Abstract
The growth arrest and DNA damage-inducible protein, GADD34, associates with protein phosphatase 1 (PP1) and promotes in vitro dephosphorylation of the α subunit of eukaryotic translation initiation factor 2, (eIF-2α). In this report, we show that the expression of human GADD34 in cultured cells reversed eIF-2α phosphorylation induced by thapsigargin and tunicamycin, agents that promote protein unfolding in the endoplasmic reticulum (ER). GADD34 expression also reversed eIF-2α phosphorylation induced by okadaic acid but not that induced by another phosphatase inhibitor, calyculin A (CA), which is a result consistent with PP1 being a component of the GADD34-assembled eIF-2α phosphatase. Structure-function studies identified a bipartite C-terminal domain in GADD34 that encompassed a canonical PP1-binding motif, KVRF, and a novel RARA sequence, both of which were required for PP1 binding. N-terminal deletions of GADD34 established that while PP1 binding was necessary, it was not sufficient to promote eIF-2α dephosphorylation in cells. Imaging of green fluorescent protein (GFP)-GADD34 proteins showed that the N-terminal 180 residues directed the localization of GADD34 at the ER and that GADD34 targeted the α isoform of PP1 to the ER. These data provide new insights into the mode of action of GADD34 in assembling an ER-associated eIF-2α phosphatase that regulates protein translation in mammalian cells.
Eukaryotic cells routinely monitor protein synthesis and folding in the endoplasmic reticulum (ER) to exercise quality control over the nearly one-third of cellular proteins that are synthesized and processed in the ER (30). Aberrations in ER function result in the accumulation of unfolded proteins and activate signal transduction pathways that trigger a complex transcriptional and translational response known as the unfolded protein response (UPR) (30). A hallmark of the UPR is increased phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF-2α) on serine-51 (44). Phosphorylated eIF-2α inhibits the guanine nucleotide exchange activity of eIF-2B and attenuates global protein synthesis, allowing cells the opportunity to clear misfolded proteins from the ER (40). The phosphorylation of eIF-2α, while inhibiting translation of most mRNAs, increases translation of a few selected transcripts such as that encoding activating transcription factor 4 (21, 27). Activating transcription factor 4 in turn induces the transcription of downstream genes, such as CHOP/GADD153, that may be critical for the overall cellular response to ER stress (21).
Recent work suggests that the PKR-like ER-associated kinase (PERK) is the major UPR-activated eIF-2α kinase in mammalian cells (22, 23, 47). Phosphorylation of eIF-2α by PERK or GCN2, a distinct eIF-2α kinase that is activated by nutrient deprivation (47), promotes the expression of GADD34, which then assembles an eIF-2α phosphatase that functions in a negative feedback loop to reverse eIF-2α phosphorylation and suppress UPR (36). GADD34 is member of a family of GADD genes that are induced by DNA damage, growth factor deprivation, and other forms of cell stress (54) and has been shown to bind the eukaryotic serine/threonine phosphatase protein phosphatase 1 (PP1) to direct eIF-2α dephosphorylation in vitro (15, 36). However, the mode of action of GADD34 in the assembly and function of an eIF-2α phosphatase in cells remains unclear.
Initial insights into the identity and mechanism of a cellular eIF-2α phosphatase were obtained from studies of a viral GADD34-related gene product, ICP34.5, which represents an essential virulence factor for herpes simplex virus type 1 (HSV-1) (24-26). In HSV-1-infected cells, ICP34.5 binds to host cell PP1α and generates a highly active eIF-2α phosphatase that overwhelms the actions of PKR, an eIF-2α kinase activated upon viral infection (25). This allows the virus to override the host cell-mediated shutoff of protein synthesis and the ensuing programmed cell death (25). The C terminus of ICP34.5 contains a region of 84 amino acids that confers PP1 binding and is highly conserved in mammalian GADD34. Deletion of these C-terminal sequences in ICP34.5 generates a virus that is unable to replicate in human neurons (12). However, substitution of the homologous C-terminal sequence from mouse GADD34/MyD116 restores ICP34.5 function, promoting protein synthesis and viral replication in cells infected with the mutant HSV-1 (24). This suggests a common function of mammalian and viral GADD34-related proteins in the assembly of an eIF-2α phosphatase.
GADD34 overexpression is known to facilitate apoptosis in mammalian cells following DNA damage and other cell stress (1, 29). This apoptotic activity requires N-terminal sequences in GADD34 that are not conserved in ICP34.5, which, in contrast to GADD34, displays antiapoptotic activity (1, 24). Recent studies suggest that N-terminal sequences in both GADD34 and ICP34.5 dictate their subcellular localization (1, 9, 33), raising the possibility that differences in localization contribute to the differing functions of the mammalian and viral GADD34-related proteins.
To investigate the physiological role of human GADD34, specifically in the control of eIF-2α dephosphorylation, we established a cell-based assay that monitors the phosphorylation of eIF-2α on serine-51 without eliciting UPR or apoptosis in cultured mammalian cells. These studies identified a unique C-terminal bipartite domain essential for GADD34 binding to PP1α. Furthermore, we showed that the N terminus of GADD34 targeted the GADD34/PP1 complex to the ER to promote eIF-2α dephosphorylation. These studies also established key differences in the modes of action of viral and mammalian GADD34 proteins that recruit PP1 to promote protein translation in mammalian cells. Implications of these findings for the physiological control of protein synthesis are discussed below.
MATERIALS AND METHODS
Reagents and antibodies.
Lipofectamine was obtained from Invitrogen Life Technologies; anti-FLAG M2 affinity gel, protein G-Sepharose 4B, tunicamycin, triethylenediamine, and poly-d-lysine were obtained from Sigma; CNBr-activated Sepharose was obtained from Amersham Pharmacia Biotech; protein A-agarose was obtained from Bio-Rad; microcystin-LR, okadaic acid (OA), calyculin A (CA), and thapsigargin were obtained from Alexis Corporation; and DiOC6 stain was obtained from Molecular Probes Inc.
The following antibodies were utilized at the dilutions shown in brackets: anti-FLAG M2 monoclonal antibody (1:1,000) from Sigma; anti-GADD34 H-193 (1:250) and anti-eIF-2α (1:500) polyclonal antibodies from Santa Cruz Biotechnology Inc.; anti-eIF-2α (phosphoserine-51) antibody (1:1,000) from Biosource International; anti-GFP monoclonal and Living Colors polyclonal antibodies (1:5,000) from Clontech; a pan anti-PP1 monoclonal antibody (1:1,000) from BD Transduction Laboratories; donkey anti-rabbit HRP-linked immunoglobulin G (IgG) secondary (1:3,000) and sheep anti-rabbit HRP-linked IgG secondary (1:3,000) antibodies from Amersham Pharmacia Biotech; and AlexaFluor 568 goat anti-rabbit IgG (1:250) from Molecular Probes Inc. The anti-translocon-associated protein (TRAP) (also known as signal sequence receptor α) polyclonal antibody (1:500) was provided by Christopher Nicchitta, Duke University (34). An anti-PP1α polyclonal antibody (1:500) (3, 16, 38) was provided by Angus Nairn, Rockefeller University. Anti-PP1β and γ1 antibodies were from Brian Wadzinski, Vanderbilt University (48).
Mutagenesis of GADD34.
Expression plasmids encoding FLAG-tagged human GADD34 [pSG-FLAG-GADD34(1-674), -GADD34(180-674), -GADD34(180-483), and -GADD34(180-610)] were kindly provided by D.C. Tkachuk (1). Plasmids encoding GFP-tagged GADD34 proteins were generated using PCR and the PCR products, digested with BglII, and subcloned into pEGFP-C1 (Clontech). C-terminal truncations of GADD34 were generated using a Quikchange site-directed mutagenesis kit (Stratagene) with pSG5-FLAG-GADD34(1-674) as the template and appropriate primers to introduce stop codons. Point mutations in FLAG-GADD34(1-674) were also introduced using the Quikchange kit. The KARA mutant was constructed by using the primer 5′CCTAAAGGCCAGAAAGGCGCGCGCCTCCGAGAAGGTCACTG3′ to substitute alanines for valine-556 and phenylalanine-558; the KK mutant was constructed by using the primer 5′CCTCACCCCTGCTGCCAAGGCCAAAGCCTGGGCACGCCTCAG3′ to substitute lysines for arginine-612 and arginine-614. Using the primers 5′CGGGCCAGAGCCTGGGCAGACCTCAGGAACCCACCTTTAG3′ and 5′CCCCTGCTGCCCGGGCCGACGCCTGGGCACGCCTCAG3′, aspartic acids were substituted for arginine-618 (R618D) and arginine-614 (R614D). All cDNAs were verified by DNA sequencing by Duke University Comprehensive Cancer Center Sequencing Facility.
Using KpnI and BamHI, a neurabin I (NrbI) cDNA encoding amino acids 286 to 552 was excised from enhanced GFP-NrbI(1-552) (37) and subcloned into EGFP-C1 (Clontech).
Expression of GADD34 in cultured mammalian cells.
Human embryonic kidney (HEK293T), African green monkey kidney (COS-7), and human lung epithelial carcinoma (A549) cells were purchased from the American Type Culture Collection and maintained in Dulbecco's modified Eagle medium (DMEM) with 10% (vol/vol) fetal bovine serum (FBS). Mouse NIH 3T3 fibroblasts, also obtained from the American Type Culture Collection, were cultured in DMEM with 10% (vol/vol) bovine calf serum. Rat pheochromocytoma PC6-3 cells, a cell line derived from PC-12 cells (43), were provided by Stefan Strack (University of Iowa) and grown in RPMI 1640 medium with 10% horse serum. All cells were grown at 37°C in an atmosphere of 5% CO2-95% air in medium containing penicillin-streptomycin-amphotericin B (Gibco-BRL). DNA transfections were performed in 6-well plates (Falcon) using 6 μl of lipofectamine and 2 μg of plasmid DNA in serum-free DMEM. After 5 h, the cells were rescued in DMEM containing 10% (vol/vol) FBS.
HEK293T cells were grown to 50 to 80% confluence in 6-well plates in DMEM containing 10% (vol/vol) FBS. To induce ER stress, the cells were treated with 5 μg of tunicamycin/ml or 500 nM thapsigargin. Control cells were treated with the vehicle, 0.1% (vol/vol) dimethyl sulfoxide (DMSO). In some experiments, the cells were treated with increasing concentrations of OA or CA for 30 to 60 min at 12 to 16 h after transfection with GADD34 plasmids. The cells were then washed in phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation buffer containing several protease inhibitors (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% [wt/vol] deoxycholic acid, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], 1% [wt/vol] NP-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 2 μg of leupeptin/ml, 1 μg of aprotonin/ml, and 1 μg of pepstatin A/ml) at 4°C. The lysates were centrifuged at 22,000 × g for 10 min at 4°C, and the supernatants were boiled in SDS sample buffer prior to SDS-polyacrylamide gel electrophoresis (PAGE). The gels were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes, which were blocked in Tris-buffered saline-Tween containing 4% (wt/vol) dried milk before incubation with antibodies diluted in the same buffer at room temperature for 1 h or overnight at 4°C. Immune complexes were detected using Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences).
Immunoprecipitations and immunoblotting.
HEK293T cells, grown to 80 to 100% confluence, were washed in PBS and lysed in NETN buffer (100 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.5% [wt/vol] NP-40, 1 mM EDTA containing a mixture of protease inhibitors), and the lysates were subjected to centrifugation at 22,000 × g for 10 min at 4°C. Supernatants containing FLAG-tagged proteins were incubated with a 25-μl bed volume of anti-FLAG M2 beads for 2 h at 4°C. GFP-containing supernatants were incubated at 4°C with 2 μl of polyclonal Living Colors anti-GFP antibody for 1 h followed by the addition of slurry containing a 1:1 mixture of protein A- and protein G-conjugated beads for an additional hour. The beads were washed three to four times with NETN buffer at 4°C, and bound proteins were eluted in 50 μl of SDS sample buffer. The samples were boiled for 5 min prior to SDS-PAGE. The gels were electrophoretically transferred to PVDF membranes and immunoblotted with appropriate antibodies.
Cellular PP1 complexes were also affinity isolated using microcystin-Sepharose as previously described (7).
Immunohistochemistry.
COS-7 cells were grown on poly-d-lysine-coated glass coverslips. At 16 hours after transfection, cells were washed in PBS, fixed for 10 min in PBS containing 4% (wt/vol) paraformaldehyde, permeabilized for 2 to 5 min in PBS containing 0.2% (wt/vol) Triton X-100, and blocked with PBS containing 2% (wt/vol) bovine serum albumin for 1 h. The cells were incubated with primary antibodies diluted in PBS containing 2% (wt/vol) bovine serum albumin for 1.5 h at room temperature, washed three times in PBS, and then incubated with AlexaFluor 568 goat anti-rabbit IgG for 1 h at room temperature. The coverslips were washed four to five times for 2 min each time in PBS prior to mounting in Mount A solution (PBS containing 50% [vol/vol] glycerol and 2.5% [wt/vol] triethylenediamine). DiOC6 (3,3′-dihexyloxacarbocyanine iodide) and DAPI (4′,6-diamino-2-phenylindol) staining was performed after the final wash following incubation of cells with secondary antibody. The slides were analyzed using an Olympus IX70 inverted system microscope with an UltraVIEW confocal imaging system (Perkin-Elmer Life Sciences).
Two approaches established the specificity of cell staining by the anti-GADD34 antibodies. First, we preincubated the anti-GADD34 antibodies with recombinant glutathione transferase (GST)-GADD34(513-674). The immunocomplexes were adsorbed on glutathione-Sepharose, and the flowthrough or immunodepleted antibody was used for cell staining as described above. The recombinant PP1-binding polypeptide, GST-NrbI(436-479), was used as a control. In other experiments, anti-GADD34 antibodies were incubated with either GST-GADD34(513-674) or GST-NrbI(436-479) (at GST protein concentrations of 0.5 mg/ml) and presented to cells during immunostaining in continued presence of the competing antigen.
RESULTS
Phosphorylation-dephosphorylation of eIF-2α in HEK293T cells.
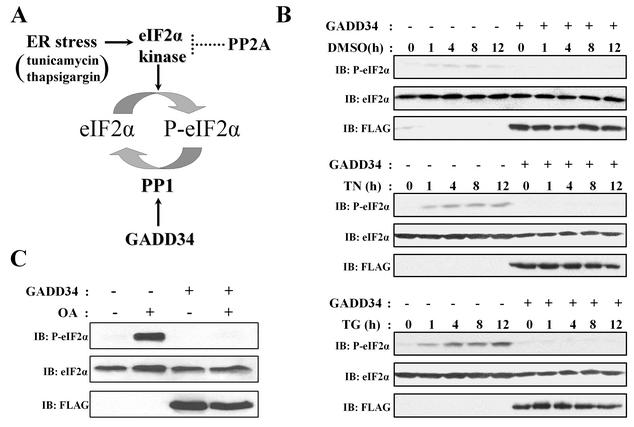
The phosphorylation of eIF-2α at serine-51 is increased by a variety of cellular stresses, including ER stress induced by defects in protein folding (44). ER stress can be experimentally induced by treatment of cells with tunicamycin, which inhibits protein glycosylation, or thapsigargin, which depletes intracellular calcium stores. This activates PERK and possibly other eIF-2α kinases (Fig. 1A) and leads to a phosphorylation of eIF-2α that peaks after several hours (Fig. 1B) (23). Little or no eIF-2α phosphorylation was induced by the vehicle, DMSO. Expression of FLAG-tagged human GADD34 abolished the eIF-2α phosphorylation induced by both tunicamycin and thapsigargin in HEK293T cells (Fig. 1B), as previously reported (36).
FIG. 1.
Regulation of eIF-2α phosphorylation in HEK293T cells. (A) A model for ER stress signaling induced by tunicamycin and thapsigargin. These stimuli activate PERK (23) and other eIF-2α kinases, such as PKR (45), which phosphorylate eIF-2α at serine-51 and inhibit protein translation. GADD34 expression in cells enhances PP1 activity, which dephosphorylates eIF-2α. The inactivation of eIF-2α kinase(s) is most likely mediated by non-GADD34-associated PP2A-like phosphatases. (B) Treatment of HEK293T cells with DMSO (0.1% vol/vol) had little effect on eIF-2α phosphorylation, as monitored by immunoblotting (IB) with an anti-phosphoserine-51-eIF-2α antibody. In contrast, treatment with 5 μg of tunicamycin (TN)/ml or 500 nM thapsigargin (TG) resulted in a time-dependent (measured in hours) increase in eIF-2α phosphorylation. This increase was severely attenuated by the expression of FLAG-GADD34. (C) Treatment of cells with 1 μM OA (OA) for 1 h resulted in a significant increase in eIF-2α phosphorylation that was also blocked by expression of FLAG-GADD34. Total eIF-2α and FLAG-GADD34 levels were monitored by immunoblotting (IB) with anti-eIF-2α and anti-FLAG antibodies.
eIF-2α phosphorylation of much greater rapidity and robustness was induced by treatment of cells with the cell-permeable phosphatase inhibitor OA. eIF-2α phosphorylation induced by OA peaked within 1 h and was 10 to 15-fold higher than that achieved after 4- to 12-h exposures to tunicamycin or thapsigargin (Fig. 1C). Expression of FLAG-GADD34 also abolished eIF-2α phosphorylation induced by OA (Fig. 1C). Similar results were obtained with COS-7, NIH/3T3, and PC6-3 cells (data not shown). In contrast to the programmed cell death elicited by ER stress (39), OA elicited no detectable death of HEK293T cells over the time course of our experiments (data not shown). Furthermore, while GADD34 overexpression has been reported to promote apoptosis (28, 29), all cells analyzed in this study showed no programmed cell death resulting from GADD34 expression alone. This established a simple and reproducible in vivo assay for analyzing the function of GADD34 in promoting eIF-2α dephosphorylation.
Phosphatase inhibitors increase eIF-2α phosphorylation.
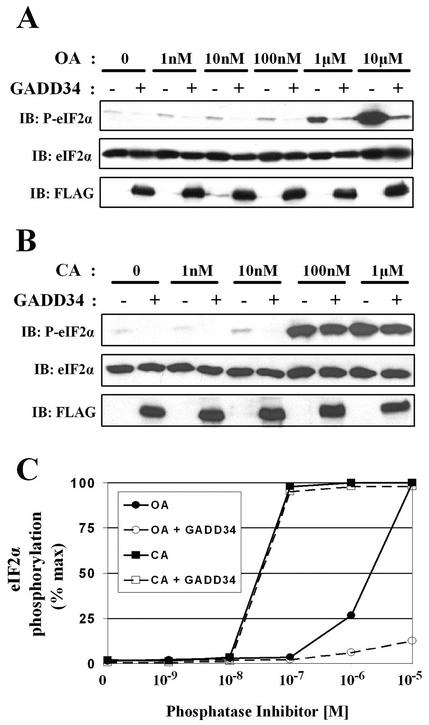
The major cellular protein serine/threonine phosphatases, PP1 and PP2A, have both been implicated in the regulation of eIF-2α function by either reversing the phosphorylation of eIF-2α itself or inactivating upstream eIF-2α kinases (8, 18, 42, 49, 50, 52). To evaluate the role of protein phosphatases in regulating eIF-2α phosphorylation, we treated control and GADD34-expressing HEK293T cells for 30 min with increasing concentrations of OA, which inhibits PP2A (50% inhibitory dose [IC50], 1 to 10 nM) more potently than PP1 (IC50, 100 nM to 1 μM) (20) (Fig. 2A). Immunoblotting with an anti-phosphoserine-51-eIF-2α antibody showed a modest increase in eIF-2α phosphorylation induced by low concentrations of OA (1 to 100 nM) and much higher phosphorylation of eIF-2α by 1 to 10 μM OA (Fig. 2A). At all OA concentrations, GADD34 expression largely reversed eIF-2α phosphorylation (Fig. 2A).
FIG. 2.
Cell-permeable phosphatase inhibitors increase eIF-2α phosphorylation. (A) eIF-2α phosphorylation was increased by treatment of HEK293T cells for 30 min with increasing concentrations of OA. FLAG-GADD34 expression attenuated the eIF-2α phosphorylation elicited by all concentrations of OA. (B) Treatment of HEK293T cells for 30 min with increasing concentrations of CA also promoted eIF-2α phosphorylation. However, FLAG-GADD34 expression failed to reverse eIF-2α phosphorylation induced by the presence of CA at concentrations above 100 nM. (C) Representative dose-response curves for OA (circles)- and CA (squares)-induced eIF-2α phosphorylation in the presence (open shapes) and absence (filled shapes) of GADD34 expression are shown. eIF-2α phosphorylation, monitored by laser densitometry analysis of anti-phosphoserine-51-eIF-2α antibody immunoblots, is expressed as a percentage of maximum response.
CA is a more potent PP1 inhibitor (IC50, 1 to 10 nM) than OA. Treatment of HEK293T cells with CA yielded a different dose-response profile for eIF-2α phosphorylation (Fig. 2B). While a small increase in eIF-2α phosphorylation was seen with 10 nM CA, a more dramatic increase was observed at higher CA concentrations (Fig. 2B). In contrast to OA-induced eIF-2α phosphorylation, GADD34 expression failed to suppress eIF-2α phosphorylation induced by CA at concentrations above 100 nM (Fig. 2B). Comparison of dose-response curves for OA- and CA-elicited eIF-2α phosphorylation in the presence and absence of GADD34 (Fig. 2C) strongly pointed to PP1 as a component of the cellular eIF-2α phosphatase assembled by GADD34.
GADD34 promotes eIF-2α dephosphorylation.
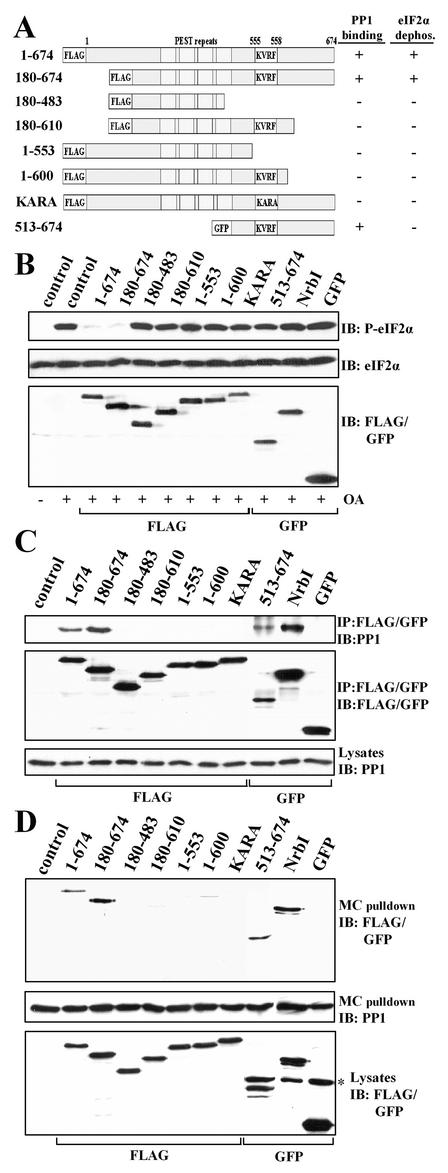
To define structural determinants in GADD34 that promote eIF-2α dephosphorylation in mammalian cells, we expressed several FLAG- and GFP-tagged GADD34 proteins in HEK293T cells (Fig. 3A). Immunoblotting with an anti-phospho-eIF-2α antibody showed that OA-induced eIF-2α phosphorylation was attenuated by the expression of wild-type (WT) GADD34(1-674) and GADD34(180-674) to levels approximating those in untreated HEK293T cells (Fig. 3B, lane 1). Mutation of the highly conserved KVRF PP1-binding sequence (residues 555 to 558) in full-length GADD34 generated KARA, which failed to reverse eIF-2α phosphorylation. Other GADD34 proteins that lack the conserved PP1-binding motif, including GADD34(180-483) and -(1-553), were also ineffective in suppressing OA-induced eIF-2α phosphorylation. This established that the PP1-binding motif in GADD34 was essential for promoting eIF-2α dephosphorylation in cells. To exclude the possibility that overexpression of GADD34 indirectly modified eIF-2α phosphorylation by disrupting endogenous PP1 complexes, we expressed another PP1-binding peptide, NrbI(286-552), representing a fragment of the neuronal actin-binding protein NrbI (37). Expression of this PP1-binding peptide had no effect on eIF-2α phosphorylation elicited by OA (Fig. 3B), demonstrating that GADD34 played a direct and active role in assembling an eIF-2α phosphatase.
FIG. 3.
Structure-function analysis of GADD34 as a component of a cellular eIF-2α phosphatase. (A) A schematic representation of theGADD34 mutants analyzed in this study, with blocks highlighting the N-terminal tag, the central PEST repeats, and the consensus KVRF PP1-binding motif. The presence and absence of eIF-2α phosphatase activity (panel B) and PP1 binding (panels C and D) displayed by GADD34 proteins are summarized at the right side of panel A: + indicates a positive function and − indicates an absence of function. All proteins were FLAG tagged with the exception of GFP-tagged GADD34(513-674). (B) HEK293T cells expressing the GADD34 proteins were treated with 1 μM OA for 1 h, and eIF-2α phosphorylation was assayed by immunoblotting with an anti-phosphoserine-51-eIF-2α antibody. NrbI represents a fragment of the neuronal actin-binding protein (amino acids 286 to 552) known to bind PP1. Immunoblotting for total eIF-2α demonstrated equal protein loading, and anti-FLAG/GFP immunoblots (IB) showed equivalent expression of GADD34 proteins. Control, lysates of untransfected cells. (C) The GADD34 proteins were immunoprecipitated (IP) using anti-FLAG M2 beads or an anti-GFP polyclonal antibody. The immunoprecipitates were subjected to SDS-PAGE, transferred to a PVDF membrane, and immunoblotted (IB) with anti-FLAG, anti-GFP, and anti-PP1 antibodies. Control, lysates of untransfected cells. (D) Cellular PP1 complexes were affinity isolated on microcystin-LR-Sepharose as described in Materials and Methods. The microcystin-bound proteins were subjected to SDS-PAGE and subjected to immunoblotting (IB) with anti-FLAG, anti-GFP, and anti-PP1 antibodies. The asterisk indicates a nonspecific band recognized by anti-GFP antibody. Control, lysates of untransfected cells.
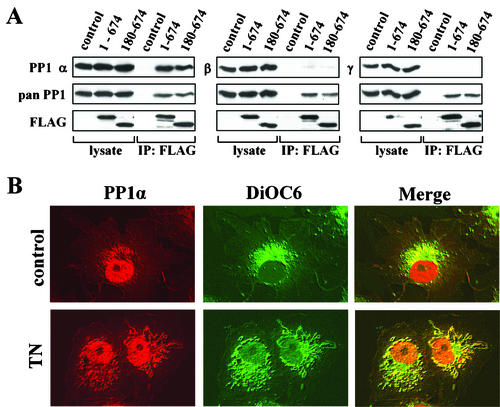
An unexpected result was that GADD34(180-610), -(1-600), and -(513-674), all of which retain the conserved PP1-binding motif, failed to reverse OA-induced eIF-2α phosphorylation. Immunoprecipitation with anti-GFP or anti-FLAG antibodies, followed by immunoblotting with a monoclonal anti-PP1 antibody, established that WT GADD34(1-674) and -(180-674) and NrbI(286-552), which contain the PP1-binding motif, indeed bound PP1, while GADD34(180-483) and -(1-553) and KARA, which lack the PP1-binding motif, failed to bind PP1 (Fig. 3C). To our surprise, GADD34(180-610) and -(1-600) proteins, which contain an intact KVRF motif, also failed to bind PP1, explaining their inability to dephosphorylate eIF-2α (Fig. 3B). The most remarkable finding was that GADD34(513-674), which encompasses the entire region of sequence homology with HSV-1 ICP34.5, bound PP1 similarly to WT GADD34 but failed to promote eIF-2α dephosphorylation. Similar results were obtained with COS-7 and PC6-3 cells (data not shown).
Using affinity isolation on immobilized microcystin-LR, a known PP1 inhibitor, we also demonstrated the GADD34-PP1 association in the reverse direction by quantitatively extracting PP1 complexes from cells expressing the GADD34 proteins (Fig. 3D). Immunoblotting with anti-GFP or anti-FLAG antibodies established that all the GADD34 proteins were expressed at nearly equivalent levels in HEK293T cells and were immunoprecipitated with equal efficiency (Fig. 3B, C, and D). As summarized in Fig. 3A, both approaches (coimmunoprecipitation and affinity isolation) emphasized that the conserved KVRF motif in GADD34 was necessary but not sufficient for PP1 binding. Furthermore, the inability of the PP1-binding GADD34(513-674) peptide to promote eIF-2α dephosphorylation suggested that factors in addition to PP1 binding were required to reverse eIF-2α phosphorylation in cells.
A novel sequence in GADD34 required for PP1 binding.
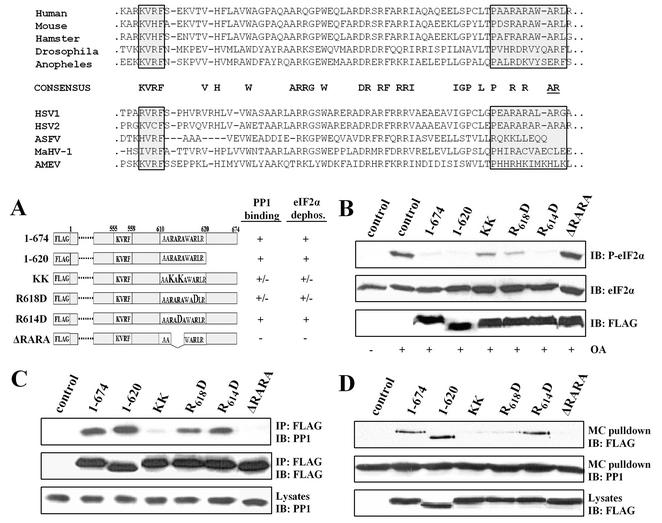
The ability of WT GADD34(1-674) but not GADD34(1-600) to bind PP1 in the coimmunoprecipitation and affinity isolation assays suggested that the C terminus of GADD34 between amino acids 600 to 674 was crucial for PP1 binding. Comparison of this region in several mammalian GADD34 homologues and the GADD34-related viral proteins highlighted clusters of conserved amino acids C terminal to the KVXF motif (Fig. 4). Thus, we undertook further analysis of the C terminus of human GADD34 (Fig. 4A). In contrast to GADD34(1-600), GADD34(1-620) functioned essentially like WT GADD34(1-674) to reverse eIF-2α phosphorylation induced by OA (Fig. 4B). Consistent with this, GADD34(1-620) bound PP1 in both coimmunoprecipitation (Fig. 4C) and affinity isolation (Fig. 4D) assays. These data narrowed the domain required for PP1 binding to residues between 600 and 620, a stretch that contains several arginine-alanine repeats conserved in mammalian and viral GADD34-like proteins.
FIG. 4.
Identification of a novel PP1-binding domain in GADD34. (Top panel) Alignment of C-terminal sequences of GADD34 proteins from human, hamster, mouse, fruit fly (Drosophila), and mosquito (Anapholes) sources is shown along with alignment of GADD34-related proteins from HSV-1 and HSV-2, African swine fever virus (ASFV), macropodid herpesvirus (MaHV-1), and amsacta moorei entomopoxvirus (AMEV) sources. Highly conserved residues are highlighted in bold letters. The consensus KVRF PP1-binding motif and an arginine- and alanine-rich (AlaArg) region are highlighted by boxes shaded in gray. Conserved arginine and alanine residues specifically implicated in ICP34.5-mediated assembly of an eIF-2α phosphatase complex (11) are underlined. (A) A schematic of FLAG-GADD34 proteins with the amino acids (shown in bold letters) mutated within the full-length GADD34(1-674). The eIF-2α phosphatase and PP1-binding activities associated with individual GADD34 proteins are summarized on the right side of the panel: +, positive function; −, absence of function; +/−, reduced function. ΔRARA represents an internal deletion of residues 612-615 from FLAG-GADD34. (B) HEK293T cells expressing individual GADD34 proteins were treated with OA, and eIF-2α phosphorylation, total eIF-2α, and FLAG-GADD34 were analyzed by immunoblotting (IB) as described above. Control, lysates of untransfected cells. (C) Immunoprecipitation of GADD34 proteins expressed in HEK293T cells was undertaken using anti-FLAG M2 beads, and the immunoprecipitates (IP) were subjected to immunoblotting (IB) with anti-FLAG and anti-PP1 antibodies. Control, lysates of untransfected cells. (D) Cellular PP1 complexes were affinity isolated using microcystin-LR-Sepharose, and the bound proteins were subjected to immunoblotting (IB) with anti-FLAG and anti-PP1 antibodies. Control, lysates of untransfected cells.
Previous studies of the viral ICP34.5 protein suggested that these alanine- and arginine-rich sequences, termed the “AlaArg motif,” were dispensable for PP1 binding by ICP34.5 but were required for the generation of an active, high-molecular-weight eIF-2α phosphatase complex (11). To analyze this region of human GADD34, we introduced point mutations at selected arginines and alanines (Fig. 4A) between residues 600 and 620. The mutant proteins were expressed in HEK293T cells and analyzed for eIF-2α dephosphorylation and PP1 binding. KK represents a full-length GADD34 protein in which two lysines were substituted in place of arginines in adjacent RA repeats. Compared to WT GADD34(1-674), KK showed decreased ability to facilitate eIF-2α dephosphorylation induced by OA in HEK293T cells (Fig. 4B). Immunoprecipitation (Fig. 4C) and microcystin affinity isolation also showed that KK exhibited reduced PP1 binding compared to WT GADD34 (Fig. 4D). Substitution of an aspartic acid in place of arginine-618 to yield R618D also reduced eIF-2α phosphatase activity (Fig. 4B) and modestly diminished PP1 binding (Fig. 4C and D). In contrast, a similar substitution at arginine-614 yielded R614D, which bound PP1 similarly to that of WT GADD34(1-674) (Fig. 4C and D) and effectively promoted eIF-2α dephosphorylation (Fig. 4B). Finally, the deletion of adjacent arginine-alanine repeats generated ΔRARA, which failed to bind PP1 (Fig. 4C and D) and did not promote eIF-2α dephosphorylation in cells (Fig. 4B). These studies suggested that the AlaArg residues identified in the HSV-1 ICP34.5 protein (Fig. 4) functioned very differently in human GADD34. Specifically, our studies defined a novel RARA sequence in GADD34 that, together with the canonical KVRF motif, was essential for PP1 binding and eIF-2α dephosphorylation in mammalian cells.
GADD34 localization in mammalian cells.
While mammalian GADD34 and viral ICP34.5 both function in eIF-2α dephosphorylation, they display distinct localizations in mammalian cells that may be determined at least in part by their unique N termini (9, 33). Deletion of N-terminal sequences from ICP34.5 redistributed the viral protein from nucleus and nucleoli to the cytoplasm (9) but did not impair its regulation of protein translation (12). In contrast, mouse GADD34 exhibited a perinuclear distribution in CHO cells (36) and deletion of its N-terminal sequences resulted in its broader distribution throughout the cytoplasm while having little effect on its ability to dephosphorylate eIF-2α in vitro (36).
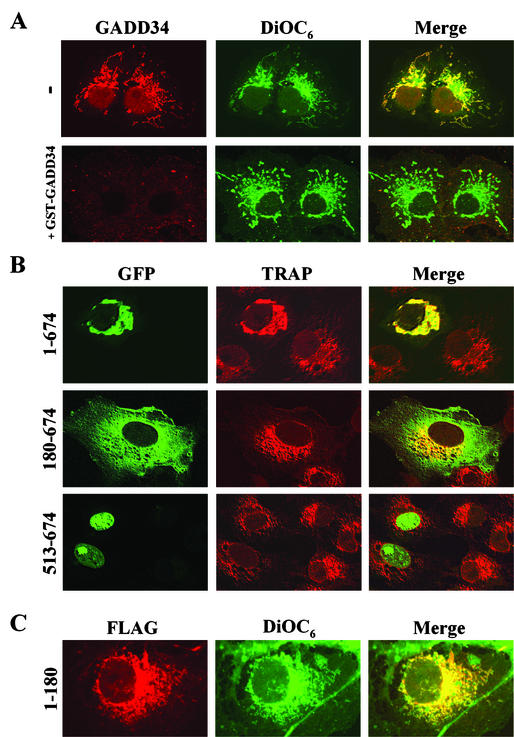
To investigate the role of GADD34 localization in cells, we utilized immunohistochemistry to examine the distribution of endogenous GADD34 in COS-7 cells. Immunostaining with an anti-GADD34 antibody showed an overlapping pattern of GADD34 with that of the ER dye, DiOC6 (Fig. 5A). Similar but weaker GADD34 staining was seen in NIH 3T3 and A549 cells (data not shown). Staining in the presence of excess recombinant GST-GADD34(513-674) essentially abolished the signal from the anti-GADD34 antibody (Fig. 5A), and immunodepletion of the anti-GADD34 antibody by using GST-GADD34(513-674) bound to glutathione-Sepharose yielded similar results (data not shown). In contrast, blocking or immunodepletion with a control peptide, GST-NrbI(436-479), had no effect on cell staining by the anti-GADD34 antibody. Notably, immunostaining with additional anti-GADD34 antibodies revealed some nuclear staining in addition to the pronounced ER staining. These data, gathered using various antibodies and cell types, indicated that endogenous GADD34 was principally associated with the ER.
FIG. 5.
Subcellular localization of GADD34 in COS-7 cells. (A) COS-7 cells were immunostained using an anti-GADD34 antibody (red) as described in Materials and Methods. The cells were also stained with the ER dye, DiOC6 (green), and the two images were merged (yellow) to allow examination of their colocalization. In the bottom row (+GST-GADD34), the anti-GADD34 antibody was blocked by the presence of excess recombinant GST-GADD34(513-674) and the cells were incubated with the antibody in the continued presence of antigen. (B) WT GFP-GADD34(1-674), GFP-GADD34(180-674), and GFP-GADD34(513-674) (shown in green) expressing COS-7 cells were immunostained using an anti-TRAP antibody (red) to identify the ER. The cells were analyzed using scanning laser confocal microscopy, and images were merged (yellow) as described in Materials and Methods. (C) Cells expressing FLAG-GADD34(1-180) were stained using the anti-FLAG antibody (red) and DiOC6 (green).
To define the structural determinants that dictate the subcellular localization of GADD34, we expressed GFP-tagged WT and mutant GADD34 proteins in COS-7 cells. WT GFP-GADD34(1-674), like the endogenous protein, was largely associated with the ER, as evidenced by immunostaining with an antibody against the ER-associated protein TRAP (Fig. 5B). Compared to the fine reticular structure of the ER in untransfected COS-7 cells, GFP-GADD34(1-674) expression resulted in a significantly dilated and enlarged ER. The N-terminally truncated GFP-GADD34(180-674) protein was widely distributed, extending into the cytoplasm and areas of plasma membrane, and had no discernible effect on ER morphology. Notably, GFP-GADD34(180-674) was also found in the nucleus in some cells. Immunostaining of cells expressing FLAG-GADD34(1-180) with an anti-FLAG antibody established that this N-terminal region of GADD34 was sufficient for localization at the ER (Fig. 5C). Interestingly, GFP-GADD34(513-674), which encompassed the ICP34.5 homology domain, was almost exclusively nuclear, with high concentrations of the protein seen in nucleoli in several cells. This suggested that the mislocalization of GADD34(513-674), which bound PP1 essentially as did WT GADD34(1-674), accounted for its inability to promote eIF-2α dephosphorylation in cells (Fig. 3). Similar results were obtained for localization of GADD34 proteins in NIH 3T3 and A549 cells (data not shown).
To exclude the possibility that GFP fusion influenced GADD34 localization in cells, we analyzed numerous FLAG-tagged GADD34 proteins expressed in several different cell lines. These immunohistochemical studies established that WT GADD34(1-674), GADD34(1-553), GADD34(1-600), KARA, and ΔRARA proteins, which shared common N-terminal sequences, localized at the ER (data not shown). In contrast, GADD34(180-483) and GADD34(180-610) proteins, which lacked N-terminal sequences, were more widely distributed (data not shown). This supported a role for the N-terminal 180 amino acids in dictating the ER localization of GADD34 and suggested that the C-terminal ICP34.5 homology domain played a minor role in the subcellular targeting of mammalian GADD34.
GADD34 recruits PP1α to ER.
The prevailing paradigm for PP1 regulation suggests that the physiological functions of PP1 are dictated by regulatory subunits that bind and target specific isoforms of PP1 catalytic subunits to unique subcellular compartments (2, 5, 14, 46). To evaluate the function of GADD34 as a PP1-targeting subunit, we first determined the PP1 isoforms that interacted with GADD34 in mammalian cells. Anti-FLAG immunoprecipitates containing WT FLAG-GADD34(1-674) and FLAG-GADD34(180-674) were subjected to immunoblotting for the three isoforms of PP1 (α, β, and γ1). While all three PP1 isoforms were highly expressed in HEK293T cells, WT GADD34(1-674) and GADD34(180-674) preferentially bound PP1α (Fig. 6A). As PP1α-specific binding was seen with both ER-localized GADD34(1-674) and the cytoplasmic GADD34(180-674), this suggested that specificity for PP1α was an intrinsic property of GADD34 and was not influenced by subcellular localization.
FIG. 6.
GADD34 binds the α isoform of PP1 and dictates its subcellular distribution. (A) Immunoprecipitation (IP) of FLAG-GADD34(1-674) and FLAG-GADD34(180-674) from HEK293T cells was undertaken using anti-FLAG M2 beads. The lysates and immunoprecipitates were subjected to immunoblotting with antibodies specific for the α, β, and γ1 isoforms of PP1, as well as with anti-FLAG and anti-PP1 (pan) antibodies, to assess levels of FLAG-GADD34 and total PP1. (B) Immunostaining untreated COS-7 cells (control) and cells treated with 5 μg of tunicamycin (TN)/ml for 8 h with anti-PP1α (red). The ER was costained using DiOC6 (green), and the panels were merged as described in Materials and Methods.
Prior studies showed that endogenous PP1α was localized primarily in nuclei of cultured cells (51). Immunohistochemistry analysis of control COS-7 cells by using an anti-PP1α antibody confirmed that PP1α was largely localized in the nucleus and excluded from nucleoli, although some PP1α staining was also seen in a perinuclear reticular pattern that was identified (using the dye DiOC6) as ER (Fig. 6B). Treatment of COS-7 cells with tunicamycin for 8 h did not result in any measurable change in total endogenous GADD34 protein (data not shown) but did cause expansion of the ER and a significant enhancement of PP1α localization at the ER (Fig. 6B).
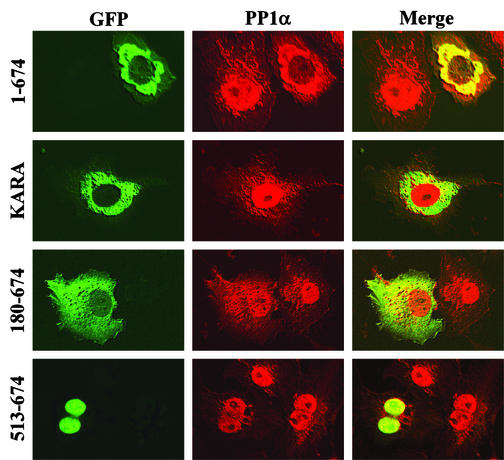
Expression of GFP-GADD34(1-674) in COS-7 cells resulted in even greater redistribution of PP1α to the ER, where it colocalized with GFP-GADD34(1-674) (Fig. 7). In some cells, overexpression of GADD34(1-674) nearly vacated the nuclear pool of PP1α. The KARA mutant, which localizes to the ER similarly to WT GADD34 but lacks a functional PP1-binding site, failed to redistribute PP1α, which remained primarily nuclear. GFP-GADD34(180-674), which showed a broader distribution than WT GADD34, promoted a less pronounced redistribution of PP1α to the cytoplasm. PP1α remained nuclear in cells expressing GFP-GADD34(513-674), although several cells showed a concentration of PP1α in nucleoli, where it colocalized with the truncated GADD34 protein. These data established that GADD34 functioned as a bona-fide PP1-targeting subunit that recruits PP1α to the ER and facilitates eIF-2α dephosphorylation.
FIG. 7.
GADD34-mediated targeting of PP1α in mammalian cells. COS-7 cells expressing either WT GFP-GADD34(1-674), the non-PP1-binding mutant GFP-KARA, GFP-GADD34(180-674), or GFP-GADD34(513-674) (shown in green) were immunostained with an anti-PP1α antibody (red). The cells were analyzed using scanning laser confocal microscopy, and the images were merged (yellow) as described in Materials and Methods.
DISCUSSION
Phosphorylation of eIF-2α in mammalian cells is a well-characterized response to cell stress, including hypoxia, nutrient and growth factor starvation, UV irradiation, ER overload, and viral infection (13, 17, 31, 35). Four protein kinases, HRI, GCN2, PKR, and PERK, decode different stress signals to phosphorylate eIF-2α at serine-51 and inhibit protein translation (23, 40). PERK, in particular, is activated by ER stress and functions as a critical component of the integrated translational and transcriptional response known as the UPR (23).
The protein phosphatases that reinitiate protein synthesis upon cellular recovery from stress are still poorly understood. Initial insights into the cellular eIF-2α phosphatase were obtained from studies of the viral virulence factor, ICP34.5, which overrides the host cell defense mechanism that blocks general protein synthesis in HSV-1-infected cells (12). The C terminus of ICP34.5, which is essential for viral infectivity, binds to host cell PP1α and targets it to dephosphorylate eIF-2α (25). Furthermore, mutations in a conserved PP1-binding motif in ICP34.5 that abolished PP1 binding also attenuate HSV-1 infection (10). These studies highlighted the importance of an ICP34.5/PP1 complex in promoting eIF-2α dephosphorylation and facilitating viral replication. As mammalian GADD34 shares sequence homology with the C terminus of ICP34.5 and can substitute for these viral sequences to maintain ICP34.5 function and facilitate HSV-1 infection (24), this suggests that mammalian GADD34 can also assemble an eIF-2α phosphatase. This was confirmed by in vitro studies showing that mammalian GADD34 bound PP1 and promoted eIF-2α dephosphorylation (15, 36). Interestingly, GADD34 mRNA levels were increased following activation of the eIF-2α kinases PERK and GCN2, and the overexpression of GADD34 has been shown to attenuate the UPR (36). These studies suggested that the eIF-2α phosphatase assembled by GADD34 functions in a negative feedback loop to facilitate the recovery of cells from UPR.
Both PP1 and PP2A can modulate the phosphorylation of eIF-2α in mammalian cells by either directly dephosphorylating eIF-2α or inactivating eIF-2α kinases (8, 18, 42, 50, 52). We demonstrated that the two cell-permeable PP1/PP2A inhibitors, OA and CA, both induced eIF-2α phosphorylation in mammalian cells but only eIF-2α phosphorylation elicited by OA was effectively reversed by expression of GADD34. The inability of GADD34 to override the effects of the more potent PP1 inhibitor CA strongly supported a role for PP1 in the GADD34-mediated assembly of a cellular eIF-2α phosphatase (4, 18, 52).
Pharmacological agents, such as thapsigargin and tunicamycin, routinely used to induce eIF-2α phosphorylation in mammalian cells also induce endogenous UPR and apoptotic responses (41). By comparison, short-term (30 to 60 min) treatment of cells with OA induced 10- to 15-fold-higher eIF-2α phosphorylation (without eliciting UPR or apoptosis) than treatment with either thapsigargin or tunicamycin. This provided a simple and reproducible cell-based assay for analyzing GADD34-mediated eIF-2α dephosphorylation in mammalian cells. Using this assay, we established a clear link between PP1 binding at the C-terminal sequences conserved in ICP34.5 and the dephosphorylation of eIF-2α. In addition, we showed that the KVRF consensus PP1-binding motif was necessary but not sufficient for PP1 binding and identified a C-terminal RARA motif that was also required for GADD34 association with PP1. While this motif is conserved in all mammalian and insect GADD34 proteins as well as in HSV ICP34.5, it is significantly modified in the GADD34-related proteins from African swine fever virus, amsacta moorei entomopoxvirus, and macropodid herpesvirus, suggesting that viral GADD34-related proteins have evolved other mechanisms for stabilizing PP1 association. This was consistent with the finding that the RARA sequence in HSV-1 ICP34.5 was not required for PP1 binding but an adjacent stretch (residues 253 and 258) containing an alanine-arginine pair was necessary to assemble a high-molecular-weight eIF-2α phosphatase complex (11). Our studies examined the comparable residues in human GADD34, particularly arginine-618 (Fig. 4), and suggested that this region strengthens PP1 binding. Together, the studies established that the PP1-binding regions conserved in mammalian and viral GADD34-related proteins may function differently to control eIF-2α dephosphorylation.
Earlier structure-function studies showed that, despite possessing equivalent eIF-2α phosphatase activity in vitro, WT mouse GADD34(1-657) was less effective than the truncated GADD34(241-657) in suppressing tunicamycin-induced expression of the UPR marker CHOP (36). This prompted the speculation that WT GADD34, which showed a distinct perinuclear localization, was sequestered at an inactive site by its N-terminal sequences. In contrast, our cell-based studies showed that WT human GADD34(1-674) produced the most effective eIF-2α phosphatase (Fig. 3B and 4C). Additionally, we showed that the N-terminally truncated GADD34(513-674) bound PP1 similarly to WT GADD34 but failed to dephosphorylate eIF-2α in cells (Fig. 4). This contrasted with earlier studies in which a similar fragment from mouse GADD34(485-657) demonstrated eIF-2α phosphatase activity equivalent to that of WT GADD34 in vitro (36). Notably, these earlier studies monitored eIF-2α dephosphorylation in vitro using lysates of cells expressing GADD34 and thus likely ignored potential contributions of subcellular localization to GADD34 function. In this regard, our in vivo studies showed that WT human GADD34(1-674) was localized at the ER and generated an effective eIF-2α phosphatase but that GADD34(513-674) was confined to the nucleus and was ineffective in promoting eIF-2α dephosphorylation. On the other hand, differences were seen in the abilities of some mutant GADD34 proteins to facilitate eIF-2α dephosphorylation (this study) and suppress UPR-induced CHOP expression (35), potentially hinting at additional functions for GADD34, other than the control of eIF-2α, during ER stress signaling in mammalian cells.
Our analysis of PP1α in mammalian cells showed its enhanced localization at the ER following ER stress. A similar but more pronounced redistribution of cellular PP1α was observed upon overexpression of WT GADD34, which targeted PP1α to the ER to generate an active eIF-2α phosphatase. These data support a role of GADD34 as a PP1 regulatory subunit that directs substrate recognition and the appropriate localization of PP1, specifically following ER stress. Notably, immunocytochemistry of endogenous GADD34 also suggested that it exists in the nucleus in addition to the ER. While our studies failed to demonstrate a nuclear localization for WT GFP-GADD34(1-674), it is interesting that GFP-GADD34(180-674) and particularly GADD34(513-674), which lack N-terminal sequences directing ER localization, showed nuclear localization in several cell lines. These proteins encompassed a potential nuclear localization sequence identified in ICP34.5 that contributes to its nuclear cytoplasmic shuttling in mammalian cells (9). Whether a similar mechanism targets GADD34 to nuclei remains unknown. The broad subcellular distribution of the viral ICP34.5 protein and the more discrete ER localization of mammalian GADD34 raise the intriguing possibility that while both proteins assemble an eIF-2α phosphatase, they regulate distinct pools of eIF-2α and thereby promote synthesis of different proteins that account for their functional differences in mammalian cells.
Physiological signals regulate both eIF-2α kinases and phosphatases (6, 15, 22, 32, 40). Previous studies suggested that the assembly and disassembly of a cellular eIF-2α phosphatase, composed of PP1 and GADD34 (15, 36), is regulated in mammalian tissues (15). GADD34 also binds the PKA-activated PP1 regulator, inhibitor 1 (15), potentially generating a heterotrimeric complex that can regulate protein synthesis in response to hormones that elevate cyclic AMP. GADD34 also undergoes covalent modification by an associated protein tyrosine kinase, Lyn, resulting in suppression of its apoptotic activity (19). Notably, this apoptotic activity of GADD34 shows the same requirement for the N- and C-terminal sequences detailed in this study as required for assembly of an ER-localized eIF-2α phosphatase (1, 53). This raises the intriguing possibility that ER localization and eIF-2α phosphatase activity, shown in this study to be directed by the N- and C-terminal domains, respectively, may also be required for the apoptotic function of GADD34. Finally, GADD34-binding proteins such as SNF5/INI1, a nuclear protein involved in chromatin remodeling, stimulate the activity of GADD34-bound PP1 (53). Thus, the structure-function studies described above provide a solid foundation for investigating the cellular mechanisms that regulate the GADD34/PP1 complex and control protein translation in mammalian cells.
Acknowledgments
This work was supported by the National Institutes of Health award RO1-DK52054 (to S.S.). D.C.W. is supported by a Department of Defense breast cancer predoctoral training fellowship, DAMD17-02-1-0378.
We thank Christopher Nicchitta and Anshu Jain for critical reagents and assistance in some experiments and Susan Walsh for helpful discussion of the manuscript.
REFERENCES
- 1.Adler, H. T., R. Chinery, D. Y. Wu, S. J. Kussick, J. M. Payne, A. J. Fornace, Jr., and D. C. Tkachuk. 1999. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol. Cell. Biol. 19:7050-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggen, J. B., A. C. Nairn, and R. Chamberlin. 2000. Regulation of protein phosphatase-1. Chem. Biol. 7:R13-R23. [DOI] [PubMed] [Google Scholar]
- 3.Andreassen, P. R., F. B. Lacroix, E. Villa-Moruzzi, and R. L. Margolis. 1998. Differential subcellular localization of protein phosphatase-1 α, γ1, and δ isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 141:1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu, S. V., and K. V. Ramaiah. 1996. Type 1 phosphatase inhibitors reduce the restoration of guanine nucleotide exchange activity of eukaryotic initiation factor 2B inhibited reticulocyte lysates rescued by hemin. Arch. Biochem. Biophys. 327:201-208. [DOI] [PubMed] [Google Scholar]
- 5.Bollen, M. 2001. Combinatorial control of protein phosphatase-1. Trends Biochem. Sci. 26:426-431. [DOI] [PubMed] [Google Scholar]
- 6.Brostrom, C. O., and M. A. Brostrom. 1998. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 58:79-125. [DOI] [PubMed] [Google Scholar]
- 7.Campos, M., P. Fadden, G. Alms, Z. Qian, and T. A. Haystead. 1996. Identification of protein phosphatase-1-binding proteins by microcystin-biotin affinity chromatography. J. Biol. Chem. 271:28478-28484. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S. C., G. Kramer, and B. Hardesty. 1989. Isolation and partial characterization of an Mr 60,000 subunit of a type 2A phosphatase from rabbit reticulocytes. J. Biol. Chem. 264:7267-7275. [PubMed] [Google Scholar]
- 9.Cheng, G., M.-E. Brett, and B. He. 2002. Signals that dictate nuclear, nucleolar, and cytoplasmic shuttling of the γ134.5 protein of herpes simplex virus type 1. J. Virol. 76:9434-9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, G., M. E. Brett, and B. He. 2001. Val193 and Phe195 of the gamma 1 34.5 protein of herpes simplex virus 1 are required for viral resistance to interferon-alpha/beta. Virology 290:115-120. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, G., M. Gross, M.-E. Brett, and B. He. 2001. AlaArg motif in the carboxyl terminus of the γ134.5 protein of herpes simplex virus type 1 is required for the formation of a high-molecular-weight complex that dephosphorylates eIF-2α. J. Virol. 75:3666-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, J., and B. Roizman. 1994. Herpes simplex virus 1 γ134.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc. Natl. Acad. Sci. USA 91:5247-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens, M. J. 1997. PKR—a protein kinase regulated by double-stranded RNA. Int. J. Biochem. Cell Biol. 29:945-949. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, P. T. 2002. Protein phosphatase 1—targeted in many directions. J. Cell Sci. 115:241-256. [DOI] [PubMed] [Google Scholar]
- 15.Connor, J. H., D. C. Weiser, S. Li, J. M. Hallenbeck, and S. Shenolikar. 2001. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell. Biol. 21:6841-6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Cruz e Silva, E. F., C. A. Fox, C. C. Ouimet, E. Gustafson, S. J. Watson, and P. Greengard. 1995. Differential expression of protein phosphatase 1 isoforms in mammalian brain. J. Neurosci. 15:3375-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, J., H. Harding, B. Raught, A. Gingras, J. Berlanga, D. Scheuner, R. Kaufman, D. Ron, and N. Sonenberg. 2002. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr. Biol. 12:1279-1286. [DOI] [PubMed] [Google Scholar]
- 18.Ernst, V., D. H. Levin, J. G. Foulkes, and I. M. London. 1982. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc. Natl. Acad. Sci. USA 79:7092-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grishin, A. V., O. Azhipa, I. Semenov, and S. J. Corey. 2001. Interaction between growth arrest-DNA damage protein 34 and Src kinase Lyn negatively regulates genotoxic apoptosis. Proc. Natl. Acad. Sci. USA 98:10172-10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, V., A. K. Ogawa, X. Du, K. N. Houk, and R. W. Armstrong. 1997. A model for binding of structurally diverse natural product inhibitors of protein phosphatases PP1 and PP2A. J. Med. Chem. 40:3199-3206. [DOI] [PubMed] [Google Scholar]
- 21.Harding, H. P., I. I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 22.Harding, H. P., Y. Zhang, A. Bertolotti, H. Zeng, and D. Ron. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5:897-904. [DOI] [PubMed] [Google Scholar]
- 23.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271-274. [DOI] [PubMed] [Google Scholar]
- 24.He, B., J. Chou, D. A. Liebermann, B. Hoffman, and B. Roizman. 1996. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the γ134.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J. Virol. 70:84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He, B., M. Gross, and B. Roizman. 1998. The γ134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 27.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 28.Hollander, M. C., M. S. Sheikh, K. Yu, Q. Zhan, M. Iglesias, C. Woodworth, and A. J. Fornace, Jr. 2001. Activation of Gadd34 by diverse apoptotic signals and suppression of its growth inhibitory effects by apoptotic inhibitors. Int. J. Cancer. 96:22-31. [DOI] [PubMed] [Google Scholar]
- 29.Hollander, M. C., Q. Zhan, I. Bae, and A. J. Fornace, Jr. 1997. Mammalian GADD34, an apoptosis- and DNA damage-inducible gene. J. Biol. Chem. 272:13731-13737. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman, R. J. 1999. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13:1211-1233. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman, R. J., D. Scheuner, M. Schroder, X. Shen, K. Lee, C. Y. Liu, and S. M. Arnold. 2002. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3:411-421. [DOI] [PubMed] [Google Scholar]
- 32.Kimball, S. R., D. A. Antonetti, R. M. Brawley, and L. S. Jefferson. 1991. Mechanism of inhibition of peptide chain initiation by amino acid deprivation in perfused rat liver. Regulation involving inhibition of eukaryotic initiation factor 2 alpha phosphatase activity. J. Biol. Chem. 266:1969-1976. [PubMed] [Google Scholar]
- 33.Mao, H., and K. S. Rosenthal. 2002. An N-terminal arginine-rich cluster and a proline-alanine-threonine repeat region determine the cellular localization of the herpes simplex virus type 1 ICP34.5 protein and its ligand, protein phosphatase 1. J. Biol. Chem. 277:11423-11431. [DOI] [PubMed] [Google Scholar]
- 34.Migliaccio, G., C. V. Nicchitta, and G. Blobel. 1992. The signal sequence receptor, unlike the signal recognition particle receptor, is not essential for protein translocation. J. Cell Biol. 117:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munoz, F., M. E. Martin, J. Manso-Tomico, J. Berlanga, M. Salinas, and J. L. Fando. 2000. Ischemia-induced phosphorylation of initiation factor 2 in differentiated PC12 cells: role for initiation factor 2 phosphatase. J. Neurochem. 75:2335-2345. [DOI] [PubMed] [Google Scholar]
- 36.Novoa, I., H. Zeng, H. P. Harding, and D. Ron. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153:1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver, C. J., R. T. Terry-Lorenzo, E. Elliott, W. A. Christensen Bloomer, S. Li, D. L. Brautigan, R. J. Colbran, and S. Shenolikar. 2002. Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology. Mol. Cell. Biol. 22:4690-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouimet, C. C., E. F. da Cruz e Silva, and P. Greengard. 1995. The alpha and gamma 1 isoforms of protein phosphatase 1 are highly and specifically concentrated in dendritic spines. Proc. Natl. Acad. Sci. USA 92:3396-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oyadomari, S., E. Araki, and M. Mori. 2002. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis 7:335-345. [DOI] [PubMed] [Google Scholar]
- 40.Pain, V. M. 1996. Initiation of protein synthesis in eukaryotic cells. Eur. J. Biochem. 236:747-771. [DOI] [PubMed] [Google Scholar]
- 41.Patil, C., and P. Walter. 2001. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13:349-355. [DOI] [PubMed] [Google Scholar]
- 42.Petryshyn, R., D. H. Levin, and I. M. London. 1982. Regulation of double-stranded RNA-activated eukaryotic initiation factor 2 alpha kinase by type 2 protein phosphatase in reticulocyte lysates. Proc. Natl. Acad. Sci. USA 79:6512-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pittman, R. N., S. Wang, A. J. DiBenedetto, and J. C. Mills. 1993. A system for characterizing cellular and molecular events in programmed neuronal cell death. J. Neurosci. 13:3669-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prostko, C. R., M. A. Brostrom, and C. O. Brostrom. 1993. Reversible phosphorylation of eukaryotic initiation factor 2 alpha in response to endoplasmic reticular signaling. Mol. Cell. Biochem. 127-128:255-265. [DOI] [PubMed] [Google Scholar]
- 45.Prostko, C. R., J. N. Dholakia, M. A. Brostrom, and C. O. Brostrom. 1995. Activation of the double-stranded RNA-regulated protein kinase by depletion of endoplasmic reticular calcium stores. J. Biol. Chem. 270:6211-6215. [DOI] [PubMed] [Google Scholar]
- 46.Shenolikar, S. 1994. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu. Rev. Cell Biol. 10:55-86. [DOI] [PubMed] [Google Scholar]
- 47.Sood, R., A. C. Porter, K. Ma, L. A. Quilliam, and R. C. Wek. 2000. Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346:281-293. [PMC free article] [PubMed] [Google Scholar]
- 48.Strack, S., M. A. Barban, B. E. Wadzinski, and R. J. Colbran. 1997. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 68:2119-2128. [DOI] [PubMed] [Google Scholar]
- 49.Szyszka, R., W. Kudlicki, G. Kramer, B. Hardesty, J. Galabru, and A. Hovanessian. 1989. A type 1 phosphoprotein phosphatase active with phosphorylated Mr = 68,000 initiation factor 2 kinase. J. Biol. Chem. 264:3827-3831. [PubMed] [Google Scholar]
- 50.Tan, S. L., S. U. Tareen, M. W. Melville, C. M. Blakely, and M. G. Katze. 2002. The direct binding of the catalytic subunit of protein phosphatase 1 to the PKR protein kinase is necessary but not sufficient for inactivation and disruption of enzyme dimer formation. J. Biol. Chem. 277:36109-36117. [DOI] [PubMed] [Google Scholar]
- 51.Trinkle-Mulcahy, L., J. E. Sleeman, and A. I. Lamond. 2001. Dynamic targeting of protein phosphatase 1 within the nuclei of living mammalian cells. J. Cell Sci. 114:4219-4228. [DOI] [PubMed] [Google Scholar]
- 52.Wek, R. C., J. F. Cannon, T. E. Dever, and A. G. Hinnebusch. 1992. Truncated protein phosphatase GLC7 restores translational activation of GCN4 expression in yeast mutants defective for the eIF-2 alpha kinase GCN2. Mol. Cell. Biol. 12:5700-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, D. Y., D. C. Tkachuck, R. S. Roberson, and W. H. Schubach. 2002. The human SNF5/INI1 protein facilitates the function of the growth arrest and DNA damage-inducible protein (GADD34) and modulates GADD34-bound protein phosphatase-1 activity. J. Biol. Chem. 277:27706-27715. [DOI] [PubMed] [Google Scholar]
- 54.Zhan, Q., K. A. Lord, I. Alamo, Jr., M. C. Hollander, F. Carrier, D. Ron, K. W. Kohn, B. Hoffman, D. A. Liebermann, and A. J. Fornace, Jr. 1994. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 14:2361-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]