulate insulin-induced glucose transport, suggesting that although they exhibit similar phosphatase activities, different phosphoinositide phosphatases carry out different functions (48). Our results indicate that SKIP functions preferentially in the regulation of glucose transport and actin cytoskeletal rearrangement. SKIP partly inhibited the incorporations of thymidine and bromodeoxyuridine (data not shown). In contrast, SKIP strongly inhibited insulin-induced GLUT4 translocation to the plasma membrane as well as membrane ruffle formation. These effects are similar to those caused by SHIP2, but not PTEN, expression.
Membrane ruffle formation is dependent on the generation of PI(3,4,5)P3 and the subsequent activation of the small GTP-binding protein Rac (7, 35). SKIP dephosphorylated PI(3,4,5)P3, which is generated at the plasma membrane in response to insulin. Therefore, membrane ruffle formation is thought to be inhibited by a decrease in PI(3,4,5)P3 levels. Such effective inhibition of membrane ruffling may be induced by the translocation of SKIP to areas of membrane ruffling in response to insulin. In contrast, PI(4,5)P2 levels were only partly suppressed and actin stress fiber formation, which correlates with PI(4,5)P2 generation, was not affected by SKIP expression. Insulin-induced calcium mobilization, another biological effect, was not strongly inhibited by the expression of SKIP. Thus, SKIP predominantly influences PI(3,4,5)P3 changes and slightly influences PI(4,5)P2 levels in response to insulin, thereby regulating the biological actions caused through PI(3,4,5)P3.
Regulation of glucose transport by SKIP.
Regulation of glucose transport involves the recruitment by insulin of the mammalian glucose transporter GLUT4 to the cell surface. The presence of GLUT4 at the cell surface is important for accelerated glucose uptake into cells. PI 3-kinase activation by insulin is necessary for glucose uptake and glycogen synthesis. Overexpression of PTEN has been reported to negatively regulate glucose transport and membrane ruffle formation (29). However, a recent report has suggested that endogenous PTEN does not play a major role in the regulation of glucose transport activity in 3T3-L1 adipocytes (31). On the other hand, mice heterozygous for the SHIP2 gene exhibit increased insulin sensitivity and glucose tolerance (4), suggesting that SHIP2 is a potent negative regulator of insulin signaling. SKIP is a PI(3,4,5)P3 phosphatase and is highly expressed in insulin-responsive tissues, such as skeletal muscle. In the present study, we showed that insulin-induced translocation of GLUT4 is substantially inhibited by SKIP expression. GLUT4 is localized mainly to intracellular compartments under resting conditions and moves to the plasma membrane in response to insulin. Similarly, insulin mobilized SKIP from intracellular compartments to the cell surface. Phosphatase-negative mutant SKIP did not affect GLUT4 movement but was also recruited to the plasma membrane, which was similar to what was seen with GLUT4. These results strongly suggest that SKIP plays a role in insulin-stimulated GLUT4 translocation. There are two possible explanations for SKIP translocation by insulin stimulation. First, the translocation might be PI 3-kinase dependent. SKIP may directly bind to PI(3,4,5)P3 or form a complex with molecules located downstream of PI 3-kinase. Second, it might be PI 3-kinase independent. Insulin induces Cbl phosphorylation and IRS-1/Grb2/Shc complex formation, which is inhibited by SHIP2, in a PI 3-kinase-independent manner. SKIP may also bind to other molecules involved in the insulin signaling pathway and translocate to the plasma membrane.
Khayat et al. have reported that insulin-dependent formation of actin structures facilitates the association of PI 3-kinase with GLUT4 vesicles and the recruitment of GLUT4 to the cell surface (16). They also suggested that activation of Rac by PI 3-kinase is necessary for GLUT4 translocation to the cell surface. In addition, Tong et al. have recently reported that insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles (44). As mentioned above, since SKIP strongly inhibited cortical actin polymerization as well as membrane ruffle formation, it is possible that SKIP plays an important role in GLUT4 translocation through the regulation of actin filament reorganization. SKIP is ubiquitously expressed but, in particular, is highly expressed in the insulin-responsive tissues, such as skeletal muscle. Thus, SKIP is likely to be involved in insulin signaling and may play a role in the negative regulation of glucose transport. The establishment of a SKIP-deficient cell line will clarify the involvement of SKIP in glucose metabolism and insulin signaling in mammals.
Acknowledgments
We thank Tomoichiro Asano and Hideyuki Sakoda, University of Tokyo, and Yousuke Ebina, Tokushima University, for the CHO cell line and technical assistance with the assay.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang, S.-H., A. C. Baumann, M. Kanzaki, C. D. Thurmond, T. R. Watson, L. C. Neudauer, G. I. Macara, E. J. Pessin, and R. A. Saltiel. 2000. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410:944-948. [DOI] [PubMed] [Google Scholar]
- 3.Chung, J., T. C. Grammer, K. P. Lemon, A. Kazlauskas, and J. Blenis. 1994. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature 370:71-75. [DOI] [PubMed] [Google Scholar]
- 4.Clement, S., U. Krause, F. Desmondt, J.-F. Tanti, J. Behrends, X. Pesesse, T. Sasaki, J. Penninger, M. Doherty, W. Malaisse, E. J. Dumont, L. Y. Marchand-Brustel, C. Erneux, L. Hue, and S. Schurmans. 2001. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409:92-96. [DOI] [PubMed] [Google Scholar]
- 5.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 6.Filippa, N., C. L. Sable, B. A. Hemmings, and E. Van Obberghen. 2000. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol. Cell. Biol. 20:5712-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558-560. [DOI] [PubMed] [Google Scholar]
- 8.Haruta, T., A. J. Morris, D. W. Rose, J. G. Nelson, P. Vollenweider, M. Mueckler, and J. M. Olefsky. 1995. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J. Biol. Chem. 270:27991-27994. [DOI] [PubMed] [Google Scholar]
- 9.Haruta, T., A. J. Morris, P. Vollenweider, J. G. Nelson, D. W. Rose, M. Mueckler, and J. M. Olefsky. 1998. Ligand-independent GLUT4 translocation induced by guanosine 5′-O-(3-thiotriphosphate) involves tyrosine phosphorylation. Endocrinology 139:358-364. [DOI] [PubMed] [Google Scholar]
- 10.He, T. C., S. Zhou, T. L. da Costa, W. K. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu, Q., A. Klippel, A. J. Muslin, W. J. Fantl, and L. T. Williams. 1995. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol 3-kinase. Science 268:100-102. [DOI] [PubMed] [Google Scholar]
- 12.Ijuin, T., Y. Mochizuki, K. Fukami, M. Funaki, T. Asano, and T. Takenawa. 2000. Identification and characterization of a novel inositol polyphosphate 5-phosphatase. J. Biol. Chem. 275:10870-10875. [DOI] [PubMed] [Google Scholar]
- 13.Janmey, A. P., W. Xian, and A. L. Flanagan. 1999. Controlling cytoskeleton structure by phosphoinositide-protein interactions: phosphoinositide binding protein domains and effects of lipid packing. Chem. Phys. Lipids 101:93-107. [DOI] [PubMed] [Google Scholar]
- 14.Jhun, B. H., D. W. Rose, B. L. Seely, L. Rameh, L. Cantley, A. R. Saltiel, and J. M. Olefsky. 1994. Microinjection of the SH2 domain of the 85-kilodalton subunit of phosphatidylinositol 3-kinase inhibits insulin-induced DNA synthesis and c-fos expression. Mol. Cell. Biol. 14:7466-7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapeller, R., and L. C. Cantley. 1994. Phosphatidylinositol 3-kinase. Bioessays 16:565-576. [DOI] [PubMed] [Google Scholar]
- 16.Khayat, Z. A., P. Tong, K. Yaworsky, R. J. Bloch, and A. Klip. 2000. Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J. Cell Sci. 113:279-290. [DOI] [PubMed] [Google Scholar]
- 17.Kisseleva, M. V., L. Cao, and W. P. Majerus. 2002. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/PKB phosphorylation and leads to apoptotic cell death. J. Biol. Chem. 22:6266-6272. [DOI] [PubMed] [Google Scholar]
- 18.Kohn, A. D., S. A. Summers, M. J. Birnbaum, and R. A. Roth. 1996. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271:31372-31378. [DOI] [PubMed] [Google Scholar]
- 19.Kotani, K., K. Hara, K. Kotani, K. Yonezawa, and M. Kasuga. 1995. Phosphoinositide 3-kinase as an upstream regulator of the small GTP-binding protein Rac in the insulin signaling of membrane ruffling. Biochem. Biophys. Res. Commun. 208:985-990. [DOI] [PubMed] [Google Scholar]
- 20.Kutateladze, T. G., K. D. Ogburn, W. T. Watson, T. de Beer, S. D. Emr, C. G. Burd, and M. Overduin. 1999. Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol. Cell 3:805-811. [DOI] [PubMed] [Google Scholar]
- 21.Lavan, B. E., V. R. Fantin, E. T. Chang, W. S. Lane, S. R. Keller, and G. E. Lienhard. 1997. A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. J. Biol. Chem. 272:21403-21407. [DOI] [PubMed] [Google Scholar]
- 22.Lavan, B. E., W. S. Lane, and G. E. Lienhard. 1997. The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J. Biol. Chem. 272:11439-11443. [DOI] [PubMed] [Google Scholar]
- 23.Li, J., C. Yen, D. Liaw, K. Podsypanina, S. Bose, S. I. Wang, J. Puc, C. Miliaresis, L. Rodgers, R. McCombie, S. H. Bigner, B. C. Giovanella, M. Ittmann, B. Tycko, H. Hibshoosh, M. H. Wigler, and R. Parsons. 1997. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943-1947. [DOI] [PubMed] [Google Scholar]
- 24.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375-13378. [DOI] [PubMed] [Google Scholar]
- 25.Martin, S. S., T. Haruta, A. J. Morris, A. Klippel, L. T. Williams, and J. M. Olefsky. 1996. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J. Biol. Chem. 271:17605-17608. [DOI] [PubMed] [Google Scholar]
- 26.Martin, S. S., D. W. Rose, A. Saltiel, L. T. Klippel, L. T. Williams, and J. M. Olefsky. 1996. Phosphatidylinositol 3-kinase is necessary and sufficient for insulin-stimulated stress fiber breakdown. Endocrinology 137:5045-5054. [DOI] [PubMed] [Google Scholar]
- 27.Ming, X. F., B. M. Burgering, S. Wennström, L. Claesson-Welsh, C. H. Heldin, J. L. Bos, S. C. Kozma, and G. Thomas. 1994. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature 371:426-429. [DOI] [PubMed] [Google Scholar]
- 28.Myers, M. G., Jr., L. M. Wang, X. J. Sun, J. H. Pierce, J. Blenis, and M. F. White. 1994. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J. Biol. Chem. 269:28783-28789. [PubMed] [Google Scholar]
- 29.Nakashima, N., M. P. Sharma, T. Imamura, R. Bookstein, and M. J. Olefsky. 2000. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J. Biol. Chem. 275:12889-12895. [DOI] [PubMed] [Google Scholar]
- 30.Nie, L., M. Kanzaki, H. Shibata, and I. Kojima. 1998. Activation of calcium permeable cation channel by insulin in Chinese hamster ovary cells expressing human insulin receptors. Endocrinology 139:179-188. [DOI] [PubMed] [Google Scholar]
- 31.Ono, H., H. Katagiri, M. Funaki, M. Anai, K. Inukai, Y. Fukushima, H. Sakoda, T. Ogihara, Y. Onishi, M. Fujishiro, M. Kikuchi, Y. Oka, and T. Asano. 2001. Regulation of phosphoinositide metabolism, Akt phosphorylation, and glucose transport by PTEN (phosphatase and tensin homolog deleted on chromosome 10) in 3T3-L1 adipocytes. Mol. Endocrinol. 15:1411-1422. [DOI] [PubMed] [Google Scholar]
- 32.Pearson, R. B., P. B. Dennis, J. W. Han, N. A. Williamson, S. C. Kozma, R. E. Wettenhall, and G. Thomas. 1995. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 14:5279-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pessin, J. E., D. C. Thurmond, J. S. Elmendorf, K. J. Coker, and S. Okada. 1999. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 274:2593-2596. [DOI] [PubMed] [Google Scholar]
- 34.Prescott, S. M. 1999. A thematic series on kinases and phosphatases that regulate lipid signaling. J. Biol. Chem. 274:8345-8346. [DOI] [PubMed] [Google Scholar]
- 35.Sander, E. E., S. van Delft, J. P. ten Klooster, T. Reid, R. A. van der Kammen, F. Michiels, and J. G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheid, M. P., M. Huber, J. E. Damen, M. Hughes, V. Kang, P. Neilsen, G. D. Prestwich, G. Krystal, and V. Duronio. 2002. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J. Biol. Chem. 277:9027-9035. [DOI] [PubMed] [Google Scholar]
- 37.Serunian, A. L., K. R. Auger, and L. C. Cantley. 1991. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 198:78-87. [DOI] [PubMed] [Google Scholar]
- 38.Sharma, P. M., K. Egawa, Y. Huang, J. L. Martin, I. Huver, G. R. Boss, and J. M. Olefsky. 1998. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. 273:18528-18537. [DOI] [PubMed] [Google Scholar]
- 39.Stephens, L., K. Anderson, D. Stokoe, H. Erdjument-Bromage, G. F. Painter, A. B. Holmes, P. R. J. Gaffney, C. B. Reese, F. McCormick, P. Tempst, J. Coadwell, and P. T. Hawkins. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate dependent activation of protein kinase B. Science 279:710-714. [DOI] [PubMed] [Google Scholar]
- 40.Stokoe, D., L. R. Stephens, T. Copeland, P. R. J. Gaffney, C. B. Reese, G. F. Painter, A. B. Holmes, F. McCormock, and P. T. Hawkins. 1997. Dual role of phosphatidylinositol 3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570. [DOI] [PubMed] [Google Scholar]
- 41.Sun, X. J., L.-M. Wang, Y. Zhang, L. Yenush, M. G. Myers, Jr., E. Glasheen, W. S. Lane, J. H. Pierce, and M. F. White. 1995. Role of IRS-2 in insulin and cytokine signaling. Nature 377:173-177. [DOI] [PubMed] [Google Scholar]
- 42.Thakker, J. K., R. DiMaychi, K. MacDonald, and J. F. Caro. 1989. Effect of insulin and insulin-like growth factors I and II on phosphatidylinositol and phosphatidylinositol 4,5-bisphosphate breakdown in liver from humans with and without type II diabetes. J. Biol. Chem. 264:7169-7175. [PubMed] [Google Scholar]
- 43.Toker, A., and L. C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387:673-676. [DOI] [PubMed] [Google Scholar]
- 44.Tong, P., Z. A. Khayat, C. Huang, N. Patel, A. Ueyama, and A. Klip. 2001. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Investig. 108:371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vollenweider, P., M. Clodi, S. S. Martin, T. Imamura, W. M. Kavanaugh, and J. M. Olefsky. 1999. An SH2 domain-containing 5′ inositol phosphatase inhibits insulin-induced GLUT4 translocation and growth factor-induced actin filament rearrangement Mol. Cell. Biol. 19:1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wada, T., T. Sasaoka, M. Ishiki, H. Hori, T. Haruta, H. Ishihara, and M. Kobayashi. 1999. Role of the Src homology 2 (SH2) domain and C-terminus tyrosine phosphorylation sites of SH2-containing inositol phosphatase (SHIP) in the regulation of insulin-induced mitogenesis. Endocrinology 140:4585-4594. [DOI] [PubMed] [Google Scholar]
- 47.Wada, T., T. Sasaoka, M. Funaki, H. Hori, S. Murakami, M. Ishiki, T. Haruta, T. Asano, W. Ogawa, H. Ishihara, and M. Kobayashi. 2001. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5′-phosphatase catalytic activity. Mol. Cell. Biol. 21:1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, L., H. Hayashi, K. Kishi, L. Huang, A. Hagi, K. Tamaoka, P. T. Hawkins, and Y. Ebina. 2000. Gi-mediated translocation of GLUT4 is independent of p85/p110α and p110γ phosphoinositide 3-kinase but might involve the activation of Akt kinase. Biochem. J. 345:543-555. [PMC free article] [PubMed] [Google Scholar]
- 49.Waters, S. B., D. Chen, A. W. Kao, S. Okada, K. H. Holt, and J. E. Pessin. 1996. Insulin and epidermal growth factor receptors regulate distinct pools of Grb2-SOS in the control of Ras activation. J. Biol. Chem. 271:18224-18230. [DOI] [PubMed] [Google Scholar]
- 50.Weng, L. P., W. M. Smith, J. L. Brown, and C. Eng. 2001. PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum. Mol. Genet. 10:605-616. [DOI] [PubMed] [Google Scholar]
- 51.Weng, Q. P., M. Kozlowski, C. Belham, A. Zhang, M. J. Comb, and J. Avruch. 1998. Regulation of the p70 S6 kinase by phosphorylation in vivo. J. Biol. Chem. 273:16621-16629. [DOI] [PubMed] [Google Scholar]
- 52.White, M. F., and C. R. Kahn. 1994. The insulin signaling system. J. Biol. Chem. 269:1-4. [PubMed] [Google Scholar]