Abstract
The membrane-bound form of Fas ligand (FasL) signals apoptosis in target cells through engagement of the death receptor Fas, whereas the proteolytically processed, soluble form of FasL does not induce cell death. However, soluble FasL can be rendered active upon cross-linking. Since the minimal extent of oligomerization of FasL that exerts cytotoxicity is unknown, we engineered hexameric proteins containing two trimers of FasL within the same molecule. This was achieved by fusing FasL to the Fc portion of immunoglobulin G1 or to the collagen domain of ACRP30/adiponectin. Trimeric FasL and hexameric FasL both bound to Fas, but only the hexameric forms were highly cytotoxic and competent to signal apoptosis via formation of a death-inducing signaling complex. Three sequential early events in Fas-mediated apoptosis could be dissected, namely, receptor binding, receptor activation, and recruitment of intracellular signaling molecules, each of which occurred independently of the subsequent one. These results demonstrate that the limited oligomerization of FasL, and most likely of some other tumor necrosis factor family ligands such as CD40L, is required for triggering of the signaling pathways.
Cytokines of the tumor necrosis factor (TNF) family are mainly implicated in the development, function, and homeostasis of the immune system but also play a role in other tissues such as bone, mammary gland, and epidermal appendages (21). Presently, the TNF family comprises 18 genes, some of which are expressed in several splice variants. These ligands contain a C-terminal TNF homology domain that associates as homotrimers and less frequently heterotrimers and mediates interaction with receptors of the TNF family (5). The TNF homology domain shows structural homology to the C-terminal globular and trimeric domain of ACRP30, a member of the complement C1q family (5, 33). ACRP30 (also called adiponectin or AdipoQ) is a serum protein secreted by adipocytes that stimulates fatty acid combustion and synergizes with insulin to regulate glycemia (4, 50).
Receptors of the TNF family are activated by ligand-mediated oligomerization and can principally engage one of two key signaling pathways (46). Receptors that signal survival, proliferation and/or differentiation recruit TNF receptor-associated factor (TRAF) family members and typically activate transcription factors such as NF-κB and AP1. Alternatively, receptors that signal cell death recruit and activate proapoptotic caspases. Some receptors have the dual capacity of activating either survival or death pathways, depending on the status of the cell (2).
The proapoptotic TNF family member Fas ligand (FasL) signals cell death by engagement of its cognate receptor, Fas. The intracellular portion of Fas contains a domain of about 90 amino acid residues, the death domain (DD), which interacts with the DD of a bipartite adaptor molecule called FADD (6, 9). FADD in turn recruits procaspase 8 and procaspase 10 via death effector domain (DED)-mediated interactions (17, 25, 47). Procaspases 8 and 10 are activated in the death-inducing signaling complex (DISC), leading to the release of their activated forms that initiate the apoptotic cascade (45).
FasL plays an important role in the effector function of cytotoxic T lymphocytes and also regulates their homeostasis (19). Genetic mutations that inactivate either FasL or Fas are associated with autoimmune lymphoproliferative syndrome, a hereditary condition characterized by the accumulation of atypical lymphocytes and by the development of autoimmune manifestations (19, 37). Fas interacts with itself via a N-terminal portion called the preligand association domain (PLAD). Its preassociation, which is required for efficient signaling, is disrupted by a dominant-negative form of Fas described in a patient with autoimmune lymphoproliferative syndrome (34).
Membrane-bound FasL is processed to a soluble form and shed by the action of a metalloprotease. The processed, soluble form of FasL has not only lost its activity but can even inhibit the action of membrane-bound FasL (31, 38, 40). Interestingly, cross-linking of soluble FasL restores its proapoptotic activity (31).
In this study, we report that a hexamer of FasL, consisting of two trimers held in close proximity, represents the minimal ligand structure required to signal apoptosis. We also dissect three early steps in the formation of the signaling complex, namely, ligand binding, receptor activation, and recruitment of signaling molecules, each of which occurs independently of the next one. The implications of these results regarding our understanding of the molecular mechanism of Fas signaling are detailed in the discussion.
MATERIALS AND METHODS
Reagents.
Antibodies and reagents were purchased from the following sources: anti-Flag M2 antibody and M2-agarose (Sigma), anti-FADD (Transduction Laboratories, Lexington, Ky.), anti-caspase 8 and anti-Fas ZB4 (MBL, Naka-ku Nagoya, Japan), anti-Fas C-20 (Santa Cruz Biotechnology, Santa Cruz, Calif.), ACTIVE JNK antibody (Promega Corp., Madison, Wis.), Z-VAD-fmk, Fas:Fc, and TRAILR2:Fc (Apotech Corp., Epalinges, Switzerland), annexin V (Nexin Research, Kattendijke, The Netherlands), phytohemagglutinin (Murex Diagnostics, Chatillon, France), Complete protease inhibitors (Roche), and protein A-Sepharose, protein G-Sepharose, and Na[125I] (all from Amersham Pharmacia).
Cells.
All cell culture reagents were purchased from Invitrogen. Dulbecco's modified Eagle's medium (DMEM)-Nutrient Mix F12 (1:1) was supplemented with 2% heat-inactivated fetal calf serum, and DMEM and RPMI media were supplemented with 10% fetal calf serum. All media were supplemented with penicillin and streptomycin at 10 μg/ml each. HEK-293 human embryonic kidney cells were cultured in DMEM:Nutrient Mix F12 (1:1). 293T, 293T-6, and HeLa cells were cultured in DMEM. BJAB, Raji, A431, Kym-1, Jurkat (clone JA3), Jurkat FADD-deficient (clone I.21), and Jurkat caspase 8-deficient (clone I.92) were cultured in RPMI medium. Jurkat and Jurkat-deficient clones were a kind gift of J. Blenis (Harvard Medical School, Boston, Mass.). 293T-6 cells originate from a clone of 293T cells selected for its sensitivity to FasL and were kindly provided by T. Takaoka (Tokyo Institute of Technology; Yokohama, Japan).
T cells were purified from human peripheral blood leukocytes by negative selection on anti-CD11b and anti-CD20 using the miniMACS magnetic cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified T cells were cultured for 24 h in RPMI medium supplemented with 5% human serum and were then activated for 2 days with phytohemagglutinin (0.5 μg/ml) and interleukin 2 (20 U/ml).
Recombinant FasL, CD40L, and Tweak.
Cloning, expression, and purification of recombinant proteins were performed essentially as described previously (30). Expression constructs for ACRP:FasL, ACRP:CD40L, and ACRP:Tweak were generated according to standard molecular biology protocols and cloned in the PCR-3 vector (Invitrogen, Leek, The Netherlands). The constructs encode the signal peptide of hemagglutinin (MAIIYLILLFTAVRG), the Flag sequence (DYKDDDDK), a linker (GPGQVQLQ), the collagen domain of muACRP30 (amino acids [aa] 18 to 111) and the C-terminal portion of the ligand of interest, namely, hFasL (aa 139 to 281), muCD40L (aa 38 to 262), or hTweak (aa 106 to 249). For Flag-tagged ligands, the sequence of ACRP30 was omitted. In ACRPΔ:FasL, the sequence of muACRP30 was truncated (aa 41 to 111), and in ACRP C39S:FasL, the sequence of muACRP30 was mutated (aa 18 to 111, Cys39Ser).
The Fc:FasL expression construct was cloned in the PCR-3 vector and encodes the hemagglutinin signal peptide, the Fc portion of human immunoglobulin G (IgG) (aa 108 to 338 of accession number AAC82527, excluding the stop codon), a linker sequence (RSPQPQPKPQPKPEPEGSLQ), and hFasL (aa 139 to 281).
Proteins were expressed transiently in 293T cells in serum-free Optimem-1 medium, and supernatants were concentrated 20× before use. Recombinant proteins were also expressed from stable HEK-293 clones and were purified by affinity on M2-agarose and protein A-Sepharose for Flag-tagged and Fc-tagged proteins, respectively (30).
Gel permeation chromatography.
Purified FasL, ACRP:FasL, and Fc:FasL (200 μg in 200 μl) were loaded onto a Superdex-200 size exclusion column (Amersham Pharmacia) and were eluted in PBS at 0.5 ml/min. Protein content was monitored at 280 nm with an online UV detector and quantified in fractions with the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Peak fractions were used directly for cytotoxic assays and, after concentration on Centricon 30 (Millipore), for electron microscopy.
Electron microscopy.
Rotary shadowing was performed as previously described (11). Briefly, proteins were mixed with one-third the volume of glycerin and were sprayed on freshly prepared mica. The probe was dried at 10−5 atm for 4 h, and rotary shadowing was performed by vaporizing platinum at an angle of 9°. After stabilization by a coat of vaporized coal (90°), the replica was detached from the mica support at a water-air interface, fished on a grid, dried, and analyzed by electron microscopy.
Cytotoxicity tests.
Tests were performed as described (30). Recombinant forms of FasL or Tweak were sequentially diluted in flat-bottom 96-well plates containing 50 μl per well of the appropriate medium in the presence or absence of 2 μg of cross-linking anti-Flag M2 antibody/ml. Cells (2 × 104 to 5 × 104 per well in 50 μl of the appropriate medium, without M2) were added and cultured for 16 h. For protection experiments, HeLa cells were incubated with ACRP:FasL at a final concentration of 200 ng/ml in the presence of various amounts of FasL. Cell viability was quantified by the PMS/MTS test (Promega), and absorbance at 490 nm was monitored after suitable color development (usually 1 to 4 h).
Purified, activated T cells were treated for 4 h with the indicated concentrations of FasL, stained with annexin V-fluorescein isothiocyanate, and analyzed by flow cytometry. The number of live, annexin V-negative cells was recorded.
JNK assay.
293T-6 cells were transfected with a Flag-c-Jun N-terminal kinase (JNK) expression plasmid. Twenty-four hours after transfection, cells were stimulated for 0, 15, 30, 60, 120, or 240 min with 50 ng of FasL or ACRP:FasL per ml in the presence or absence of 1 μg of anti-Flag M2 antibody/ml. Cells were harvested, lysed, and analyzed by Western blotting by using anti-phospho-JNK and anti-Flag M2 antibodies.
B-cell proliferation assay.
Splenic B cells from C57BL/6 mice were purified on anti-B220-coated beads by using the miniMACS magnetic cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany). B cells were seeded in triplicates at 70,000 cells per well in flat-bottomed 96-well plates in a final volume of 200 μl of RPMI medium containing 5% fetal calf serum, 0.1 mM 2-mercaptoethanol, and the indicated concentration of muCD40L, ACRP:muCD40L, heat-inactivated (5 min at 95°C) ACRP:muCD40L, or lipopolysaccharide (LPS). When present, anti-Flag M2 was added at a final concentration of 1 μg/ml. After 36 h of incubation, cells were labeled for 10 h with [3H]thymidine (0.5 μCi/well) and harvested. Incorporated radioactivity was monitored with a TopCount scintillation counter (Packard).
Fas-FasL interaction ELISA.
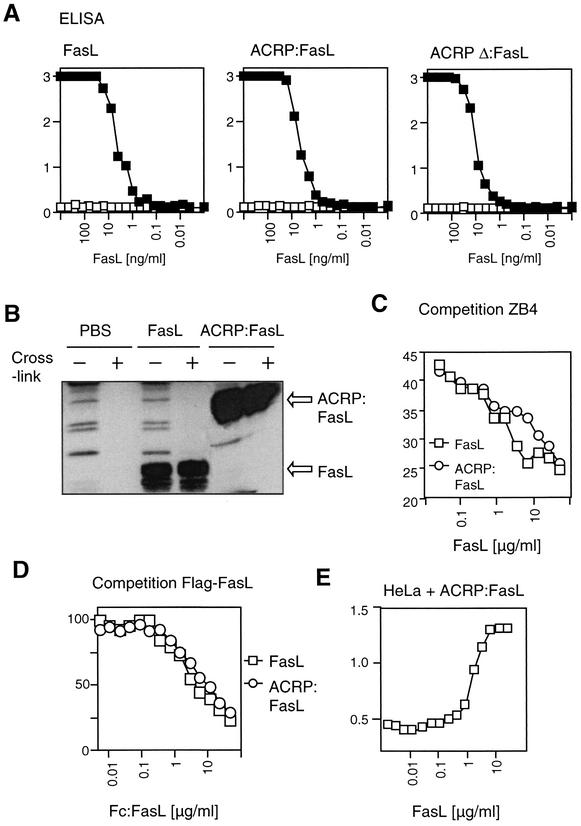
Enzyme-linked immunosorbent assay (ELISA) was performed essentially as described before (30). ELISA plates were coated with Fas:Fc or TRAILR2:Fc at 1 μg/ml in PBS. After, blocking, dilutions of FasL, ACRP:FasL, and ACRPΔ:FasL were added and bound FasL was revealed with anti-Flag M2 antibody and horseradish peroxidase-conjugated secondary antibody. For competition experiments, FasL and ACRP:FasL were used at a concentration of 200 ng/ml. After 30 min of incubation, dilutions of Fc:FasL were added and incubated for a further 60 min. Bound FasL was revealed with biotinylated M2 antibody and horseradish peroxidase-coupled streptavidin.
Fas binding competition experiment.
The anti-Fas ZB4 monoclonal antibody was labeled with Na[125I] to a specific activity of 1.5 μCi/μg by using the IodoGen method (Pierce), and unincorporated Na[125I] was removed by chromatography on a Sephadex G-25 column (Amersham Pharmacia). A fixed amount of labeled ZB4 antibody (50,000 cpm) was incubated for 1 h at 37°C with 2 × 105 BJAB cells in a final volume of 1 ml and in the presence of various concentrations of FasL or ACRP:FasL. The minimal number of BJAB cells allowing maximum binding of ZB4 was 2 × 105. After three washings with ice-cold PBS containing 1 mg of bovine serum albumin/ml, the radioactivity associated with the cells was counted in a gamma counter and was expressed as percentage of bound counts per minute.
DISC analysis.
Jurkat cells (wild type, FADD deficient, and caspase 8 deficient) or Raji cells (n = 108) were harvested, resuspended in 500 μl of RPMI medium, and treated or not treated for 15 min at 37°C with FasL or ACRP:FasL (each at 1 μg/ml) in the presence or absence of cross-linking anti-Flag M2 antibody (1 μg/ml). Subsequent steps were performed on ice. Reactions were stopped by addition of 10 ml of ice-cold PBS, and cells were harvested, washed with PBS, and lysed for 10 min in 1 ml of lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 0.2% NP-40, and Complete protease inhibitors). The insoluble material was removed by centrifugation (twice for 10 min at 10,000 × g), and lysates were precleared for 2 h on Sepharose 6B. An aliquot of soluble cell extracts was collected at that time. Samples that had not been treated with cross-linking antibody (or all samples in certain experiments) were supplemented with 0.5 μg of anti-Flag M2 antibody. In some experiments, extracts of untreated cells were also supplemented with 0.5 μg of FasL at this stage. Anti-Flag antibody and associated proteins (FasL, Fas, and signaling proteins) were immunoprecipitated with protein G-Sepharose for 1 to 4 h at 4°C. Beads were washed four times with lysis buffer and analyzed by Western blotting.
RESULTS
Generation of hexameric FasL fusion proteins.
In order to investigate the minimal stoichiometry of FasL that is required to induce cell death, we generated chimeric constructs between the trimeric TNF homology domain of FasL and selected oligomerization domains (Fig. 1A).
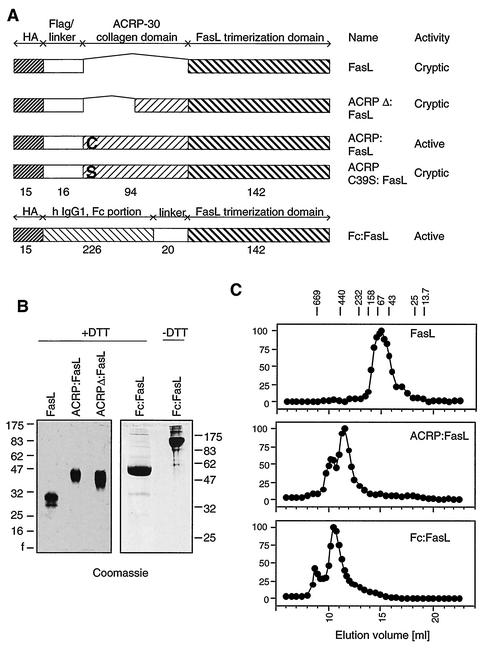
FIG. 1.
Determination of the molecular masses of various FasL fusion proteins. (A) Schematic representation of the different FasL constructs used in this study. The number of amino acid residues in each domain is indicated. “Cryptic” means that the cytotoxic activity remains cryptic until the ligand is cross-linked. (B) SDS-PAGE analysis of purified recombinant FasL. Proteins (5 μg per lane) were analyzed under reducing conditions. Fc:FasL was also analyzed under nonreducing conditions. Gels were stained with Coomassie blue. Molecular mass markers are in kilodaltons. (C) Gel permeation chromatography of purified recombinant FasL. Ligands (200 μg) were applied to a Superdex-200 column and eluted in PBS. Cytotoxic activity was measured on Jurkat cells in the presence of anti-Flag antibodies and is expressed as the percentage of maximal response. The elution positions of molecular mass markers are indicated at the top of the figure (molecular masses in kilodaltons).
In a first construct, called Fc:FasL, FasL was fused at the C terminus of the dimerization domain of IgG1 (Fc domain). The fusion of a dimeric structure to a trimeric one is expected to give rise to a hexameric structure and possibly higher multimers.
In a second construct, coined ACRP:FasL, FasL was fused to the C terminus of the collagen domain of the serum protein ACRP30. ACRP30 belongs to the C1q family of proteins, whose C-terminal domain is structurally related to that of FasL (29, 33). Its collagen domain is predicted to form a “bouquet-like” structure similar to that found in C1q. In ACRP:FasL, the number of trimeric FasLs in the fusion protein should be equal to the number of collagen triple helices forming the bouquet structure.
In a control construct, called FasL, the TNF homology domain of FasL is fused to a Flag tag that functions as an inducible aggregation domain upon cross-linking with an anti-Flag monoclonal antibody (31). A Flag tag is also present in the ACRP:FasL construct.
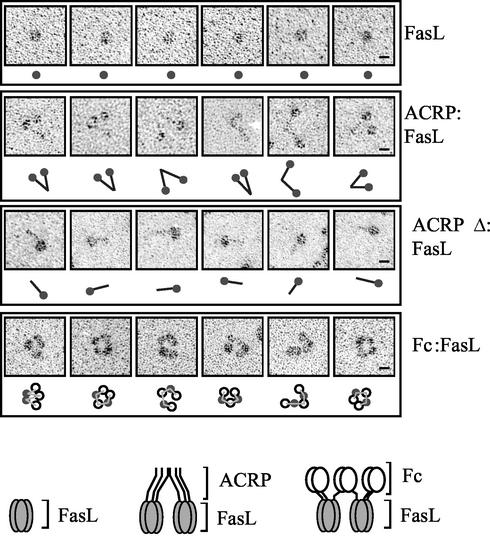
The molecular masses of affinity-purified recombinant proteins were determined under denaturing (Fig. 1B) and native (Fig. 1C) conditions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and gel permeation chromatography, respectively. Because the latter method is useful for globular proteins but overestimates masses of asymmetric proteins, the main protein species of each elution profile was also examined by electron microscopy (Fig. 2). The calculated stoichiometry of FasL (2.9-mer) and its globular appearance by electron microscopy are entirely compatible with its expected trimeric structure. Elution profiles of ACRP:FasL and Fc:FasL both contained a major peak and a minor, larger species that still eluted in the included volume of the column. The calculated stoichiometries of the main species of ACRP:FasL and Fc:FasL are 8.5 and 7.7, respectively, but these numbers are likely overestimates in view of the asymmetric shape of these proteins. The collagen domain of ACRP:FasL has a rodlike structure that is clearly apparent on electron micrographs (Fig. 2). Surprisingly, the collagen triple helices of ACRP30 do not form a hexameric bouquet-like structure as found in C1q (22) but rather associate as dimers via their N-terminal portion. Interestingly, the dimerization point appears to be quite flexible. Taken together, these data indicate that ACRP:FasL is a hexameric structure or more precisely a dimer of trimeric FasL. Electron micrographs of Fc:FasL most often show ring- or wormlike structures, in which five globular domains can be distinguished (Fig. 2). These domains most probably correspond to three dimeric Fc domains and two trimeric FasLs. We conclude that we have generated two independent and well-defined FasL oligomers, ACRP:FasL and Fc:FasL, in which two trimeric FasLs are kept in close proximity.
FIG. 2.
Rotary shadowing electron microscopy of recombinant FasL. Six representative images of FasL, ACRP:FasL, ACRPΔ:FasL, and Fc:FasL preparations are shown, with a schematized interpretation of the picture. Filled circles, trimeric FasL; black rods, collagen domain of ACRP or ACRPΔ; open circles, dimeric Fc portion of IgG1; thin lines, possible connectivity between FasL and Fc domains. For Fc:FasL, alternative interpretations are possible regarding the identification of FasL and Fc domains and regarding the connectivity between domains. Pictures were taken at a magnification of ×150,000, and a scale of 10 nm is indicated (bars). Schematic representations of the hexameric forms of ACRP:FasL and Fc:FasL are shown at the bottom of the figure.
Hexameric FasL is a potent inducer of apoptosis.
The cytotoxic activity of the various recombinant FasLs was quantified on several FasL-sensitive cell lines. Fc:FasL and ACRP:FasL turned out to be potent inducers of cell death with a 50% inhibitory concentration of 0.5 to 5 ng/ml as measured on the Jurkat lymphoblastoma T- cell line (Fig. 3A and B). Because of the heterogeneous size of Fc:FasL, we tested the activity of the low- and high-molecular-weight fractions and found that they had roughly identical specific activities (Fig. 3A). The same was true for high- and low- molecular-weight fractions of ACRP:FasL (data not shown). We conclude that higher multimers of Fc:FasL and ACRP:FasL (possibly 12-mer) are not more active than hexameric forms.
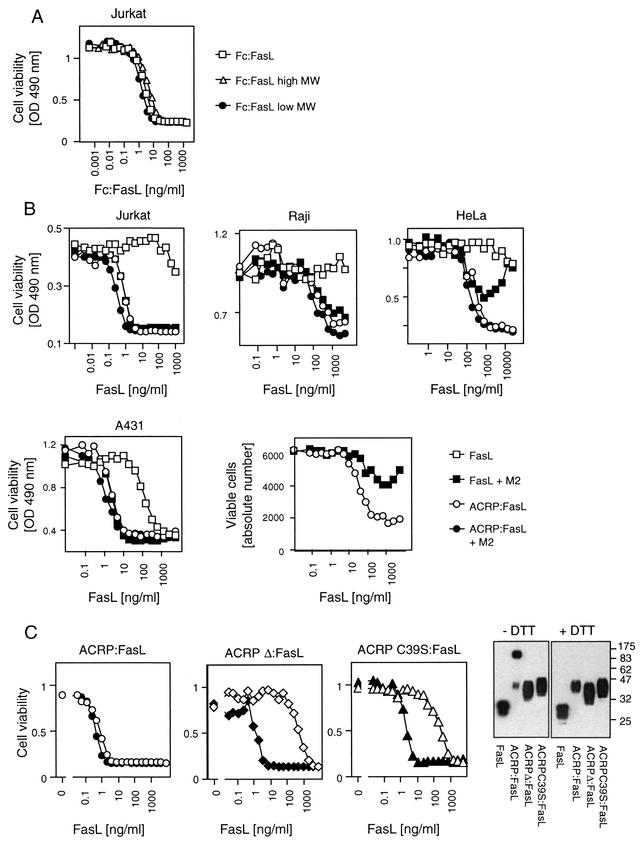
FIG. 3.
Cytotoxicity of various recombinant FasLs. OD, optical density. (A) The T-lymphoblastoma Jurkat cell line was exposed to the indicated amount of purified Fc:FasL (total preparation) or to the high- and low-molecular-weight (high MW and low MW) fractions of Fc:FasL (fractions 7 and 15 of Fig. 1C, respectively). The reducing capacity of mitochondria was monitored with PMS/MTS reagents as a measure of cell viability. (B) Raji Burkitt lymphoma, HeLa epitheloid cervix carcinoma, A431 epidemoid carcinoma, and Jurkat cell lines and primary human T cells from peripheral blood activated for 2 days were exposed to recombinant FasL (squares) or ACRP:FasL (circles) at the indicated concentration, in the presence (filled symbols) or absence (open symbols) of cross-linking anti-Flag M2 monoclonal antibody. For cell lines, viability was measured with PMS/MTS reagents. Primary T cells were stained with annexin V, and viable cells were quantified by fluorescence-activated cell sorter analysis. (C) Left three panels: the cytotoxic activity of the indicated FasL constructs was monitored on Jurkat cells in the presence (filled symbols) or absence (open symbols) of anti-Flag M2 antibody. Cell viability was quantified with PMS/MTS reagents. Right panel: anti-Flag Western blot of various FasL preparations separated by SDS-PAGE under reducing (+DTT) or nonreducing (−DTT) conditions.
In contrast to Fc:FasL and ACRP:FasL, FasL is generally not or only poorly cytotoxic. However, cross-linking via the Flag tag enhances its activity by at least 2 or 3 orders of magnitude, allowing it to reach the same specific activity as ACRP:FasL (Fig. 3B) (31). Cross-linking of ACRP:FasL does not significantly enhance its cytotoxicity, indicating that hexameric FasL is a unit fully capable of activating the Fas pathway. It is noteworthy that the intrinsic cross-linked nature of ACRP:FasL circumvents problems linked to an inadequate ratio of ligand to cross-linking antibody. Indeed, cytotoxicity is diminished at a high FasL-to-antibody ratio, probably because cross-linked trimers are either not formed (antibodies may preferentially bind two epitopes within a single trimer) or because their action is antagonized by the excess of uncross-linked FasL (Fig. 3B, HeLa; also see Fig. 4B) (data not shown).
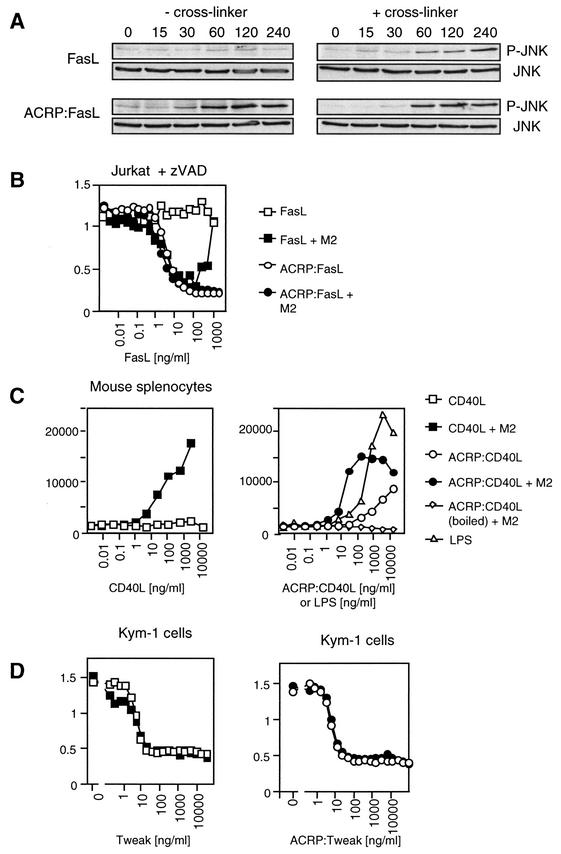
FIG. 4.
Biological activity of ACRP:FasL, ACRP:CD40L, and ACRP:Tweak. (A) FasL-sensitive 293T-6 cells transfected with a Flag-JNK expression plasmid were treated for the indicated period of time with FasL or ACRP:FasL in the presence or absence of cross-linking antibody. Cell lysates were analyzed by Western blotting using an anti-phospho-JNK antibody. Equal expression levels of JNK were confirmed by reprobing the membrane with anti-Flag antibody. (B) Jurkat cells were treated with FasL and ACRP:FasL as described in the Fig. 3B legend, except that the caspase inhibitor Z-VAD-fmk was added to reveal caspase-independent cell death. (C) Purified murine splenic B cells were incubated for 48 h with the indicated concentration of murine CD40L (squares) or ACRP:CD40L (circles) in the presence (filled symbols) or absence (open symbols) of cross-linking anti-Flag M2 antibody. Proliferation was measured by [3H]thymidine incorporation. Controls include LPS (triangles) and heat-inactivated ACRP:CD40L (diamonds). Figure shows the mean plus or minus standard deviation of triplicates. (D) The Kym-1 rhabdomyosarcoma cell line was treated with Tweak and ACRP:Tweak, as indicated. Cell viability was monitored using PMS/MTS reagents.
SDS-PAGE analysis of ACRP:FasL under reducing and nonreducing conditions indicates the presence of an interchain disulfide bridge involving the unique cysteine residue located in the N-terminal portion of the collagen domain of ACRP30 (Fig. 3C). Mutation of this cysteine residue (ACRP C39S:FasL) or deletion of the N-terminal portion of the collagen domain containing this cysteine residue (ACRPΔ:FasL) reduces the activity of ACRP:FasL by 2 or 3 orders of magnitude (Fig. 3C). In addition, hexameric structures were never observed in the preparation of ACRPΔ:FasL (Fig. 2). This further demonstrates that the hexameric structure of FasL, and not its fusion to a collagen domain, is necessary for its biological activity.
Hexameric ligands of the TNF family are efficient inducers of cellular responses.
In addition to apoptosis, Fas can signal activation of the transcription factor c-Jun, raising the question of whether hexameric FasL is also required for the activation of this nonapoptotic signal. In accord with previous results (44), activation of JNK was absent with trimeric FasL but was clearly induced by hexameric or antibody cross-linked trimeric FasL (Fig. 4A). A caspase-independent, Fas-dependent cell death pathway is active in T cells and in Jurkat cells, which can be evidenced in the presence of the pan-caspase inhibitor Z-VAD-fmk (14). Again, trimeric FasL was unable to induce caspase-independent cell death, whereas ACRP:FasL efficiently did so (Fig. 4B). These results indicate that at least three different signals mediated by Fas require triggering by hexameric FasL.
CD40L is another member of the TNF family, which is well known to be active in a membrane-bound form. Proliferation of splenic murine B cells was monitored in response to murine CD40L or ACRP:CD40L. CD40L was virtually inactive, whereas cross-linked CD40L induced a robust B-cell proliferation, comparable to that obtained with bacterial LPS (Fig. 4C). As expected, ACRP:CD40L alone induced B-cell proliferation. However, in contrast to what we observed with FasL, cross-linking of ACRP:CD40L led to a further significant increase of activity (Fig. 4C). Taken together, these results strongly suggest that the requirement of two or more ligands is not unique to FasL but is possibly applicable to all TNF family ligands whose activity is dependent on membrane association, such as FasL, CD40L, CD30L, and others. In contrast, ligands that are known to display full activity in their soluble form, such as Tweak, TNF, or BAFF, showed no further enhancement of their activity when fused to ACRP30, as exemplified by the death-inducing activity of Tweak on Kym-1 cells (Fig. 4D).
Trimeric FasL and hexameric FasL bind equally well to Fas.
We next addressed the question of whether the poor biological activity of trimeric FasL was simply due to a decreased ability to bind to Fas. However, FasL, ACRP:FasL, and the deletion mutant of ACRP:FasL (ACRPΔ:FasL) bound equally well to a recombinant form of human Fas (Fas:Fc) (Fig. 5A). Also, when FasL and ACRP:FasL were incubated with Fas-positive Raji cells, comparable amounts of ligand associated with the cells, regardless of whether cross-linking antibody was added or not, demonstrating that trimeric and hexameric FasL can bind endogenous Fas and not only recombinant forms of soluble Fas (Fig. 5B). Binding of ligands to cells was not only specific but also occurred with similar affinities as demonstrated by the ability of both FasL and ACRP:FasL to compete for the binding of a monoclonal anti-Fas antibody to Fas-positive cells (Fig. 5C). In a similar way, Fc:FasL displaced Flag-tagged FasL and Flag-tagged ACRP:FasL from recombinant Fas:Fc with comparable efficacy (Fig. 5D). To rule out the formal possibility that FasL and ACRP:FasL engage different subsets of receptors varying on their signaling capacity, HeLa cells were exposed to a lethal dose of ACRP:FasL in the presence of increasing amounts of FasL. FasL abolished the cytotoxic activity of ACRP:FasL in a dose-dependent manner, clearly indicating that FasL and ACRP:FasL engage the same set of receptors, despite their differences in biological activity (Fig. 5E). Taken together, the disparity of activity between trimeric and hexameric FasL cannot be explained by a difference in receptor binding, because both ligands bind to Fas equally well.
FIG. 5.
FasL and ACRP:FasL bind Fas equally well. (A) The interaction between Flag-tagged FasL, ACRP:FasL, and ACRPΔ:FasL on the one hand and coated Fas:Fc (filled symbols) or TRAILR2:Fc (open symbols, negative control) on the other hand was monitored by ELISA. (B) Burkitt lymphoma Raji cells were incubated for 15 min with FasL, ACRP:FasL, or PBS alone. Washed cells were lysed and FasL bound to the cells was detected by anti-Flag Western blotting. Cross-linking antibody was added during the incubation (+) or after cell lysis (−). (C) The binding of [125I]-labeled ZB4 anti-Fas antibody to BJAB cells was competed with the indicated concentrations of recombinant FasL (squares) or ACRP:FasL (circles). (D) The binding of Flag-tagged FasL or ACRP:FasL (at 200 ng/ml) to recombinant Fas:Fc was competed with the indicated amounts of untagged Fc:FasL. Bound ligands were detected via the Flag tag. (E) HeLa cells were incubated for 16 h with a constant, lethal concentration of ACRP:FasL in the presence of various amounts of FasL. Cell viability was monitored with the PMS/MTS reagent.
Trimeric FasL fails to induce a DISC.
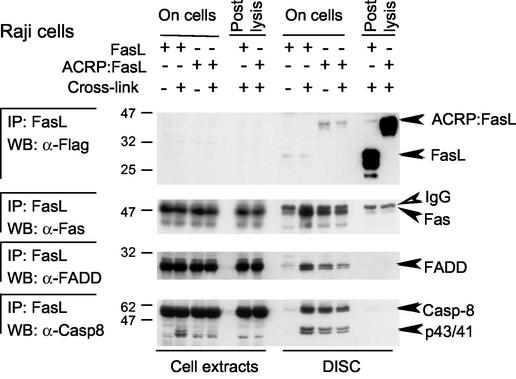
Upon triggering by FasL or agonistic antibodies, Fas recruits FADD and procaspase 8, a cysteine-protease zymogen that is processed to its mature, proapoptotic form at the level of the DISC. Fas, FADD, and procaspase 8 were all present in the DISC triggered by ACRP:FasL or by cross-linked FasL, but no DISC was formed with FasL alone in Raji (Fig. 6) and Jurkat (Fig. 7A) cells and in other cell types (data not shown). As FasL and ACRP:FasL bind Fas in a similar way, the inability of FasL to immunoprecipitate Fas was somewhat surprising. However, Fas was also very poorly immunoprecipitated by an excess of FasL or ACRP:FasL added after cell lysis (Fig. 6). This suggests that all forms of FasL are unable to immunoprecipitate Fas efficiently under our experimental conditions, unless Fas is incorporated into the DISC. This can be explained if Fas oligomerizes in the DISC, thereby increasing its avidity for FasL. In this respect, it is noteworthy that Fas present in the DISC migrated not only at its expected size of about 45 kDa but also as a 150-kDa species that is not present in total cellular extracts (16) (Fig. 7), supporting the hypothesis that a proportion of Fas gets modified upon engagement by active FasL. The presence of a DISC correlated precisely with the cytotoxic activity of the different FasL preparations. In particular, the poorly cytotoxic trimeric FasL recruited little Fas and no detectable FADD and caspase 8. The inability of trimeric FasL to induce a DISC is sufficient to explain its lack of biological activity.
FIG. 6.
DISC analysis in Raji cells. Raji cells were treated for 15 min with 0.5 μg of FasL or ACRP:FasL or with PBS alone in the presence (+) or absence (−) of cross-linking anti-Flag M2 antibody and were then washed in PBS. After cell lysis and sampling of soluble cell extracts, lysates of untreated cells were supplemented with 0.5 μg of FasL or ACRP:FasL (postlysis). Anti-Flag antibody was added to all samples to allow immunoprecipitation (IP) of Flag-tagged FasL. Immunoprecipitates and cell extracts were analyzed by Western blotting (WB) for Fas, FADD, caspase 8, and Flag-tagged FasL. IgG, heavy chain of the immunoprecipitating antibody. Molecular masses are indicated in kilodaltons.
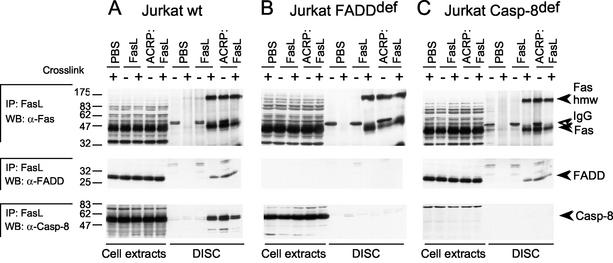
FIG. 7.
DISC analysis in wild-type (wt) and mutant Jurkat cells. Jurkat cells (wild type, FADD deficient [def], and caspase 8 deficient) were treated for 15 min with FasL, ACRP:FasL, or PBS alone, in the presence (+) or absence (−) of cross-linking anti-Flag M2 antibody, and were then washed in PBS. After lysis, samples treated without cross-linker were supplemented with anti-Flag antibody and ligands were immunoprecipitated and analyzed by Western blotting for Fas, FADD, and caspase 8. For each condition (except PBS alone), 1% of the postnuclear extract was loaded for comparison. The migration positions of procaspase 8 (Casp-8), FADD, and Fas and the high-molecular-mass modification of Fas (Fas hmw) are indicated by black arrowheads. The open arrowhead points to the heavy chain of the immunoprecipitating antibody (IgG).
Recruitment of Fas by hexameric FasL does not require FADD and caspase 8.
The fact that trimeric FasL is unable to immunoprecipitate Fas efficiently, although it can bind to it, suggests that the interaction of FasL with Fas is stabilized in the DISC. We wondered whether one or more of the following events would be required for stable Fas-FasL interaction in the DISC: (i) the high-molecular-mass modification of Fas, (ii) the recruitment of FADD, and (iii) the recruitment of procaspase 8. We took advantage of FADD-deficient and caspase 8-deficient Jurkat cell lines to address this issue. In FADD-deficient and caspase 8-deficient cells treated with hexameric FasL or cross-linked FasL, Fas was recruited and modified as efficiently as in wild-type cells, indicating that recruitment of FADD and caspase 8 is dispensable for the high-molecular- mass modification of Fas (Fig. 7). FADD was recruited to the DISC in caspase 8-deficient cells, indicating that caspase 8 is not required for FADD recruitment to the DISC (Fig. 7C). Taken together, these results indicate that the high-molecular-mass modification of Fas, the recruitment of FADD, and the recruitment of caspase 8 are sequential events during DISC formation. In addition, the high-molecular-mass modification of Fas can only be induced by hexameric FasL and not by trimeric FasL.
DISCUSSION
In cytotoxic T lymphocytes, FasL is inactivated by a proteolytic cleavage that generates a soluble form of FasL. Soluble FasL can still bind to Fas but cannot activate its downstream signaling events. In this study we provide evidence that this activity is restored if two soluble FasLs are physically linked. We therefore hypothesize that a similar mechanism may apply to membrane-bound FasL, with the membrane playing the role of a cross-linker. This effect may be especially pronounced at the contact site with the target cell, where high concentrations of membrane-bound FasL may exist. In addition, one may envision that trimers of membrane-bound FasL interact physically with each other, possibly via their intracellular domains and/or via the region that links the transmembrane domain to the TNF homology domain. In this respect, it is of interest that a recombinant version of murine FasL, comprising the full extracellular domain, displayed increased cytotoxicity compared to a shorter version lacking the stalk region (39). Even if these putative stalk-stalk interactions may not be strong enough to generate aggregates in solution, they may suffice to induce transient association of FasL trimers bound to a target cell and therefore promote apoptosis.
As previously demonstrated (38), the biologically inactive form of soluble FasL can efficiently compete with the biologically active, aggregated form of FasL, indicating that both forms bind to the same set of receptors. Trimeric FasL therefore represents a candidate inhibitor of apoptosis, which may prove useful in the treatment of diseases with deregulated expression of FasL, such as stroke, toxic epidermal necrolysis, Hashimoto thyroiditis, or hepatitis (18, 23, 36, 43). Because soluble FasL targets Fas, which is readily accessible at the cell surface, it may represent an alternative to anti-FasL agents whose efficiency may be hampered by the restricted exposure of FasL at the surface of cytotoxic T cells (7).
The stoichiometry and the respective arrangement of Fas, FADD, caspase 8, caspase 10, and FLIP within the DISC of Fas are poorly defined, and even less is known about other potential binding partners, such as FAP-1, RIP, and Ubc9, for example (14, 35, 49, 51). The fact that hexameric FasL is necessary and sufficient to activate the apoptotic pathway in Fas-sensitive cells has implications regarding the mechanism of DISC formation. First, engagement of Fas by FasL is necessary but insufficient to induce signaling. In fact, two trimers of FasL need to act in concert to trigger apoptosis. Second, hexameric but not trimeric FasL apparently triggers a FADD-independent mechanism that results in the high-molecular-weight modification of Fas. Third, FADD and procaspase 8 are sequentially recruited to and activated at the DISC, in agreement with the results of other investigators (16, 24, 25).
Recent reports have suggested that the formation of the high-molecular-weight modification of Fas may be dependent on the lateral mobility of Fas in the membrane, itself regulated by the actin skeleton (1, 42). However, these observations do not explain the difference observed between the activities of trimeric FasL and hexameric FasL on a given cell type. In another study, cross-linked FasL induced the generation of ceramide and mediated capping of the receptor (10). Interestingly, exogenous addition of ceramide to wild-type cells rendered them sensitive to the apoptotic action of trimeric FasL, whereas acid sphingomyelinase-deficient hepatocytes were resistant to Fas-induced apoptosis (10). This suggests that hexameric FasL may trigger the generation of ceramides, which in turn are necessary for the high-molecular-weight modification of Fas, although the very rapid formation of this modification seems to preclude a complex mechanism of activation (16). The nature of the modification of Fas is presently not known but may be the result of a covalent modification, because it is resistant to SDS, reducing agents, and organic solvents (Fig. 7 and data not shown). This modification is probably necessary for capping and for the recruitment of FADD and apical caspases to the DISC. FasL-sensitive cells have been classified in two categories (type I and type II cells) depending on whether they require (type II, e.g., Jurkat) or do not require (type I, e.g., Raji) the mitochondria for execution of apoptosis (28). Our results indicate that hexameric but not trimeric FasLs trigger DISC formation in both cell types.
We have shown that other signaling pathways downstream of Fas, namely, phosphorylation of JNK and activation of a caspase-independent, RIP-dependent cell death pathway, are also activated by hexameric but not by trimeric FasL. These results were anticipated, as both of these pathways have been shown to be dependent on FADD (14, 15) and because FADD recruitment is dependent on hexameric FasL (Fig. 7). In addition, our results allow us to rule out the hypothesis that one trimeric FasL binds to one preassociated trimer of Fas in order to transmit a signal (34).
Recently, a structure-based model of the Fas-FADD interaction has been proposed (48). This model predicts that DDs can interact via three different interfaces (and not just one as suggested by available structures). Three DDs of Fas may thus recruit three DDs of FADD in their center, in a geometry that is compatible with that of the ligand-receptor complex (48). Similarly, the three DEDs of FADD could form a trimeric structure ready to recruit the DED of procaspase 8. By homology with procaspase 9, procaspase 8 is likely to exist as a monomer at physiological concentrations and to be activated upon dimerization (27). Indeed, aggregation of procaspase 8 is both necessary and sufficient to induce its spontaneous activation (52). In contrast, effector caspases such as caspase 7 exist as dimers that are activated by proteolytic processing (8). A trimeric structure of FADD may not provide enough docking sites to recruit two procaspase 8 proteins, especially if both DEDs of procaspase 8 participate in the interaction or if their relative positioning is important. These requirements may only be possible on two closely packed trimers of FADD DED, as would be recruited by a hexameric FasL. In the absence of FasL, the DD of Fas may be kept in a conformation that precludes interaction with FADD, possibly because of the way Fas self-associates via its PLAD domain. Rearrangement of the DD in an active conformation could be favored by “squeezing” PLAD-associated receptors between two ligands and possibly by locking the active conformation by the high-molecular-weight modification of Fas.
Curiously, productive signaling via CD40 also appears to be refractive to trimeric CD40L and efficiently triggered by cross-linked or hexameric CD40L. Similar conclusions have been reached previously using a dodecameric CD40L construct (13). Although Fas and CD40 make use of a different set of signaling molecules that trigger different cellular responses, they nevertheless share some geometrical homology. Indeed, TRAF molecules form homotrimers that can be recruited between three intracellular tails of CD40 in a geometry that closely resembles that of the proposed Fas-FADD complex (26, 48). Aggregation of the N-terminal portion of TRAFs is sufficient to induce activation of downstream kinases leading, among other things, to the activation of the transcription factor NF-κB (3). Thus, activation of signaling pathways downstream of CD40 may require the presence of two or more trimers of TRAF in close proximity, as would be recruited by a hexameric CD40L or cross-linked CD40L.
In our ACRP:FasL and ACRP:CD40L constructs, two trimeric ligands are artificially linked via a collagen domain. It is noteworthy that a similar organization is found naturally in one ligand of the TNF family called EDA. Unlike FasL, EDA must be solubilized in order to be active, which is easily understandable in view of the geographically distinct expression patterns of EDA and of its receptor EDAR (20, 41). The collagen domain of EDA may thus serve the purpose of aggregating soluble EDA trimers. This hypothesis is supported by the observation that mutations in the collagen domain of EDA impair its biological activity (32).
Finally, our results indicate that ACRP30 is most probably a hexamer. Recent studies have indicated that both the full-length protein and the trimeric globular domain of ACRP30 are biologically active (4, 12, 50). However, expression of a full-length form of biologically active ACRP30 requires a eukaryotic expression system, probably because posttranslational modifications such as hydroxyprolination and/or disulfide bridge formation are required (4). It remains to be determined whether the specific activity of ACRP30 is higher than that of the globular domain, as is predicted from our results. In any case, the fact that both immunoglobulins and ACRP30 are serum proteins is a good indication that ACRP:ligands and Fc:ligands might prove useful reagents for systemic use.
Acknowledgments
We are grateful to Takao Takaoka for providing 293T-6 cells and to Peter Juo and John Blenis for the gift of the FADD and caspase 8-deficient Jurkat clones. We thank Heike Voigt and Yazmin Hauyon for their assistance in the B-cell proliferation experiment and Kimberley Burns for careful reading of the manuscript.
This work was supported by grants from the Swiss National Science Foundation (to P.S., M.K.B., and J.T.).
P.S. and J.T. share senior coauthorship.
REFERENCES
- 1.Algeciras-Schimnich, A., L. Shen, B. C. Barnhart, A. E. Murmann, J. K. Burkhardt, and M. E. Peter. 2002. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 22:207-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baud, V., and M. Karin. 2001. Signal transduction by TNF and its relatives. Trends Cell Biol. 11:372-377. [DOI] [PubMed] [Google Scholar]
- 3.Baud, V., Z. G. Liu, B. Bennett, N. Suzuki, Y. Xia, and M. Karin. 1999. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 13:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, A. H., T. P. Combs, X. Du, M. Brownlee, and P. E. Scherer. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7:947-953. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer, J. L., P. Schneider, and J. Tschopp. 2002. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27:19-26. [DOI] [PubMed] [Google Scholar]
- 6.Boldin, M. P., E. E. Varfolomeev, Z. Pancer, I. L. Mett, J. H. Camonis, and D. Wallach. 1995. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270:7795-7798. [DOI] [PubMed] [Google Scholar]
- 7.Bossi, G., and G. M. Griffiths. 1999. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 5:90-96. [DOI] [PubMed] [Google Scholar]
- 8.Chai, J., Q. Wu, E. Shiozaki, S. M. Srinivasula, E. S. Alnemri, and Y. Shi. 2001. Crystal structure of a procaspase-7 zymogen: mechanisms of activation and substrate binding. Cell 107:399-407. [DOI] [PubMed] [Google Scholar]
- 9.Chinnaiyan, A. M., K. O'Rourke, M. Tewari, and V. M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505-512. [DOI] [PubMed] [Google Scholar]
- 10.Cremesti, A., F. Paris, H. Grassme, N. Holler, J. Tschopp, Z. Fuks, E. Gulbins, and R. Kolesnick. 2001. Ceramide enables fas to cap and kill. J. Biol. Chem. 276:23954-23961. [DOI] [PubMed] [Google Scholar]
- 11.Engel, J. 1994. Electron microscopy of extracellular matrix components. Methods Enzymol. 245:469-488. [DOI] [PubMed] [Google Scholar]
- 12.Fruebis, J., T. S. Tsao, S. Javorschi, D. Ebbets-Reed, M. R. Erickson, F. T. Yen, B. E. Bihain, and H. F. Lodish. 2001. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 98:2005-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haswell, L. E., M. J. Glennie, and A. Al-Shamkhani. 2001. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur. J. Immunol. 31:3094-3100. [DOI] [PubMed] [Google Scholar]
- 14.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J. L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 15.Juo, P., M. S. Woo, C. J. Kuo, P. Signorelli, H. P. Biemann, Y. A. Hannun, and J. Blenis. 1999. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Differ. 10:797-804. [PubMed] [Google Scholar]
- 16.Kischkel, F. C., S. Hellbardt, I. Behrmann, M. Germer, M. Pawlita, P. H. Krammer, and M. E. Peter. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kischkel, F. C., D. A. Lawrence, A. Tinel, H. LeBlanc, A. Virmani, P. Schow, A. Gazdar, J. Blenis, D. Arnott, and A. Ashkenazi. 2001. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276:46639-46646. [DOI] [PubMed] [Google Scholar]
- 18.Kondo, T., T. Suda, H. Fukuyama, M. Adachi, and S. Nagata. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3:409-413. [DOI] [PubMed] [Google Scholar]
- 19.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 20.Laurikkala, J., J. Pispa, H. S. Jung, P. Nieminen, M. Mikkola, X. Wang, U. Saarialho-Kere, J. Galceran, R. Grosschedl, and I. Thesleff. 2002. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development 129:2541-2553. [DOI] [PubMed] [Google Scholar]
- 21.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 22.Lu, J., H. Wiedemann, R. Timpl, and K. B. Reid. 1993. Similarity in structure between C1q and the collectins as judged by electron microscopy. Behring Inst. Mitt. 93:6-16. [PubMed] [Google Scholar]
- 23.Martin-Villalba, A., M. Hahne, S. Kleber, J. Vogel, W. Falk, J. Schenkel, and P. H. Krammer. 2001. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 8:679-686. [DOI] [PubMed] [Google Scholar]
- 24.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 26.Ni, C. Z., K. Welsh, E. Leo, C. K. Chiou, H. Wu, J. C. Reed, and K. R. Ely. 2000. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. USA 97:10395-10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renatus, M., H. R. Stennicke, F. L. Scott, R. C. Liddington, and G. S. Salvesen. 2001. Dimer formation drives the activation of the cell death protease caspase 9. Proc. Natl. Acad. Sci. USA 98:14250-14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherer, P. E., S. Williams, M. Fogliano, G. Baldini, and H. F. Lodish. 1995. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270:26746-26749. [DOI] [PubMed] [Google Scholar]
- 30.Schneider, P. 2000. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 322:325-345. [DOI] [PubMed] [Google Scholar]
- 31.Schneider, P., N. Holler, J. L. Bodmer, M. Hahne, K. Frei, A. Fontana, and J. Tschopp. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider, P., S. L. Street, O. Gaide, S. Hertig, A. Tardivel, J. Tschopp, L. Runkel, K. Alevizopoulos, B. M. Ferguson, and J. Zonana. 2001. Mutations leading to X-linked hypohidrotic ectodermal dysplasia affect three major functional domains in the tumor necrosis factor family member ectodysplasin-A. J. Biol. Chem. 276:18819-18827. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro, L., and P. E. Scherer. 1998. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 8:335-338. [DOI] [PubMed] [Google Scholar]
- 34.Siegel, R. M., J. K. Frederiksen, D. A. Zacharias, F. K. Chan, M. Johnson, D. Lynch, R. Y. Tsien, and M. J. Lenardo. 2000. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science 288:2354-2357. [DOI] [PubMed] [Google Scholar]
- 35.Stanger, B. Z., P. Leder, T. H. Lee, E. Kim, and B. Seed. 1995. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81:513-523. [DOI] [PubMed] [Google Scholar]
- 36.Stassi, G., M. Todaro, F. Bucchieri, A. Stoppacciaro, F. Farina, G. Zummo, R. Testi, and R. De Maria. 1999. Fas/Fas ligand-driven T cell apoptosis as a consequence of ineffective thyroid immunoprivilege in Hashimoto's thyroiditis. J. Immunol. 162:263-267. [PubMed] [Google Scholar]
- 37.Straus, S. E., M. Sneller, M. J. Lenardo, J. M. Puck, and W. Strober. 1999. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann. Intern. Med. 130:591-601. [DOI] [PubMed] [Google Scholar]
- 38.Suda, T., H. Hashimoto, M. Tanaka, T. Ochi, and S. Nagata. 1997. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J. Exp. Med. 186:2045-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suda, T., M. Tanaka, K. Miwa, and S. Nagata. 1996. Apoptosis of mouse naive T cells induced by recombinant soluble Fas ligand and activation-induced resistance to Fas ligand. J. Immunol. 157:3918-3924. [PubMed] [Google Scholar]
- 40.Tanaka, M., T. Itai, M. Adachi, and S. Nagata. 1998. Downregulation of Fas ligand by shedding. Nat. Med. 4:31-36. [DOI] [PubMed] [Google Scholar]
- 41.Tucker, A. S., D. J. Headon, P. Schneider, B. M. Ferguson, P. Overbeek, J. Tschopp, and P. T. Sharpe. 2000. Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis. Development 127:4691-4700. [DOI] [PubMed] [Google Scholar]
- 42.Varadhachary, A. S., M. Edidin, A. M. Hanlon, M. E. Peter, P. H. Krammer, and P. Salgame. 2001. Phosphatidylinositol 3′-kinase blocks CD95 aggregation and caspase-8 cleavage at the death-inducing signaling complex by modulating lateral diffusion of CD95. J. Immunol. 166:6564-6569. [DOI] [PubMed] [Google Scholar]
- 43.Viard, I., P. Wehrli, R. Bullani, P. Schneider, N. Holler, D. Salomon, T. Hunziker, J. H. Saurat, J. Tschopp, and L. E. French. 1998. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 282:490-493. [DOI] [PubMed] [Google Scholar]
- 44.Villunger, A., D. C. Huang, N. Holler, J. Tschopp, and A. Strasser. 2000. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involve DAXX, RIP, or RAIDD. J. Immunol. 165:1337-1343. [DOI] [PubMed] [Google Scholar]
- 45.Walczak, H., and M. R. Sprick. 2001. Biochemistry and function of the DISC. Trends Biochem. Sci. 26:452-453. [DOI] [PubMed] [Google Scholar]
- 46.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 47.Wang, J., H. J. Chun, W. Wong, D. M. Spencer, and M. J. Lenardo. 2001. Caspase-10 is an initiator caspase in death receptor signaling. Proc. Natl. Acad. Sci. USA 98:13884-13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber, C. H., and C. Vincenz. 2001. A docking model of key components of the DISC complex: death domain superfamily interactions redefined. FEBS Lett. 492:171-176. [DOI] [PubMed] [Google Scholar]
- 49.Wright, D. A., B. Futcher, P. Ghosh, and R. S. Geha. 1996. Association of human fas (CD95) with a ubiquitin-conjugating enzyme (UBC-FAP). J. Biol. Chem. 271:31037-31043. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi, T., J. Kamon, H. Waki, Y. Terauchi, N. Kubota, K. Hara, Y. Mori, T. Ide, K. Murakami, N. Tsuboyama-Kasaoka, O. Ezaki, Y. Akanuma, O. Gavrilova, C. Vinson, M. L. Reitman, H. Kagechika, K. Shudo, M. Yoda, Y. Nakano, K. Tobe, R. Nagai, S. Kimura, M. Tomita, P. Froguel, and T. Kadowaki. 2001. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7:941-946. [DOI] [PubMed] [Google Scholar]
- 51.Yanagisawa, J., M. Takahashi, H. Kanki, H. Yano-Yanagisawa, T. Tazunoki, E. Sawa, T. Nishitoba, M. Kamishohara, E. Kobayashi, S. Kataoka, and T. Sato. 1997. The molecular interaction of Fas and FAP-1. A tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J. Biol. Chem. 272:8539-8545. [DOI] [PubMed] [Google Scholar]
- 52.Yang, X., H. Y. Chang, and D. Baltimore. 1998. Autoproteolytic activation of pro-caspases by oligomerization. Mol. Cell 1:319-325. [DOI] [PubMed] [Google Scholar]